Open Access Article
Open Access ArticleEvaluation of fluorine and sulfonic acid co-functionalized graphene oxide membranes under hydrogen proton exchange membrane fuel cell conditions†
Robin
Sandström
 a,
Alagappan
Annamalai
a,
Alagappan
Annamalai
 a,
Nicolas
Boulanger
a,
Joakim
Ekspong
a,
Alexandr
Talyzin
a,
Nicolas
Boulanger
a,
Joakim
Ekspong
a,
Alexandr
Talyzin
 a,
Inge
Mühlbacher
b and
Thomas
Wågberg
a,
Inge
Mühlbacher
b and
Thomas
Wågberg
 *a
*a
aDepartment of Physics, Umeå University, Umeå 90187, Sweden. E-mail: thomas.wagberg@umu.se
bPolymer Competence Center Leoben GmbH PCCL, Leoben 8700, Austria
First published on 8th May 2019
Abstract
The use of graphene oxide (GO) based membranes consisting of self-assembled flakes with a lamellar structure represents an intriguing strategy to spatially separate reactants while facilitating proton transport in proton exchange membranes (PEMs). Here we chemically modify GO to evaluate the effect of fluorine and sulfonic acid groups on the performance of H2/O2 based PEM fuel cells. Mild fluorination is achieved in the presence of hydrogen fluoride during oxidation and subsequent sulfonation resulted in fluorine and SO3− co-functionalized GO. Membrane electrode assembly performance under low temperature and moderate humidity conditions suggested that both functional groups contribute to reduced H2 crossover compared to appropriate reference membranes. Moreover, fluorine groups promoted an enhanced hydrolytic stability while contributing to the prevention of structural degradation after constant potential experiments, whereas sulfonic acid exerted a stabilizing effect by preserving proton conductivity.
1. Introduction
Research and development in proton exchange membrane fuel cells (PEMFCs) is well motivated by the need for fossil-free transport solutions in order to address rapidly growing concerns about climate change. The main components in a PEMFC constitute the so-called membrane electrode assembly (MEA) which is constructed from three main components: (i) a diffusion layer with appropriately tuned wettability, (ii) an active layer made of a high surface area Pt catalyst and (iii) a proton conducting membrane. Each component is based on advanced materials with suitable properties, well-matched to coexist in a MEA operating under harsh conditions. The compatibility between each component as well as the fabrication process of the MEA is therefore crucial to the final device performance.The most common PEMFC fuel is hydrogen, but alternatives including formic acid (direct formic acid fuel cells, DFAFCs)1 or methanol (direct methanol fuel cells, DMFCs)2 are promising for portable applications, demonstrating a high flexibility of the proton exchange membrane (PEM) devices. In fact, PEM technology can even be used for reverse reaction, such as the production of hydrogen through electrolysis.3,4 Often, the research and development of new MEA components of a particular PEM configuration can stimulate advances in other systems utilizing the PEM concept.
Large efforts have been devoted to the field of PEMFCs, particularly to the sluggish oxygen reduction reaction (ORR) that greatly hampers the overall cell performance.5–7 The solid membrane, acting as both an electrolyte and a separator of reactants, is however also a bottleneck with room for improvement. Requirements on the PEM are demanding, where crucial material properties include, but are not limited to, (i) chemical inertness, (ii) low permeation of reactants, (iii) proton conduction, (iv) electrical insulation, (v) stable operation in desired temperature ranges, (vi) hydrolytic stability and (vii) fabrication with low-cost environmentally sound production methods.8 So far, sulfonated polytetrafluoroethylene (Nafion®), developed in the late 1960's, is preferred under the majority of PEMFC operating conditions owing to a fine balance between the above-mentioned attributes.9,10 However, issues including high cost, high liquid fuel permeability rates (e.g. methanol and formic acid) and poor proton conductivity at high temperatures (>80 °C) and low humidity has led researchers to explore alternatives.
Perfluorinated sulfonic acid (PFSA) materials (like Nafion) obtain their chemical and physical stability mainly from the polytetrafluoroethylene (PTFE) backbone, while the channels of sulfonic acid provide charged sites to facilitate ionic transport. Consequently, polymeric alternatives such as aromatic thermoplastic poly(ether ether ketone) (PEEK) can be modified by the attachment of sulfonic acid groups (SPEEK) to enhance the proton conductivity.11,12 In addition, fluorination of this polymer (F-SPEEK) has been employed for enhanced gas separation.13,14 Inan et al.15 also produced SPEEK/poly vinylidine fluoride polymeric blends and demonstrated improved chemical stability and methanol separation properties, to the cost of lowered water uptake and proton conductivity with increased fluorocarbon content. Other examples of promising polymer membranes currently under development include poly(arylene ether sulfone) derivatives16–18 as well as multi-block polymers,19–21 where chemical modifications such as sulfonation are also considered.
As an alternative to polymer electrolytes, graphene oxide (GO) consists of two-dimensional flakes of few-layered oxidized carbon sheets and can be filtered from its colloidal dispersion into paper-like membranes with an ordered lamellar structure.22 GO is synthesized from abundant natural graphite flakes and thereby considered as an attractive low-cost option for PEMs containing inter-flake channels rich of negatively charged functionalities for ionic transport. Innovative procedures employed to use such structures as PEMs include laminating a thin reactant barrier to Nafion,23–25 Nafion or SPEEK polymeric composites for interconnecting proton conducting channels26–30 and as freestanding membranes.31,32 Recently, Bayer et al.33 used GO dispersions combined with a spray methodology to directly cover a gas diffusion electrode for MEA fabrication establishing a lamellar catalyst coated membrane structure. At a membrane thickness of 3 μm, a maximum power output of ∼80 mW cm−2 could be measured in an H2/air PEMFC, demonstrating that assembling such membranes can be industrially favorable and not limited to filtration methods.
As for polymers, sulfonation of GO membranes is also beneficial for proton transport.34,35 Fluorinated graphene derivatives on the other hand, despite their diverse and intriguing qualities, have been scarcely implemented and tested in practical applications. In fact, to the best of our knowledge, no functionalities of this sort have ever been explored for GO-based PEM applications. The main characteristics of F-modified graphene which motivate its use in PEMFCs include PTFE-like chemical inertness, electrical insulation, thermal stability and tunable wetting characteristics.36–40
Herein, fluorinated and sulfonated co-functionalized graphene oxide membranes were prepared by employing a novel method resulting in a low concentration (∼0.5%) of both fluorine and SO3− groups which was shown to have a major influence on the wetting characteristics. Fluorination also demonstrated improved hydrolytic stability and lowered H2 crossover and had a positive role in preserving the lamellar structure under PEMFC conditions, while SO3− functionalization was necessary to maintain proton conductivity. Altogether we show that GO-derived two-dimensional PFSA equivalent membranes represent a compelling prospect for PEM applications.
2. Experimental methods
2.1. Synthesis of graphene oxide
By far, the most frequently used methods in the literature to prepare graphene oxide are slightly different varieties of the so-called “modified Hummers' method”.41,42 This process commonly involves a pre-oxidation procedure of graphite with K2S2O8 and P4O10 in concentrated H2SO4, in order to facilitate improved graphite oxidation in the subsequent main oxidation step (see the ESI† for details). Such pre-oxidized graphite was used for increasing the yield of fluorinated graphene oxide, which was based on “Tours” graphene oxide, but commonly also known in the literature as the “Improved method”.430.5 g of the pre-oxidized graphite powder was placed in a Teflon beaker and dispersed in H2SO4 and H3PO4 (85%) at a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Then 10 g of ammonium fluoride (NH4F) was slowly added under stirring and placed on a hotplate heated to 60 °C (solution temperature approximately 53–54 °C). After 2 h, 2.25 g of potassium permanganate was added and the dispersion was then maintained for 12 h. The mixture was slowly diluted with 0 °C ice-cold (H2O)DI (water) while surrounded by an ice-bath, followed by addition of 1.5 ml H2O2 (30%). The white/yellow dispersion was then separated into falcon tubes and washed repeatedly (4× each) with 10% HCl, (H2O)DI and finally ethanol (99.5%). The resulting alco-gel was spread out on a Petri-dish and left to dry overnight in a well-ventilated ambient atmosphere. In addition to the fluorinated GO (denoted as F-TGO), it was deemed necessary to prepare reference samples based on the same method where NH4F was omitted (TGO*) and another one where the pre-oxidation step was also omitted (TGO). Moreover, another reference based on the modified Hummers' method (HGO) was prepared, described in detail in the ESI.† Dispersions were produced by re-dispersion of the obtained powder into (H2O)DI (0.5 mg ml−1) by 1 h sonication and centrifugation (6000 rpm) for 1 h to remove small amounts of larger un-exfoliated pieces. These dispersions were then vacuum filtered (Whatman®, alumina, 47 mm, 0.2 μm pore size) until a dry membrane could be peeled off from the filter. All membranes were stored in a desiccator (ambient temperature) and allowed to dry for at least two weeks before they were used for characterization. Schematic illustration of the entire SF-TGO fabrication can be found in Fig. 1.
1 ratio. Then 10 g of ammonium fluoride (NH4F) was slowly added under stirring and placed on a hotplate heated to 60 °C (solution temperature approximately 53–54 °C). After 2 h, 2.25 g of potassium permanganate was added and the dispersion was then maintained for 12 h. The mixture was slowly diluted with 0 °C ice-cold (H2O)DI (water) while surrounded by an ice-bath, followed by addition of 1.5 ml H2O2 (30%). The white/yellow dispersion was then separated into falcon tubes and washed repeatedly (4× each) with 10% HCl, (H2O)DI and finally ethanol (99.5%). The resulting alco-gel was spread out on a Petri-dish and left to dry overnight in a well-ventilated ambient atmosphere. In addition to the fluorinated GO (denoted as F-TGO), it was deemed necessary to prepare reference samples based on the same method where NH4F was omitted (TGO*) and another one where the pre-oxidation step was also omitted (TGO). Moreover, another reference based on the modified Hummers' method (HGO) was prepared, described in detail in the ESI.† Dispersions were produced by re-dispersion of the obtained powder into (H2O)DI (0.5 mg ml−1) by 1 h sonication and centrifugation (6000 rpm) for 1 h to remove small amounts of larger un-exfoliated pieces. These dispersions were then vacuum filtered (Whatman®, alumina, 47 mm, 0.2 μm pore size) until a dry membrane could be peeled off from the filter. All membranes were stored in a desiccator (ambient temperature) and allowed to dry for at least two weeks before they were used for characterization. Schematic illustration of the entire SF-TGO fabrication can be found in Fig. 1.
F-TGO was, for reasons discussed in the Results section, selected for sulfonation with the aryl diazonium salt of sulfanilic acid.44 First, 200 mg of sulfanilic acid was dissolved in a basic solution containing 58 mg sodium carbonate (Na2CO3) in 2.5 ml (H2O)DI by brief heating on a 100 °C hotplate until completely dissolved. Once the mixture reached room temperature, 75 mg sodium nitrite (NaNO2) was added. The prepared solution was then added dropwise into an acid solution containing 1.6 ml H2O and 0.25 ml HCl (35%) in an ice-bath under vigorous stirring. The mixture was kept under stirring for 15 min to ensure complete reaction, after which a finely divided white precipitate was observed. Sulfonation was then achieved by dropwise addition of the prepared precursor into the partially reduced GO dispersion under stirring in an ice bath. After a reaction time of 4 h, the remaining salts were removed by dialysis (3.5 kDa MWCO) for one week. The dispersion was used directly to fabricate membranes with the filtration procedure described above, henceforth denoted as SF-TGO.
2.2. Physical characterization
Thermogravimetric data were collected by using a METTLER Toledo TGA/DSC 1 setup under an N2 flow of 40 ml min−1 at a heating rate of 10 K min−1 from 30 °C to 900 °C. X-ray diffraction (XRD) was performed on membranes in both dry and wet states (H2O soaked), covered and immobilized with plastic foil, with a Panalytical X'Pert3 Powder diffractometer with a CuKα (λ = 1.5406 Å) source. XRD patterns were all intensity normalized. X-ray photoelectron spectroscopy (XPS) was performed by using a Kratos Axis Ultra DLD electron spectrometer using a 150 W monochromatic AlKα source. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra were recorded by using a Vertex 80 (Bruker), where membranes were firmly pressed against a germanium (Ge) ATR crystal. All FTIR spectra were normalized with respect to the peak centered around ∼1600 cm−1 likely corresponding to a combination of un-oxidized sp2 domains and adsorbed water.45,46 Contact angle measurements were carried out in order to estimate the hydrophobicity of the GO membranes. Small individual droplets were placed on the dry membranes and measured with an optical tensiometer from Biolin Scientific. The angles were fitted by the Young Laplace method using Attension software.2.3. Membrane electrode assembly fabrication and characterization
Hydrogen crossover measurements were performed in realistic low temperature fuel cell environments at moderate relative humidity (75% RH). The as-produced membranes were carefully assembled (free-standing) together with gas diffusion electrodes (GDEs) acquired from Fuel Cell Store Inc. (0.2 mgPt cm2, 20% Pt on Vulcan, carbon cloth). The anodes and cathodes were fed with H2 (100 ml min−1) and N2 (1000 ml min−1) respectively and the potential was swept from 0.05 V to 0.5 V vs. RE/CE (anodic ref.) with a scan rate of 50 mV s−1 using an 885 fuel cell potentiostat connected to an 850e fuel cell test station (Scribner). The measurements were performed at various temperatures ranging from 30 °C up to 70 °C with 10 °C intervals. Each LSV was recorded at least once ensuring representable curves with stable crossover-current. Stable LSVs could be achieved on all samples apart from TGO* (all temperatures) and HGO at 70 °C.Fuel cell polarization curves in an H2/O2 configuration were acquired in a non-freestanding state by assembling the MEA with a Nafion 211 membrane (N211) with an MEA area of 5 cm2 (see the ESI for the membrane pretreatment protocol and Fig. S7† for a more detailed illustration of the MEA). The membranes were sandwiched facing the anode GDE followed by N211 and finally the cathode GDE was sealed with a combination of Teflonized fiberglass and silicone rubber gaskets in a quickCONNECT cell fixture (Baltic FuelCells corp.) and connected to an 850e fuel cell test station (Scribner). All measurements were performed at 40 °C and 75% RH with an H2 and O2 flow of 100 ml min−1 at the anode and cathode respectively. Moderate humidity was chosen as a compromise to not cause the GO membranes to become too fragile22,47,48 and at the same time providing sufficient H2O supply to promote proton conductivity particularly in the Nafion components (catalyst layer and membrane) in the MEA. Owing to the diverse properties of the membranes, multiple polarization curves were recorded at different time periods of FC operation in order to get representative data of membrane behavior. First, a curve was recorded as soon as the open circuit potential (OCP) was stabilized (i) followed by another after mild conditioning for 1 h at 0.6 V (ii), a third curve after an additional 8 h at 0.5 V (iii) and lastly a final one after another 8 h at 0.5 V (iv). All curves were recorded with 25 mA intervals until reaching 0.3 V with a stabilization time of 30 s per data point.
3. Results
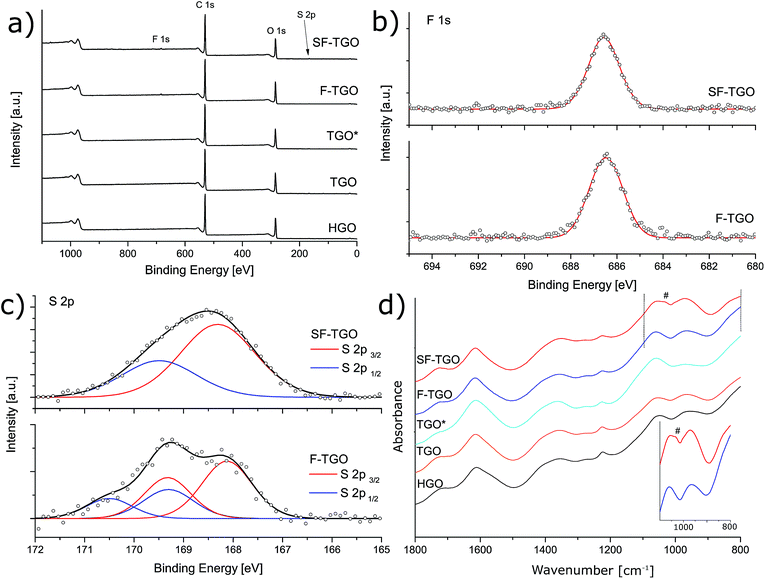
Fig. S1† depicts dispersions produced directly after the synthesis of fluorinated (F-TGO) and sulfonated/fluorinated (SF-TGO) GO as well as the three reference membranes (TGO, TGO* and HGO). A notably different visual appearance is seen for F-TGO in comparison to the reference samples. The F-TGO membranes produced via filtration were immediately noted to be significantly more stable in neutral H2O than all the other reference materials (see Fig. S2†), and could even preserve this property after being sulfonated. The property of high structural stability under high humidity conditions is desired for all MEA components within the PEMFC. The thicknesses of all membranes as measured by a micrometer tool ranged from 7 to 9 μm (Table S1†), while it was also noted that here the handling of F-TGO and SF-TGO without breakage was notably easier, suggesting enhanced mechanical robustness. This is also supported by a significantly enhanced flexibility as a result of fluorination, as shown in bend radius measurements illustrated in Fig. S3.†Thermogravimetric curves recorded under a N2 atmosphere of respective synthesized GO powder and the resulting membranes are shown in Fig. 2a and b respectively. The TGA profile of GO can be divided into three parts: desorption of surface water at T < ∼200 °C, explosive exfoliation and release of steam and CO2 around 200 °C and continued reduction of more firmly attached functional groups defined by a more shallow slope43 at T > ∼200 °C. Here, all as-synthesized GO powders followed such a characteristic trend for graphene oxide while F-TGO has a slightly higher exfoliation temperature by about 30 °C, indicating that the addition of NH4F might have altered the inter-flake attractions. The same TGA experiments performed after vacuum filtration into membranes show similar trends (Fig. 2b), albeit with slightly upshifted exfoliation temperatures (∼10–20 °C), likely related to the strength of the interlayer interactions formed during self-assembly. X-ray diffraction was performed under both dry and wet conditions on the prepared membranes as shown in Fig. 1c. In the dry phase, F-TGO had a slight increase in the interlayer distance signified by the C(002) reflection (2θ = 10.3°) compared to that of HGO and TGO (2θ = 10.9°), which can be attributed to a more repulsive effect of the F functionalities.49 Sulfonation shifted the C(002) peaks to higher angles (10.8 °C) indicating attractive interactions between the layers as a result of sulfonic acid.32 Although the interlayer spacing under dry conditions differs slightly (see Table S2†), the similar swelling behavior suggests that the maximum water uptake is nearly identical for all membranes (with the exception of TGO*), which is already documented for modified Hummers' GO.50 It is noteworthy however to recall that the stability of F-TGO and SF-TGO in the wet state is superior to that of HGO and TGO (Fig. S2†), despite the similar interlayer distances.
Comparable synthesis procedures have previously been suggested to produce F functional groups of a semi-ionic nature attached to GO flakes.39,49,51 In our case the binding energy of the F 1s band of 686.5 eV, as measured by X-ray photoelectron spectroscopy (Fig. 3a, b and Table S3†), may also suggest that such functionalities are present, where the atomic concentration of F is measured to be ∼0.5%. However, the presence of HF-type species cannot be entirely excluded owing to their similar binding energies to semi-ionic F.52 No significant changes in oxygen groups were detected (see the C 1s band in Table S3†) and the degree of oxidation was similar with a C/O ratio of 2.0–2.2, for all samples except for TGO* showing a C/O value as low as 1.7, indicating a severe over-oxidation of the latter sample also supported by TEM images showing damaged perforated flakes (Fig. S4†). As a result, TGO* based membranes were very fragile in comparison to other samples (although no change in the D/G ratio could be detected by Raman spectroscopy, Fig. S5†). The reason for avoiding over-oxidation in F-TGO can likely be attributed to the formation of ammonium hydrogen sulfate ((NH4)HSO4) in the H2SO4-rich mixture by the reaction
| NH4F + H4SO4 → (NH4)HSO4 + HF, | (1) |
F-TGO preserved the vast majority of F species throughout the whole sulfonation procedure, including thorough washing by dialysis, indicating a firm attachment of the F functionalities. Successful sulfonation is supported by a clear increase in the signal at 168.2 eV representing the S 2p3/2 core-level region,54 from 0.1–0.2% for the reference samples to nearly 0.6% after sulfonation (see Fig. 3c), thus providing a good estimate of the degree of sulfonation which is ∼0.5%. The addition of SO3− groups in the bulk membrane is further manifested by an increase in ion-exchange capacity (IEC) from ∼1.2 mmol g−1 (F-TGO) to ∼1.5 mmol g−1 (SF-TGO), as measured by the procedure shown in the ESI (eqn (S1)†). Sulfonation is also supported by ATR-FTIR (Fig. 3d) showing emerging characteristic vibrations of the disubstituted phenyl group at ∼1030 cm−1 corresponding to C–H in-plane bending accompanied by a shoulder near 840 cm−1 attributed to the out-of-plane wagging.44,55 Moreover, the FTIR spectra show signs of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (∼1720 cm−1), C–OH (∼1060 cm−1 & ∼1360 cm−1), epoxy or peroxide (∼970 cm−1) and epoxy C–O–C (∼1230 cm−1) without excluding the possibility of overlapped sulfate vibrations on the latter.45,46,56,57 The similarities in O-functional groups between the samples are thus clearly seen in the spectra, supporting the above C 1s XPS data (Table S3†). However, no clear signs of F species could be detected above the noise level by ATR-FTIR.
O (∼1720 cm−1), C–OH (∼1060 cm−1 & ∼1360 cm−1), epoxy or peroxide (∼970 cm−1) and epoxy C–O–C (∼1230 cm−1) without excluding the possibility of overlapped sulfate vibrations on the latter.45,46,56,57 The similarities in O-functional groups between the samples are thus clearly seen in the spectra, supporting the above C 1s XPS data (Table S3†). However, no clear signs of F species could be detected above the noise level by ATR-FTIR.
Contact angle measurements performed on the GO membranes are shown in Fig. 4. While all membranes prepared from conventional oxidation procedures showed contact angles of 91–95°, the F-TGO membrane was clearly more hydrophilic (75°) which is in line with previously reported studies of fluorinated graphene oxide.38,58 This phenomenon can be attributed to a lowered free surface energy at the membrane|H2O interface due to hydrogen bonding interactions between the H (in water) and the highly electronegative F atoms. Moreover, the addition of hydrophilic SO3− groups further lowered the contact angle to 40°. Zeta potential measurements were measured on the dispersions produced prior to the filtration step (Fig. S6†). Although measurement points with slightly lowered potentials in the pH region between 4 and 7 were detected for F-TGO and SF-TGO, hinting at greater flake-to-flake electrostatic repulsion, no firm conclusions can be drawn from the Zeta potential measurements owing to relatively high standard deviations.
 | ||
| Fig. 4 Contact angle measurements for wettability determination. Reference membranes (top row) all show higher hydrophobicity than the membranes prepared from chemically modified GO (bottom). | ||
The permeation of H2 through the proton exchange membrane (crossover) can be estimated directly in a single PEMFC by first feeding the cathode with an inert gas such as N2. Since the H2 flow remains at the anode, the potential can be linearly swept into a potential window where any molecular H2 present at the cathode is immediately oxidized.59,60Fig. 5a shows such a procedure performed on the free-standing as-synthesized GO membranes at 30 °C and 75% RH as well as a Nafion type 211 (N211) membrane for comparison purposes. Typically, a plateau is formed above ∼0.2 V corresponding to the rate of H2 permeation. However, whenever there is a linear increment of current with voltage, there is a sign of internal shorting, where the slope is directly related to the severity of the through-plane electrical short of the membrane. Taking this into consideration, both the crossover current near the hydrogen evolution onset at 0.2 V and the slopes formed between 0.3 and 0.5 V are presented in Fig. 5b and c respectively, for temperatures up to 70 °C, in order to represent in situ temperature dependent trends in membrane characteristics. TGO* was however significantly more fragile than the others and stable LSVs could not be recorded, presumably due to increased breakage in the compressed MEA. A similar effect was also observed for HGO at 70 °C, consistent with previously observed instability likely owing to partial reduction of Hummers' GO under PEMFC conditions with an onset near 70 °C.61 Interestingly, TGO did not have this problem, suggesting that the synthesis of GO through the Tours method is to be preferred if such conditions are required. Roughly, the H2 permeation rate below 50 °C followed a trend of TGO* > TGO ≈ HGO > F-TGO ≈ N211 > SF-TGO, while it is important to note that the commercial Nafion membrane (N211) has more than double the thickness (25 μm). These results are in line with the superior H2 blocking performance of GO membranes as observed previously,33 although they are most often measured under low-humidity conditions. Due to the severe internal short of some of the membranes as well as a fairly non-linear trend, especially at higher temperatures (possibly due to side-reactions), internal short correction for calculation of precise in situ permeability rates could not be performed. Considering that electrical short within the PEM is a contributing factor to poor performance and low OCP,62 reaching sufficient electrical insulation thus poses an additional challenge in developing GO membranes.
The performance in an H2/O2 configuration was evaluated and polarization curves after various operational time periods are shown in Fig. 6. Testing GO-based membranes under real fuel cell conditions has proven to be a challenge and typically the membranes can either be stabilized with a commercial one, laminated or used directly as a freestanding PEM but limited to a small cell area if the membrane is deemed durable enough. Here, the GO membranes, covering the whole 5 cm2 cell, are backed with a N211 facing the cathode protecting the direct contact of H2 with O2 in the case of pinhole formation, as illustrated in detail in Fig. S7.† In fact, brief experiments where freestanding membranes were used showed a sharp drop in the OCP down to ∼0 V at random points during operation, indicating a failure of barricading the H2 from the cathode in short to medium term operation under these conditions. This N211-backed MEA configuration was thus necessary in order to get a meaningful view of the temporal evolution of fuel cell performance, where the relative ohmic drops signify the differences in through-plane proton conductivity. As expected, the total proton conductivity due to the added GO membrane clearly decreased, as can be clearly seen by comparing the stable MEA performance of a single N211 membrane (Fig. 6f). Note however that TGO* was excluded from these measurements due to the extreme brittleness that caused it to break immediately when contacting the heavily humidified N211 membrane.
As shown in the polarization profiles in Fig. 6b–e with corresponding current vs. time curves presented in Fig. S8,† all membranes had diverse performances. Overall, the performance evolved from initially TGO < HGO ≈ SF-TGO < F-TGO into F-TGO ≈ HGO < SF-TGO < TGO after 1 h at 0.6 V plus an additional 16 h at 0.5 V. First, we note that sulfonation of F-TGO had a stabilizing effect as shown by the superior stability of SF-TGO, whereas F-TGO degraded at a similar rate to that of HGO. In contrast, the only membrane that showed an increase in performance with time was TGO. Coincidentally, as shown by the cross-sectional SEM micrographs in Fig. S10 and S11,† the only membrane with notable severe structural disruption to the laminar framework after FC operation was also TGO. These observations suggest that structural degradation of the GO membrane, reducing the protonic travel path, can enhance the performance in this MEA configuration, thus highlighting that caution must be exercised when interpreting results from membranes tested with similar cell designs. Nevertheless, the results advocate that the addition of fluorine groups prevented such structural degradation implying that the performance loss of F-TGO should be assigned to a degradation of a chemical nature. In addition, the open circuit potential can normally be used as an indicator of fuel crossover since usually H2 permeation is the main contributing factor to low OCP.63 However, as shown in ESI Fig. S9,† the majority of OCP values that were recorded prior to measuring polarization curves were lower than that measured from a single N211 membrane. This observation in combination with the lack of correlation to the measurements in Fig. 5 (at 40 °C and 75% RH) implies that the main cause of lowered OCP lies elsewhere. Overall, it can be argued that the preservation of structural integrity combined with a relatively slow decline in performance implies that SF-TGO would most likely be the best performer in hypothetical freestanding equivalent experiments.
4. Conclusions
In summary, we fabricated membranes entirely based on graphene oxide through a vacuum filtration method. Mild fluorination was successfully achieved by introducing HF species during the oxidation procedure, based on the Tours method, with NH4F as an F-precursor. Subsequent sulfonation resulted in a membrane with F and SO3− co-functionalized flakes as evidenced by XPS, ATR-FTIR and contact angle measurements. The F-groups prevented membrane re-dispersion in neutral H2O, thus demonstrating promising structural stability under high-humidity conditions (hydrolytic stability) despite showing similar water uptake to reference membranes.The role of fluorine and sulfonic acid groups attached to the GO flakes was evaluated in realistic fuel cell environments by comparing them with appropriate reference materials. At low temperatures and moderate humidity, diverse properties were detected in H2 crossover in situ measurements where both F and SO3− had a beneficial influence. Moreover, multiple polarization curves under low temperature H2/O2 conditions were recorded and evaluation of the results suggests that the F-groups prevented structural degradation that was observed in the membrane prepared from the conventional Tours method. Despite demonstrating relatively high initial performance, there was a rapid loss in the performance of the F-based membrane that was in addition shown to be alleviated by sulfonation. Further optimization work including achieving an appropriate concentration and balance of F and SO3− as well as selecting appropriate oxidation methods remains to be performed.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
T. W. acknowledges support from Vetenskapsrådet (2017-04862) Energimyndigheten (45419-1), Interreg Nord, Region Västerbotten, and Ångpanneföreningen (15-483). The authors acknowledge the Umeå Core Facility for Electron Microscopy (UCEM) at Umeå University, Chemical Biological Centre (KBC). Andrey Shchukarev at Umeå University, KBC, is further acknowledged for performing the XPS measurements. I. M. acknowledges the K-Project “PolyTherm” at the Polymer Competence Center Leoben GmbH (PCCL, Austria) within the framework of the COMET-program of the Federal Ministry for Transport, Innovation and Technology and Federal Ministry for Science, Research and Economy with contributions by the Graz University of Technology (Institute for Chemistry and Technology of Materials as well as the HRSM-Soft Matter Application Lab), the Montanuniversitaet Leoben, and the Delft University of Technology. Funding was provided by the Austrian Government and the State Government of Styria.References
- X. W. Yu and P. G. Pickup, J. Power Sources, 2008, 182, 124–132 CrossRef CAS.
- V. Neburchilov, J. Martin, H. J. Wang and J. J. Zhang, J. Power Sources, 2007, 169, 221–238 CrossRef CAS.
- D. Bessarabov, H. Wang, H. Li and N. Zhao, PEM Electrolysis for Hydrogen Production: Principles and Applications, CRC Press, 2016 Search PubMed.
- M. Carmo, D. L. Fritz, J. Merge and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Appl. Catal., B, 2005, 56, 9–35 CrossRef CAS.
- V. Mehta and J. S. Cooper, J. Power Sources, 2003, 114, 32–53 CrossRef CAS.
- J. Zhang, PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications, Springer London, 2008 Search PubMed.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- B. Smitha, S. Sridhar and A. A. Khan, J. Membr. Sci., 2005, 259, 10–26 CrossRef CAS.
- M. Rikukawa and K. Sanui, Prog. Polym. Sci., 2000, 25, 1463–1502 CrossRef CAS.
- L. Unnikrishnan, S. Mohanty and S. K. Nayak, High Perform. Polym., 2013, 25, 854–867 CrossRef.
- X. Jin, M. T. Bishop, T. S. Ellis and F. E. Karasz, Br. Polym. J., 1985, 17, 4–10 CrossRef CAS.
- Z. Tahir, A. Ilyas, X. F. Li, M. R. Bilad, I. F. J. Vankelecom and A. L. Khan, J. Appl. Polym. Sci., 2018, 135(10), 45952 CrossRef.
- H. Asghar, A. Ilyas, Z. Tahir, X. F. Li and A. L. Khan, Sep. Purif. Technol., 2018, 203, 233–241 CrossRef CAS.
- T. Y. Inan, H. Doğan, E. E. Unveren and E. Eker, Int. J. Hydrogen Energy, 2010, 35, 12038–12053 CrossRef CAS.
- F. Wang, M. Hickner, Y. S. Kim, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231–242 CrossRef CAS.
- C. Y. Wang, Y. P. Zhou, C. Xu, X. Y. Zhao, J. Li and Q. Ren, Int. J. Hydrogen Energy, 2018, 43, 20739–20749 CrossRef CAS.
- M. K. Ahn, B. Lee, J. Jang, C. M. Min, S. B. Lee, C. Pak and J. S. Lee, J. Membr. Sci., 2018, 560, 58–66 CrossRef CAS.
- K. Kang and D. Kim, J. Membr. Sci., 2019, 578, 103–110 CrossRef CAS.
- W. Zheng and C. J. Cornelius, Polymer, 2016, 103, 104–111 CrossRef CAS.
- J. Yuk, S. Lee, A. F. Nugraha, H. Lee, S. H. Park, S. D. Yim and B. Bae, J. Membr. Sci., 2016, 518, 50–59 CrossRef CAS.
- A. V. Talyzin, T. Hausmaninger, S. J. You and T. Szabo, Nanoscale, 2014, 6, 272–281 RSC.
- S. J. Lue, Y.-L. Pai, C.-M. Shih, M.-C. Wu and S.-M. Lai, J. Membr. Sci., 2015, 493, 212–223 CrossRef CAS.
- K. Feng, B. B. Tang and P. Y. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 1481–1488 CrossRef CAS PubMed.
- C. W. Lin and Y. S. Lu, J. Power Sources, 2013, 237, 187–194 CrossRef CAS.
- Y. Heo, H. Im and J. Kim, J. Membr. Sci., 2013, 425–426, 11–22 CrossRef CAS.
- R. Kumar, M. Mamlouk and K. Scott, RSC Adv., 2014, 4, 617–623 RSC.
- H. C. Chien, L. D. Tsai, C. P. Huang, C. Y. Kang, J. N. Lin and F. C. Chang, Int. J. Hydrogen Energy, 2013, 38, 13792–13801 CrossRef CAS.
- I. Nicotera, C. Simari, L. Coppola, P. Zygouri, D. Gournis, S. Brutti, F. D. Minuto, A. S. Arico, D. Sebastian and V. Baglio, J. Phys. Chem. C, 2014, 118, 24357–24368 CrossRef CAS.
- A. K. Sahu, K. Ketpang, S. Shanmugam, O. Kwon, S. Lee and H. Kim, J. Phys. Chem. C, 2016, 120, 15855–15866 CrossRef CAS.
- R. Kumar, M. Mamlouk and K. Scott, Int. J. Electrochem., 2011, 2011, 7 Search PubMed.
- Ravikumar and K. Scott, Chem. Commun., 2012, 48, 5584–5586 RSC.
- T. Bayer, R. Selyanchyn, S. Fujikawa, K. Sasaki and S. M. Lyth, J. Membr. Sci., 2017, 541, 347–357 CrossRef CAS.
- Z. Jiang, Y. Shi, Z.-J. Jiang, X. Tian, L. Luo and W. Chen, J. Mater. Chem. A, 2014, 2, 6494–6503 RSC.
- H. Y. Hou, X. H. Hu, X. X. Liu, W. Hu, R. J. Meng and L. Li, Ionics, 2015, 21, 1919–1923 CrossRef CAS.
- Z. Wang, J. Wang, Z. Li, P. Gong, X. Liu, L. Zhang, J. Ren, H. Wang and S. Yang, Carbon, 2012, 50, 5403–5410 CrossRef CAS.
- A. Mathkar, T. N. Narayanan, L. B. Alemany, P. Cox, P. Nguyen, G. H. Gao, P. Chang, R. Romero-Aburto, S. A. Mani and P. M. Ajayan, Part. Part. Syst. Charact., 2013, 30, 266–272 CrossRef CAS.
- K. Samanta, S. Some, Y. Kim, Y. Yoon, M. Min, S. M. Lee, Y. Park and H. Lee, Chem. Commun., 2013, 49, 8991–8993 RSC.
- W. Feng, P. Long, Y. Y. Feng and Y. Li, Adv. Sci., 2016, 3(7), 1500413 CrossRef PubMed.
- R. R. Nair, W. C. Ren, R. Jalil, I. Riaz, V. G. Kravets, L. Britnell, P. Blake, F. Schedin, A. S. Mayorov, S. J. Yuan, M. I. Katsnelson, H. M. Cheng, W. Strupinski, L. G. Bulusheva, A. V. Okotrub, I. V. Grigorieva, A. N. Grigorenko, K. S. Novoselov and A. K. Geim, Small, 2010, 6, 2877–2884 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS PubMed.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342–3347 CrossRef CAS.
- C. K. Chua and M. Pumera, J. Mater. Chem. A, 2013, 1, 1892–1898 RSC.
- S. Stankovich, D. A. Dikin, O. C. Compton, G. H. B. Dommett, R. S. Ruoff and S. T. Nguyen, Chem. Mater., 2010, 22, 4153–4157 CrossRef CAS.
- S. Sun, C. Wang, M. Chen and M. Li, Chem. Phys. Lett., 2013, 561–562, 166–169 CrossRef CAS.
- O. Jankovsky, P. Simek, D. Sedmidubsky, S. Matejkova, Z. Janousek, F. Sembera, M. Pumera and Z. Sofer, RSC Adv., 2014, 4, 1378–1387 RSC.
- B. Lian, S. De Luca, Y. You, S. Alwarappan, M. Yoshimura, V. Sahajwalla, S. C. Smith, G. Leslie and R. K. Joshi, Chem. Sci., 2018, 9, 5106–5111 RSC.
- A. R. Thiruppathi, B. Sidhureddy, W. Keeler and A. Chen, Electrochem. Commun., 2017, 76, 42–46 CrossRef CAS.
- K. Shimizu, A. Shchukarev, P. A. Kozin and J.-F. Boily, Langmuir, 2013, 29, 2623–2630 CrossRef CAS PubMed.
- X. Yang, X. Jia and X. Ji, RSC Adv., 2015, 5, 9337–9340 RSC.
- M. Wahlqvist and A. Shchukarev, J. Electron Spectrosc. Relat. Phenom., 2007, 156, 310–314 CrossRef.
- N. B. Colthup, L. H. Daly and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy, Elsevier Science, 1990 Search PubMed.
- C. Hontoria-Lucas, A. J. López-Peinado, J. d. D. López-González, M. L. Rojas-Cervantes and R. M. Martín-Aranda, Carbon, 1995, 33, 1585–1592 CrossRef CAS.
- G. I. Titelman, V. Gelman, S. Bron, R. L. Khalfin, Y. Cohen and H. Bianco-Peled, Carbon, 2005, 43, 641–649 CrossRef CAS.
- Y. Wang, W. C. Lee, K. K. Manga, P. K. Ang, J. Lu, Y. P. Liu, C. T. Lim and K. P. Loh, Adv. Mater., 2012, 24, 4285–4290 CrossRef CAS PubMed.
- S. S. Kocha, J. D. L. Yang and J. S. Yi, AIChE J., 2006, 52, 1916–1925 CrossRef CAS.
- K. R. Cooper, In situ pem fuel cell fuel: Fuel crossover and electrical short circuit measurement, 2008 Search PubMed.
- T. Bayer, S. R. Bishop, M. Nishihara, K. Sasaki and S. M. Lyth, J. Power Sources, 2014, 272, 239–247 CrossRef CAS.
- W. Vielstich, H. A. Gasteiger and H. Yokokawa, Handbook of Fuel Cells: Fundamentals Technology and Applications: Advances in Electrocatalysis, Materials, Diagnostics and Durability, Wiley-Blackwell, 2009 Search PubMed.
- S. A. Vilekar and R. Datta, J. Power Sources, 2010, 195, 2241–2247 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional synthesis and characterization experimental details, photographic images, thickness measurements, supporting XRD table, supporting XPS table detailing chemical species, Raman analysis, TEMs, zeta potential measurements, MEA illustration, reference polarization curve (Nafion) and cross-sectional SEM images. See DOI: 10.1039/c9se00126c |
| This journal is © The Royal Society of Chemistry 2019 |