Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrocarbon-soluble, hexaanionic fulleride complexes of magnesium†
Samuel R.
Lawrence
a,
C. André
Ohlin
 b,
David B.
Cordes
b,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a and
Andreas
Stasch
a and
Andreas
Stasch
 *a
*a
aEaStCHEM School of Chemistry, University of St Andrews, North Haugh, St Andrews, KY16 9ST, UK. E-mail: as411@st-andrews.ac.uk
bDepartment of Chemistry, Umeå University, Linnaeus väg 10, Umeå, 907 36, Sweden
First published on 9th October 2019
Abstract
The reaction of the magnesium(I) complexes [{(Arnacnac)Mg}2], (Arnacnac = HC(MeCNAr)2, Ar = Dip (2,6-iPr2C6H3), Dep (2,6-Et2C6H3), Mes (2,4,6-Me3C6H2), Xyl (2,6-Me2C6H3)) with fullerene C60 afforded a series of hydrocarbon-soluble fulleride complexes [{(Arnacnac)Mg}nC60], predominantly with n = 6, 4 and 2. 13C{1H} NMR spectroscopic studies show both similarities (n = 6) and differences (n = 4, 2) to previously characterised examples of fulleride complexes and materials with electropositive metal ions. The molecular structures of [{(Arnacnac)Mg}nC60] with n = 6, 4 and 2 can be described as inverse coordination complexes of n [(Arnacnac)Mg]+ ions with C60n− anions showing predominantly ionic metal–ligand interactions, and include the first well-defined and soluble complexes of the C606− ion. Experimental studies show the flexible ionic nature of the {(Arnacnac)Mg}+⋯C606− coordination bonds. DFT calculations on the model complex [{(Menacnac)Mg}6C60] (Menacnac = HC(MeCNMe)2) support the formulation as an ionic complex with a central C606− anion and comparable frontier orbitals to C606− with a small HOMO–LUMO gap. The reduction of C60 to its hexaanion gives an indication about the reducing strength of dimagnesium(I) complexes.
Introduction
Dimagnesium(I) compounds LMgMgL, where L is a sterically demanding monoanionic ligand, have been employed as soluble, selective, strong, stoichiometric and safe reducing agents towards a range of organic, organometallic and inorganic substrates.1 They have, for example, been employed as reductants to afford compounds with magnesium(I),2 zinc(I) and zinc(0),3 aluminium(I),4 and germanium(0) centres,5 as well as reduced anthracene to its dianion, benzophenone to the ketyl radical,6 reversibly added to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of selected alkenes,7 and after activation reductively coupled CO.8 Thus, they can be regarded as strong to very strong reducing agents,9 although the reduction potentials of these reagents have not yet been experimentally determined due to decomposition reactions.1a Reactions of dimagnesium(I) compounds towards substrates with a known series of reduction potentials could give some indication about the reducing strength of these complexes.
C bonds of selected alkenes,7 and after activation reductively coupled CO.8 Thus, they can be regarded as strong to very strong reducing agents,9 although the reduction potentials of these reagents have not yet been experimentally determined due to decomposition reactions.1a Reactions of dimagnesium(I) compounds towards substrates with a known series of reduction potentials could give some indication about the reducing strength of these complexes.
Buckminsterfullerene, C60, shows five degenerate orbitals at the HOMO level of hu symmetry, and three degenerate orbitals at the LUMO (t1u) and LUMO+1 (t1g) level, respectively.10–12 Fullerenes are generally difficult to oxidise but are readily reduced multiple times.10,13 In line with having a triply degenerate LUMO level, C60 shows six reduction waves from cyclic voltammetry experiments, for example giving E1/2 of −1.03, −1.44, −1.94, −2.42, −2.91, and −3.28 V relative to Fc0/+ (approximately −0.38 V vs. SCE or −0.62 V vs. SHE)14 in toluene/acetonitrile,15 with almost equidistant potentials (ca. 0.45 V) between reduction steps.13 A large number of materials involving fullerene anions (fullerides, fullerenides) have been studied and those with polyanionic fullerides are typically paired with electropositive metals such as alkali metals, alkaline earth metals or selected lanthanoids.16,17 Alkali metals have especially been used to afford phases such as M6C60 (M = alkali metal) and even some of composition M12C60, and others with differing metal-to-fullerene ratios.13,16,17 The C606− anion is a symmetric and diamagnetic species due to the fully filled C60 LUMO (t1u) level. For the rare C6012−, the former LUMO+1 (t1g) level is filled,10,13,16,17 although complete charge transfer from the metal to the fulleride cannot be certain in some materials. Some reduced fullerene states offer the possibility of different spin states, e.g. a singlet versus a triplet state for C602−.12,13 A non-symmetric occupation of these orbital levels can effect a Jahn–Teller distortion in the C60 framework and this has been observed for all C60n− ions with 1 ≤ n ≤ 5. This is even the case for n = 3, which could be expected to prefer a symmetric structure with a degenerate t1u level in a quartet state, but instead forms a slightly distorted species with a lower doublet spin state.13,16 The M3C60 class materials have received considerable attention because some phases with M = K, Rb, Cs are superconductors.16,17 Other fulleride materials with s-block metal ions can show structures with C–C bonded polymeric C60 units that demonstrate superionic conductivity and are of interest for battery materials.18
In addition to insoluble solid state materials, many well-defined exohedral19 metal complexes involving metal coordination to neutral fullerenes, fullerides with low charges or fullerene derivatives are known predominantly with transition metals.20 Soluble alkali metal complexes with polyanionic fullerides have been prepared and structurally characterised, typically using potassium, rubidium or caesium metal involving solution state chemistry in coordinating solvents and, in some cases, the addition of donor ligands such as crown ethers, cryptands etc.21–23 These experiments formed well-defined isolated complexes with fulleride anions up to C604−.21,22 Furthermore, potassium complexes of C605− have been studied in liquid ammonia24 and the reaction of lithium metal with C60 in THF afforded solutions showing a sharp 13C NMR signal for the C606− anion.25 In this work we report on the facile formation of soluble fulleride complexes from reactions of dimeric magnesium(I) compounds LMgMgL with C60.
Results and discussion
Synthesis
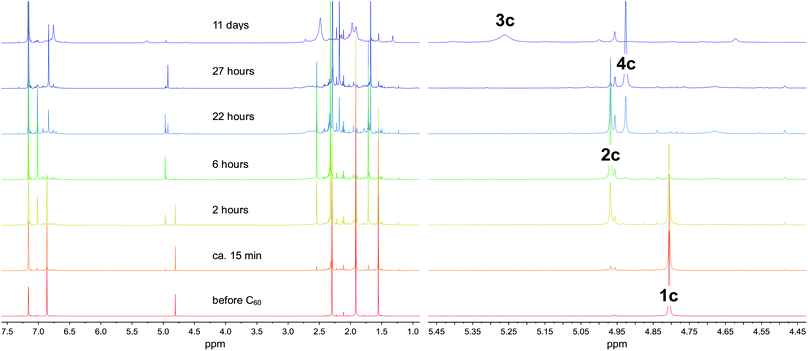
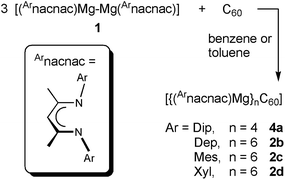
The most easily accessible dimagnesium(I) compounds are the β-diketiminate complexes [{(Arnacnac)Mg}2] 1, where Arnacnac = HC(MeCNAr)2, and Ar = Dip (2,6-diisopropylphenyl) 1a,26 Dep (2,6-diethylphenyl) 1b,27 Mes (mesityl, 2,4,6-trimethylphenyl) 1c,28 and Xyl (xylyl, 2,6-dimethylphenyl) 1d.1c,4 Treating a partially dissolved mixture of C60 in deuterated benzene with [{(Arnacnac)Mg}2] 1 at room temperature leads in all cases to a change in colour from the characteristic light purple of the fullerene, to dark brown owing to the formation of [{(Arnacnac)Mg}nC60] species. The reaction mixtures can further change over time as judged by 1H NMR and 13C{1H} NMR spectroscopy, depending on the number of equivalents of 1 used and on the steric demand of the Arnacnac-ligand in 1. When an excess of [{(Mesnacnac)Mg}2] 1c, e.g. six equivalents, is reacted with C60, new resonances for a single new product, [{(Mesnacnac)Mg}6C60] 2c, gradually grow in 1H NMR spectra next to those of unreacted 1c. Reacting only 0.5 equivalents of [{(Mesnacnac)Mg}2] 1c with C60 in deuterated benzene initially also leads to resonances for the formation of [{(Mesnacnac)Mg}6C60] 2c (Fig. 1), e.g. after six hours, but the composition further changes over time to mixtures of other fulleride complexes [{(Mesnacnac)Mg}nC60], 1 ≤ n ≤ 5. Of these, [{(Mesnacnac)Mg}2C60] 3c and [{(Mesnacnac)Mg}4C60] 4c were found to be dominant species, see Fig. 1, and these were also obtained from reactions with other 1c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 ratios. The experiment presented in Fig. 1 also shows that essentially full conversion of 1c to [{(Mesnacnac)Mg}6C60] 2c can be achieved (6 hours) before it further reacts with excess C60 to afford [{(Mesnacnac)Mg}4C60] 4c (27 hours), which then slowly further converts to a mixture with a large proportion of [{(Mesnacnac)Mg}2C60] 3c (11 days). Further conversion was complicated by the poor solubility of the lower substituted fulleride complexes and long reaction times. Generally, the solubility of fulleride complexes within a ligand series increases with a higher number of decorated [(Arnacnac)Mg]+ groups. Small quantities of dark precipitates formed in some instances especially when low numbers of equivalents of 1 had been added. This is believed to cause the broadened resonances for [{(Mesnacnac)Mg}2C60] 3c in Fig. 1 due to the presence of solids in the NMR sample. Further additions of portions of 0.5 equivalents of [{(Mesnacnac)Mg}2] 1c to these mixtures followed by equilibration showed the formation of varying quantities of 2c, 4c, 3c and other species over time that ultimately formed [{(Mesnacnac)Mg}6C60] 2c when three equivalents of 1c were used (Fig. S47–S49†). Reactions carried out at elevated temperatures of 60–80 °C with an excess of 1c and C60 show that these reactions are faster but do not show easily detectable product formation beyond the composition of [{(Mesnacnac)Mg}6C60] 2c.
C60 ratios. The experiment presented in Fig. 1 also shows that essentially full conversion of 1c to [{(Mesnacnac)Mg}6C60] 2c can be achieved (6 hours) before it further reacts with excess C60 to afford [{(Mesnacnac)Mg}4C60] 4c (27 hours), which then slowly further converts to a mixture with a large proportion of [{(Mesnacnac)Mg}2C60] 3c (11 days). Further conversion was complicated by the poor solubility of the lower substituted fulleride complexes and long reaction times. Generally, the solubility of fulleride complexes within a ligand series increases with a higher number of decorated [(Arnacnac)Mg]+ groups. Small quantities of dark precipitates formed in some instances especially when low numbers of equivalents of 1 had been added. This is believed to cause the broadened resonances for [{(Mesnacnac)Mg}2C60] 3c in Fig. 1 due to the presence of solids in the NMR sample. Further additions of portions of 0.5 equivalents of [{(Mesnacnac)Mg}2] 1c to these mixtures followed by equilibration showed the formation of varying quantities of 2c, 4c, 3c and other species over time that ultimately formed [{(Mesnacnac)Mg}6C60] 2c when three equivalents of 1c were used (Fig. S47–S49†). Reactions carried out at elevated temperatures of 60–80 °C with an excess of 1c and C60 show that these reactions are faster but do not show easily detectable product formation beyond the composition of [{(Mesnacnac)Mg}6C60] 2c.
Reaction products from comparable experiments for an excess of other [{(Arnacnac)Mg}2] 1 with C60 were found to be [{(Dipnacnac)Mg}4C60] 4a for 1a, [{(Depnacnac)Mg}6C60] 2b for 1b, and [{(Xylnacnac)Mg}6C60] 2d for 1d, see Scheme 1. Titration experiments between C60 and [{(Arnacnac)Mg}2] 1, performed by sequentially adding 1 to C60 (Fig. S41–S46†), have shown a similar course to those described above for 1c with comparable dominating species 2 (although 2a was not observed), 3 and 4 observed in solution. The different composition in 4a compared to 2b–d as a final product is likely due to the large steric demand of the Dip group in the series, vide infra. A competition experiment of a freshly prepared mixture of three equivalents of [{(Dipnacnac)Mg}2] 1a and three equivalents of [{(Mesnacnac)Mg}2] 1c with one equivalent of C60 only afforded new resonances for [{(Mesnacnac)Mg}6C60] 2c soon after addition and highlights the importance of steric factors for the activation reaction.
In situ preparations of 2–4 often afforded high conversions in solution with a single dominant species only that were characterised by NMR spectroscopy. The formation of these complexes were in some instances accompanied by dark precipitates, especially for products with low solubility. These reactions have been found to be considerably faster in toluene compared with benzene, due to the higher solubility of C60 in toluene.29 The relatively low solubility of crystalline C60, especially in benzene, appears to be one of the main limiting factors for the reaction rates. Finely grinding the fullerene crystals and using sufficiently large solvent volumes accelerate product formation. [{(Depnacnac)Mg}6C60] 2b and [{(Mesnacnac)Mg}6C60] 2c were isolated as crystalline solids in around 50% yield, and [{(Depnacnac)Mg}2C60] 3b in 45% yield. In addition, crystalline samples of other compounds were isolated and structurally characterised, vide infra.
Assignment of these series of complexes was conducted by recording 13C{1H} NMR spectra at various points in the study, and through isolated examples characterised by X-ray diffraction, vide infra, as complexes of the C606− (2), C602− (3) and C604− (4) anions. Other fulleride species C60n− as well as dimerised and polymerised fulleride species with C60–C60 fragments are possibilities for other products or intermediates.16,18
Molecular structures from single crystal X-ray diffraction
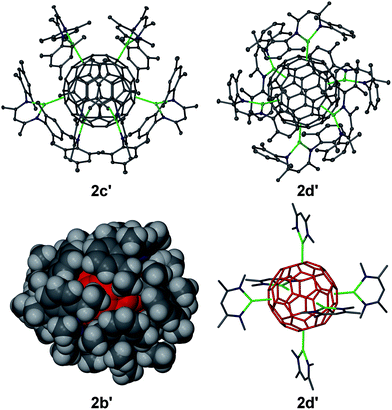
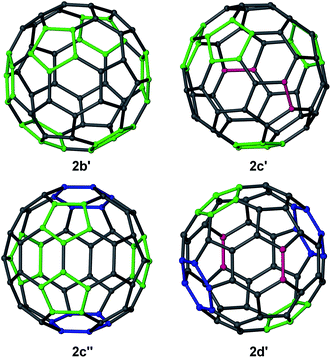
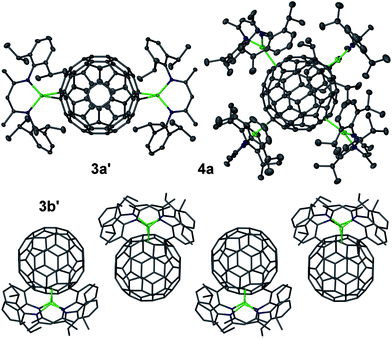
Several complexes [{(Arnacnac)Mg}6C60] 2 were structurally characterised; namely [{(Depnacnac)Mg}6C60]·4C6H142b′, [{(Mesnacnac)Mg}6C60]·7C6H62c′, [{(Mesnacnac)Mg}6C60]·4C6H142c′′ and [{(Xylnacnac)Mg}6C60]·7C6H62d′, see Fig. 2 and the ESI.† These molecular structures show the coordination of six (Arnacnac)Mg fragments around a central C60 core with various Mg⋯C60 coordination modes. The (Arnacnac)Mg coordination to C60 including their relative positions is presented in colour-coded format in Fig. 3 highlighting η2, η5, and η6 coordination modes. Compounds 2b′ and 2c′ each crystallised with a full molecule in the asymmetric unit. The most sterically crowded derivative 2b′ demonstrates that the six (Arnacnac)Mg units completely wrap-in the C60 core, see the space-filling model in Fig. 2 and S60† for that of 2c′. In 2b′, all six Mg centres coordinate in a η5 fashion to five-membered rings of the fullerene. These five-membered rings are all adjacent to each other, i.e. only separated by one [6,6] C–C bond each, and form a chiral “ribbon” on the C60 surface, see Fig. 3. Solvate 2c′ shows four η5 Mg⋯C60 interactions to five-membered carbon rings and two η2 interactions to [5,6] C–C bonds. Two of these Mg centres coordinate to adjacent five-membered rings. Complex 2c′′ crystallised with two full independent molecules in the asymmetric unit showing identical Mg⋯C60 coordination modes. 2c′′ shows four Mg centres coordinate η5 to five-membered rings, two of these being adjacent to each other, and two coordinate in an η6 fashion to six-membered carbon rings. The least sterically shielded derivative 2d′ crystallised with half a molecule in the asymmetric unit and shows two η6, η5 and η2 coordination modes each on opposite ends of the C60 core (Fig. 3). The structure of 2d′ shows a near-perfect octahedral coordination arrangement of six (Xylnacnac)Mg fragments around the central C60 unit, with orthogonal arrangements of (Xylnacnac)Mg ligand planes with respect to neighbouring ligand planes (Fig. 2 and S63†).The molecular structures 2c′ and 2d′ show the highest bond precisions in the series, well-ordered fulleride units and thus a more detailed analysis is described here. The Mg–N distances (2.006 Å mean in 2c′, 2.001 Å mean in 2d′) are short, and for example comparable to those of recently reported cationic [(Dipnacnac)Mg·arene]+ complexes,30 and those in [(Dipnacnac)MgPh] with a three-coordinate Mg centre.31 The shortest Mg–N distances [e.g. Mg(3)–N(47) 1.9805(12) Å and Mg(3)–N(51) 1.9867(12) Å in 2d′] are those for Mg centres involved in η2 (Arnacnac)Mg⋯C60 interactions, which supports anticipated ionic Mg⋯C60 bonding interactions, vide infra. The Mg⋯C distances also strongly depend on the coordination mode. For 2d′, the η6 [2.074 Å to centroid; Mg–C range: 2.4218(14)–2.6192(14) Å; mean 2.522 Å], η5 [2.094 Å to centroid; Mg–C range: 2.3656(14)–2.4799(14) Å; mean 2.429 Å], and η2 [2.140 Å to [6,6] bond midpoint; Mg(3)–C(90) 2.2551(14) Å, Mg(3)–C(97)' 2.2660(14) Å] interactions are relatively short; as expected, shorter than Mg⋯C distances in the cationic [(Dipnacnac)Mg (η6-mesitylene)]+ [2.205 Å to centroid; Mg–C range: 2.5325(17)–2.6988(16) Å; mean 2.612 Å], although longer than Mg–C single bonds, e.g. Mg–C: 2.095(3) Å in [(Dipnacnac)MgPh].30,31 The fulleride [5,6] bonds (1.444 Å mean in 2c′, 1.441 Å mean in 2d′) are slightly longer than the [6,6] bonds (1.424 Å mean in 2c′, 1.423 Å mean in 2d′); the latter are elongated compared to those in free C60.10,13 However, an overlapping range for individual bonds has been found, for example 1.431(2)–1.456(2) Å plus two outliers for [5,6] bonds, and 1.416(2)–1.441(2) Å for [6,6] bonds in 2c′. The two [5,6] outliers are somewhat longer and are the two C–C bonds that show η2-coordination to Mg centres [1.469(2) Å to Mg6 and 1.471(2) Å to Mg5]. Similarly, the longest [5,6] bonds in 2d′ [2 × 1.4556(19) Å] are involved in coordinating to Mg centres. The slight lengthening of C–C bonds of fragments that coordinate to Mg centres in comparison to similar uncoordinated fragments is also observed in the mean values of C–C bonds in five-membered carbon rings with (1.448 Å) and without (1.439 Å) η5 Mg coordination in 2c′. In 2c′ two Mg centres (Mg1, Mg2) coordinate to two five-membered rings that are only separated by one [6,6] bond and lead to a relatively short Mg⋯Mg separation of ca. 5.69 Å. Similar values are found for related motifs in the other molecular structures of 2.
Further molecular structures were determined for [{(Dipnacnac)Mg}2C60]·4C6H63a′, [{(Dipnacnac)Mg}2C60]·2.5C6H63a′′, [{(Depnacnac)Mg}2C60]·1.5C6H63b′, and [{(Dipnacnac)Mg}4C60] 4a, see Fig. 4. The molecular structures of 3a′ and 3a′′ are highly similar and only one is presented. The complexes 3a′ and 3a′′ each crystallized with a full molecule in the asymmetric unit and the molecular structures show essentially two terminal (η1) Mg⋯C60 coordinations with an average Mg–C interaction of 2.212 Å across 3a′ and 3a′′ (cf. Mg–C of 2.095(3) Å in [(Dipnacnac)MgPh]) and an average sum of the angles around each three-coordinate Mg centre of 360° [359.9(5)°, 360.0(5)°, 359.7(4)°, 359.8(3)°]. The Mg–N bonds (1.975 Å average for 3a′ and 3a′′) are short. No close C60⋯C60 interactions are found in the packing of 3a′/3a′′. In contrast, while the molecular structure of 3b′ shows similar bond lengths to those found for 3a′/3a′′, the two (Depnacnac)Mg units are coordinated to the C60 core in a more acute fashion as illustrated by comparison between the Mg⋯C60-centre⋯Mg angles in 3b′ (ca. 79°) and 3a′/3a′′ (ca. 126°). This allows for some relatively close C60⋯C60 interactions from π-stacked one-dimensional fulleride chains along the b-axis, arising from short intermolecular C⋯C interactions (ca. 3.4 Å) between slightly offset co-planar five-membered carbon rings from neighbouring C602− moieties. The fulleride C–C bond lengths in 3a′/3a′′ do show a wide range of distances, but also a comparatively low bond precision that does not allow a detailed analysis with respect to differences between, for example, [6,6] and [5,6] bonds, coordination between Mg and fulleride unit or Jahn–Teller distortion. Analysis of the distances between fulleride carbon atoms to the fullerene centre in the C602− complex with the highest data quality, 3a′′, revealed that 58 of them all lie within 0.02–0.03 Å of the mean value (3.54 Å) and no clear and significant pattern for a fulleride distortion emerged. Jahn–Teller distortion in fullerides is relatively small, but can in some cases be observable by structural studies.13,32 We did, however, observe that the two carbon centres that terminally coordinate the Mg centres are significantly more pyramidalized, slightly protrude from the fulleride “sphere” and show C⋯C60-centroid distances that are 0.09–0.10 Å longer than the mean from the remaining 58 distances. Also, the fulleride C–C bond lengths in 3b′ cannot be analysed due to poor ordering and low bond precision.
Complex [{(Dipnacnac)Mg}4C60] 4a crystallised with half a molecule in the asymmetric unit and suffers from fulleride disorder, meaning that the Mg⋯C60 coordination modes cannot be commented on. The four (Dipnacnac)Mg units are approximately arranged in a “square planar” fashion around the C60 core, albeit with significant tetrahedral distortion. This results in two small (93° mean) and one large (155° mean) Mg⋯C60-centre⋯Mg angle for each Mg centre (see Fig. S70†).
The combined analysis of the molecular structures of 2–4 shows that many Mg⋯C60 coordination modes (η1, η2, η5, η6) are possible and that all fulleride fragments (carbon atoms, [6,6] bonds, [5,6] bonds, five-membered rings, six-membered rings) can be involved in bonding to a metal. This, together with bond distance considerations, suggest ionic Mg⋯C60 interactions, i.e. that 2–4 are coordination complexes of n [(Arnacnac)Mg]+ with central C60n− ions. These can be regarded as “inverse coordination compounds”33 where cationic LMg+ “ligands” coordinate to a central C60n− “superatom”.34 The molecular structure 2d′ shows a near-perfect octahedral coordination mode of six [(Xylnacnac)Mg]+ around the C606− unit with an orthogonal arrangements of ligand planes to those of their neighbours. This arrangement is comparable in overall structure to, for example, those in the transition metal complexes M(NMe2)6 (M = Mo, W) with inverted charges.35 Other coordination geometries can be regarded as distorted octahedral (2), distorted square-planar (4a) and bent (3).
Spectroscopic and physical properties
1H NMR spectra for [{(Mesnacnac)Mg}6C60] 2c and [{(Xylnacnac)Mg}6C60] 2d in deuterated benzene or toluene show sharp resonances for one ligand environment, respectively, e.g. see Fig. 1 (6 hours), Fig. S29 and S35.† This is expected for coordination compounds with ionic metal–ligand interactions given the various solid state coordination modes for [(Arnacnac)Mg]+ fragments coordinating to C606− ions and the expected low energy processes for interconversions between them. 1H NMR spectra obtained from dissolving crystalline samples of [{(Depnacnac)Mg}6C60] 2b, however, always afforded resonances for a mixture of species (Fig. S6, S7, S9 and S10†). This included samples that showed a satisfactory microanalysis for 2b. These spectra mainly show resonances assigned to [{(Depnacnac)Mg}6C60] 2b, [{(Depnacnac)Mg}4C60] 4b, and the tentatively assigned species [{(Depnacnac)Mg}5C60] 5b with significantly broadened resonances (e.g. Fig. S24 and S25†). At elevated temperatures in deuterated toluene, some broadened resonances only slightly sharpen and a mixture of species is still observed. Further addition of [{(Depnacnac)Mg}2] 1b to this sample and recording an 1H NMR spectrum at 100 °C showed that both compounds 2b and 1b together with other fulleride complexes (such as 5b) are present demonstrating the difficulty to fully reduce C60 to C606− using [{(Depnacnac)Mg}2] 1b. This is in line with the highly crowded ligand sphere in the molecular structure of 2b. Recording spectra of 2b immediately after dissolution showed the highest concentration of 2b, followed by growing resonances for 5b, then 4b (Fig. S6–S11†). Trace quantities of 1b were also found in these spectra though these do not account for all the (Depnacnac)Mg fragments removed from 2b. For comparison, [{(Arnacnac)Mg}2] 1 forms equilibria with selected alkenes and alkynes such as 1,1-diphenylethene to novel 1,2-dimagnesioethane species,7 and a related equilibrium process may be in operation for 2b, plus follow-on reactivity forming 5b, 4b, 1b and other species. 1H NMR spectra for [{(Arnacnac)Mg}2C60] 3 (Fig. S2, S16, S18, S31 and S37†) and [{(Arnacnac)Mg}4C60] 4 (Fig. S4, S22, S33 and S39†) complexes also show resonances for one ligand environment. Poorly soluble complexes 3c,d show somewhat broadened resonances. The addition of donor solvents such as THF to solutions of [{(Arnacnac)Mg}6C60] 2 typically leads to the precipitation of insoluble materials from solution, likely ionic complexes.13C{1H} NMR spectra of the fulleride complexes in deuterated benzene or toluene each show resonances for one ligand environment and one resonance for the fulleride ion as expected for complexes with ionic bonding interactions. The fulleride resonances of the complexes (Table 1) are all downfield shifted compared to that of neutral C60 (143 ppm).13 The C606− complexes 2b–d show a sharp resonance at around 153 ppm that is close to that observed for C602− species 3a–d (ca. 156 ppm). For comparison, previously a solution of Li6C60 could be generated in deuterated THF that showed a sharp resonance at 156.7 ppm.25a A broad resonance for C604− species 4a–d (ca. 166 ppm) appears significantly further downfield, and a very broad resonance at ca. 174 ppm was tentatively assigned to the putative C605− species 5b.
| [{(Arnacnac)Mg}nC60] | Ar = Dip (a) | Ar = Dep (b) | Ar = Mes (c) | Ar = Xyl (d) |
|---|---|---|---|---|
| n = 6 (sharp) 2 | — | 153.2 | 152.9 | 152.8 |
| n = 5 (very broad) 5 (?) | — | 174 (?) | — | — |
| n = 4 (broad) 4 | 166.2 | 167.3 | 166.2 | 166.4 |
| n = 2 (sharp to broad) 3 | 155.4 | 156.5 | 156.3 | 156.2 |
All observed fulleride complexes 2–5 show 1H and 13C{1H} NMR resonances for highly symmetric species in solution at room temperature. In addition, NMR spectra for the most soluble series (Ar = Dep), [{(Depnacnac)Mg}6C60] 2b, [{(Depnacnac)Mg}2C60] 3b, and [{(Depnacnac)Mg}4C60] 4b, were recorded at −80 °C in deuterated toluene (Fig. S12–S15, S20 and S21†) and all support symmetric solution behaviour at this temperature. 1H NMR data show as expected one ligand backbone CH resonance for each complex, though broader resonances indicate a more hindered rotation of the ethyl groups in the ligand sphere and/or a reduced solubility. The low temperature 13C{1H} NMR spectra for 2b, 3b and 4b all show a fulleride resonance that is essentially unchanged with respect to its room temperature chemical shift. Also, the sharp appearance of the 13C{1H} NMR fulleride singlet of 3b and the broad signal shape for 4b are virtually unchanged when compared to their room temperature spectra and support highly fluxional behaviour at this temperature. The 13C{1H} NMR fulleride resonance of 2b, however, is broadened or starts to split into individual resonances (Fig. S13†). This could have several reasons and could signify splitting of fulleride resonances between different coordination modes and/or coordinated versus uncoordinated carbon centres amplified by the extreme ligand crowding in the complex, or simply result from the low solubility under these experimental conditions.
The 13C NMR fulleride data found for complexes 2 is very close to those reported for M6C60 (M = alkali metal cations) solid state materials.13,16,36 The data deviates significantly for complexes 3 (by ca. 25–30 ppm) and 4 (by ca. 15–20 ppm) compared to those reported for related solid state materials and soluble ionic fulleride complexes in coordinating solvents. These generally show 13C NMR chemical shifts for C602− and C604− species of approximately 180–185 ppm; species with C60− and C603− ions can show even further downfield shifts.13,24b In general it has been suggested that a larger downfield shift can be associated with a higher paramagnetism in fullerides,13 and that species with the same number of unpaired electrons may show 13C NMR chemical shift in a similar region.13,16 Comparisons with 13C NMR chemical shifts available for solid state materials may be complicated due to contributions from the conduction electrons (Knight shifts) and magnetic coupling between fulleride anions with close contacts in the solid state.13 Comparisons to data from solid state materials or from species in coordinating solvents may in some cases be difficult due to possible signal averaging from exchange reactions between fullerides of different charge states.10,13 The fulleride complexes reported herein, at least those with higher charges such as 2 and 4, likely suppress direct C60n−⋯C60n− interactions; preventing close approach by the large organic ligand sphere as an outer separating layer (Fig. 2). This, together with the use of non-coordinating hydrocarbon solvents, will also likely suppress electron exchange reactions such as disproportionations. Thus, our reported 13C{1H} NMR resonances are likely not averages from different species and show unchanged chemical shifts for individual species in titration experiments. Comparisons of these with 13C NMR data from solid state materials or for alkali metal species in coordinating solvents may allow further conclusions to be drawn with respect to Knight shifts, electronic states or other effects in the solid or solution state.
The [{(Arnacnac)Mg}6C60] complexes 2 are diamagnetic species with sharp C606− resonances. [{(Arnacnac)Mg}nC60] complexes with uneven numbers of n are inevitably paramagnetic species where issues such as significant signal broadening and shifting of resonances in NMR spectra can be expected. For species with 2 ≤ n ≤ 4 several spin states are possible such as high and low spin configurations.10,12,13 We used Evan's method37 to shed further light on the number of unpaired electrons and the dominating spin state for these species in solution (Fig. S43–S49†). Although we could not yet study solutions of [{(Arnacnac)Mg}2C60] 3 or [{(Arnacnac)Mg}4C60] 4 that were completely free of impurities, and these fullerides may be in equilibrium with others of different charges as is known for fullerides in coordinating solvents, these experiments indicated a negligible to very low magnetic moment in solution (e.g. Fig. S47 and S48†). This suggests that these species are dominated by a diamagnetic ground state and that all electrons are paired and/or antiferromagnetically coupled in an open shell singlet state. Previous studies on C602− species suggest close singlet and triplet states that are partially populated; data for C603− is consistent with a doublet state (typically described as a low spin species), and a triplet state is suggested for C604−.13,22,38 A multiconfigurational description for some species may be warranted and results in a complicated picture. The possibility that C602− complexes 3 and C604− complexes 4 are essentially diamagnetic suggests that these complexes could be in different electronic states to those of known ionic fulleride species, and requires further investigation. In contrast and for comparison, mixtures showing resonances for the tentatively assigned species [{(Depnacnac)Mg}5C60] 5b, which has to be a paramagnetic species, show a large relative paramagnetism from similar experiments in solution (Fig. S46a†). A diamagnetic state for 3 and 4 could furthermore explain the significant difference in their 13C NMR fulleride chemical shifts to those of related reported materials with significant upfield shift; especially the resonances for 3 which are shifted by 25–30 ppm compared with known materials and are very close to those of diamagnetic C606− in 2. The further downfield shift and fulleride signal broadening of C604− species 4 could hint at a slightly higher average paramagnetic character despite only very small solution magnetic moments or hint at an open shell singlet state. In this context it is worth noting that the fulleride 13C chemical shifts and line shapes for 3b and 4b were essentially unchanged when data from room temperature and −80 °C experiments were compared.
All fulleride complexes observed herein are different shades of deep brown in solution. Complexes 2 show an orange-brown colour in solution, complexes 3 show a more green-brown colour. UV/Vis spectra of 2c (Fig. S1†) recorded in hydrocarbon solution (n-hexane or toluene) show some absorption across the visible spectrum with an absorption peak at 428 nm (ε ≈ 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 mol−1 dm3 cm−1) and a rise in absorption towards the NIR region between 700 and 800 nm; the latter being the limit of the experiment. The absorption maximum is in line with an orange tint and the rise in absorption at longer wavelengths contributes to the overall brown colour.
800 mol−1 dm3 cm−1) and a rise in absorption towards the NIR region between 700 and 800 nm; the latter being the limit of the experiment. The absorption maximum is in line with an orange tint and the rise in absorption at longer wavelengths contributes to the overall brown colour.
Computational studies
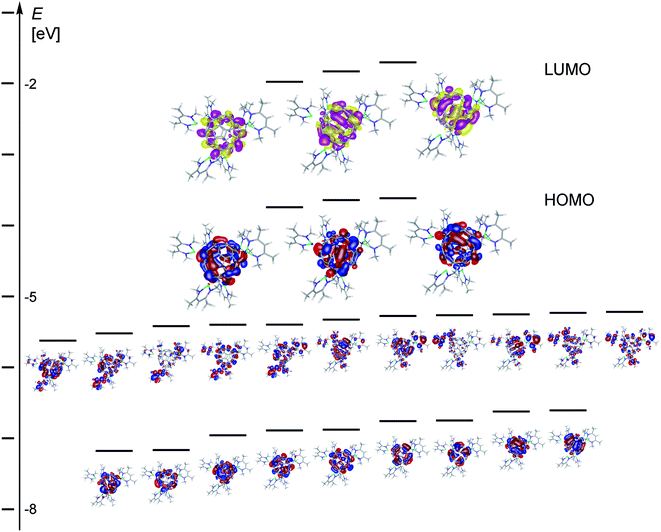
Density functional theory (DFT) studies at the pbe0/def2-svp level of theory on the model complex [{(Menacnac)Mg}6C60] 2Me (Menacnac = HC(MeCNMe)2), C60, C606− and [(Menacnac)Mg]+, were carried out to shed further light on the bonding situation of complexes 2b–d. Four isomers were optimised from core coordinates of X-ray solid state structures (Fig. S71–S74†); 2Me-1 (using 2c′ as a starting geometry), 2Me-2 (from 2b′), 2Me-3 (from 2c′′) and 2Me-4 (from 2d′). In each case, the [(Menacnac)Mg]+ groups underwent some repositioning in part due to the smaller steric demand of the ligand sphere in isomers of 2Me. Isomers 2Me-1 to 2Me-3 lie within 3 kcal mol−1 of one another and show a combination of η5 and η2 Mg coordination modes (Table S2†), whereas isomer 2Me-4 is ca. +5 kcal mol−1 above the lowest isomer 2Me-3 and shows one terminal (η1) Mg coordination mode not found in the solid state structures of 2. η6 Mg coordination modes, as observed in structures 2c′′ and 2d′, were not found in isomers of 2Me. The fulleride C–C bond lengths in isomers of 2Me show variations within the [5,6] and [6,6] bonds and are affected by Mg coordination. Bond lengths for η2 Mg coordinated C–C bonds are ca. 0.02 Å longer for [5,6] bonds in 2Me-1, and ca. 0.04 Å longer for [6,6] bonds in 2Me-4, compared to uncoordinated examples in the same respective optimised structure, and supports the trend found in the solid state structures of 2. Isomer 2Me-2 contains six five-membered carbon rings coordinated to Mg centres and six not coordinated to Mg centres. The former C–C bond lengths (mean 1.445 Å) are on average slightly longer than the latter ones (mean 1.438 Å) and these values are highly comparable to those obtained from the mean measured bond lengths in 2c′, cf. 1.448 Å and 1.439 Å, respectively. The sum of the three C–C–C angles around the carbon atom with η1 Mg coordination (ca. 341°) in 2Me-4 is ca. 6° smaller than the mean from comparable uncoordinated carbon atoms in this isomer showing the trend towards pyramidalization and echoes the findings for the carbon atoms with η1 Mg coordination in the solid state structure of the C602− species 3a′′.A partial molecular orbital diagram of 2Me-1 (Fig. 5) supports the formulation of a complex with a central C606− ion. The frontier orbital HOMO and LUMO levels of 2Me-1 are entirely C60 based and largely resemble those of C606− with three occupied (t1u in Hückel theory) and three unoccupied (t1g) molecular orbitals.10–12 The HOMO–LUMO gap in 2Me-1 is small (1.64 eV, 158 kJ mol−1, 37.8 kcal mol−1) and similar to that calculated for C606− (1.78 eV) at the same DFT level (Fig. S77†). Below the HOMO level in 2Me-1 is a band of eleven orbitals that appear to originate from mixing of five C60hu orbitals (the former C60 HOMO level or the C606− HOMO-1 level) and six [(Menacnac)Mg]+ HOMO orbitals (Fig. S78†). The band of nine orbitals below are C60 based and largely represent the four gg and five hg orbitals that occur at almost identical energy in C60 or C606−, respectively (e.g. Fig. S76 and S77†). Approximately 1 eV above the LUMO level of 2Me-1 starts a band of 14 orbitals that appear to originate from mixing eight C60 based orbitals (5 hg and 3 t2u) with six [(Menacnac)Mg]+ LUMO+1 orbitals (Fig. S75†). The gap between HOMO and LUMO+1 level approximately corresponds in energy to the UV absorption at 428 nm and the rise in absorption around 700–800 nm to the HOMO–LUMO gap. In relation to the relatively small HOMO–LUMO gap determined for 2Me-1 it is worth mentioning that the C606− anion shows a significantly higher aromatic character than C60 with strong diatropic ring currents and a huge endohedral shielding as has been determined for 3He@C606− using 3He NMR spectroscopy.10,11,25
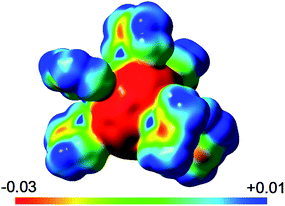
The calculated Natural Bond Orbital charges for the isomers of 2Me show some variation of charges on C60 carbon atoms that depend on nearby Mg coordination. In general, low hapticity Mg coordination leads to more localised anionic charge. For example, the terminal (η1) carbon coordinated to Mg in 2Me-4 carries a charge of −0.41; its three direct neighbours only show an average of −0.06. In 2Me-3, the average charge of an η2 coordinated carbon atom is ca. −0.28 and its four neighbours have a significantly lower charge of ca. −0.02. Less polarisation of the central C60 unit is found around η5 coordination modes. This charge accumulation in Mg-coordinated fragments is accompanied by the previously discussed C–C bond lengthening and pyramidalization of carbon centres. For comparison, the expected average charges of 0.00 for C60 and −0.10 for C606− with very little to no variation are found. The Mg ions in 2Me show a charge of approximately +1.77, similar to that in [(Menacnac)Mg]+. The sum of charges on C60 of −5.8 in 2Me-3 is close to the ideal value of −6 for a wholly ionic system, and supports the view as a contact ion complex with a central C606− ion coordinating to six [(Menacnac)Mg]+ ions. Similarly, the electrostatic potential visualises this situation (Fig. 6), and highlights the difference in potential between the highly anionic fulleride unit (red) and positive [(Menacnac)Mg]+ fragments (blue sections), especially the strongly positive Mg2+ centres, that help balance and dissipate the accumulated charges by coordination.
In order to further probe the nature of the interactions in complexes 2, we reacted [{(Mesnacnac)Mg}6C60] 2c with six equivalents of [(Xylnacnac)Li] and observed the very rapid formation of the magnesium(II) complex [(Mesnacnac)Mg(Xylnacnac)] 6, as suggested by 1H, 7Li and 13C{1H} NMR spectroscopy (Fig. S52–S56†), and the formation of an insoluble dark orange-brown precipitate, likely Li6C60. The rapid and facile breaking of the {(Mesnacnac)Mg}+⋯C606− coordination bonds in this reaction is further support for the flexible, ionic nature of these interactions and suggests that complexes 2 can serve as soluble sources of C606−.
Conclusion
Dimagnesium(I) complexes LMgMgL, L = Arnacnac, react with C60 to form a range of hydrocarbon-soluble fulleride complexes of the general formula [(LMg)nC60] and can reduce C60 up to its hexaanion if sterics permit. The combined experimental and computational studies for n = 6 support the formulation as an inverse coordination complex with flexible ionic LMg+ to C606− interactions, and a small HOMO–LUMO gap. The Mg⋯C60 interactions can show a wide range of coordination modes that are easily distorted and represent relatively weak electrostatic interactions. The anions C602−, C604− and C606− were found to be dominant species and were characterised in the solution and solid state. C606− complexes 2 are as expected diamagnetic and indication for C602− in complexes 3 and C604− in complexes 4 suggest a dominating diamagnetic ground state. The ease of reducing C60 to its hexaanion suggests that the reducing capabilities of LMgMgL compounds towards substrates are at least in the order of E0 ≤ −2.9 V (vs. SCE) or ≤−2.65 V (vs. SHE) and thus can be regarded as very strong reducing agents.9 This can approximately be compared to the reported reduction potentials of Mg0/I (Mg+ + e− → Mg: E0 = −2.70 V vs. SHE; cf. Mg2+ + 2 e− → Mg: E0 = −2.372 V vs. SHE) and Na metal (Na+ + e− → Na: E0 = −2.71 V vs. SHE);39 the latter can be used as a reducing agent in preparing LMgMgL complexes. In addition to thermodynamic considerations such as reduction potentials, kinetic factors, for example an appropriate set of sterics and a suitable mechanism, are important in the chemistry of LMgMgL complexes.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the University of St Andrews and the EPSRC (PhD studentship for SRL; EP/N509759/1). CAO thanks the Kempe Foundation for grant JCK-1719, and computational resources were provided by the High Performance Computing Centre North (HPC2N) and the National Supercomputer Centre (NSC) via grants SNIC 2018/3-253 and 2018/3-389, respectively.Notes and references
- (a) C. Jones, Nat. Rev. Chem., 2017, 1, 0059 CrossRef CAS; (b) C. Jones and A. Stasch, Top. Organomet. Chem., 2013, 45, 73–101 CrossRef CAS; (c) A. Stasch and C. Jones, Dalton Trans., 2011, 40, 5659–5672 RSC.
- A. Stasch, Angew. Chem., Int. Ed., 2014, 53, 10200–10203 CrossRef CAS PubMed.
- (a) J. Hicks, E. J. Underhill, C. E. Kefalidis, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2015, 54, 10000–10004 CrossRef CAS PubMed; (b) A. Stasch, Chem.–Eur. J., 2012, 18, 15105–15112 CrossRef CAS PubMed.
- S. J. Bonyhady, D. Collis, N. Holzmann, A. J. Edwards, R. O. Piltz, G. Frenking, A. Stasch and C. Jones, Nat. Commun., 2018, 9, 3079 CrossRef PubMed.
- A. Sidiropoulos, C. Jones, A. Stasch, S. Klein and G. Frenking, Angew. Chem., Int. Ed., 2009, 48, 9701–9704 CrossRef CAS PubMed.
- C. Jones, L. McDyre, D. M. Murphy and A. Stasch, Chem. Commun., 2010, 1511–1513 RSC.
- (a) D. Deepak, A. R. Gair, D. D. L. Jones, M. Juckel, S. Aldridge and C. Jones, Chem. Sci., 2019, 10, 3208–3216 RSC; (b) A. J. Boutland, A. Carroll, C. A. Lamsfus, A. Stasch, L. Maron and C. Jones, J. Am. Chem. Soc., 2017, 139, 18190–18193 CrossRef CAS PubMed.
- K. Yuvaraj, I. Douair, A. Paparo, L. Maron and C. Jones, J. Am. Chem. Soc., 2019, 141, 8764–8768 CrossRef CAS PubMed.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS PubMed.
- (a) F. Liu and S. Yang, Carbon: Fullerenes, in Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley & Sons, Ltd, 2014 Search PubMed; (b) A. Hirsch and M. Brettreich, Fullerenes: Chemistry and Reactions, Wiley-VCH Weinheim, 2005 Search PubMed.
- (a) G. Orlandi and F. Negri, Photochem. Photobiol. Sci., 2002, 1, 289–308 RSC; (b) M. Bühl and A. Hirsch, Chem. Rev., 2001, 101, 1153–1183 CrossRef.
- W. H. Green Jr, S. M. Gorun, G. Fitzgerald, P. W. Fowler, A. Ceulemans and B. C. Titeca, J. Phys. Chem., 1996, 100, 14892–14898 CrossRef.
- C. A. Reed and R. D. Bolskar, Chem. Rev., 2000, 100, 1075–1120 CrossRef CAS PubMed.
- V. V. Pavlishchuk and A. W. Addison, Inorg. Chim. Acta, 2000, 298, 97–102 CrossRef CAS.
- (a) G. Diao, L. Li and Z. Zhang, Talanta, 1996, 43, 1633–1637 CrossRef CAS; (b) F. Arias, Q. Xie, Y. Wu, Q. Lu, S. R. Wilson and L. Echegoyen, J. Am. Chem. Soc., 1994, 116, 6388–6394 CrossRef CAS; (c) Y. Ohsawa and T. Saji, J. Chem. Soc., Chem. Commun., 1992, 781–782 RSC; (d) Q. Xie, E. Perez-Cordero and L. Echegoyen, J. Am. Chem. Soc., 1992, 114, 3978–3980 CrossRef CAS.
- (a) Y. Iwasa and T. Takenobu, J. Phys.: Condens. Matter, 2003, 15, R495–R519 CrossRef CAS; (b) M. J. Rosseinsky, Chem. Mater., 1998, 10, 2665–2685 CrossRef CAS; (c) K. Prassides, Curr. Opin. Solid State Mater. Sci., 1997, 2, 433–439 CrossRef CAS; (d) C. H. Pennington and V. A. Stenger, Rev. Mod. Phys., 1996, 68, 855–910 CrossRef CAS.
- (a) Y. Nomura, S. Sakai, M. Capone and R. Arita, J. Phys.: Condens. Matter, 2016, 28, 153001 CrossRef PubMed; (b) K. Kamarás and G. Klupp, Dalton Trans., 2014, 43, 7366–7378 RSC; (c) O. Gunnarsson, J. E. Han, E. Koch and V. H. Crespi, Struct. Bonding, 2005, 114, 71–101 CrossRef CAS; (d) M. C. Böhm and J. Schulte, Phys. C, 1995, 252, 282–294 CrossRef.
- (a) D. Pontiroli, M. Aramini, M. Gaboardi, M. Mazzani, A. Gorreri, M. Ricco, I. Margiolaki and D. Sheptyakov, Carbon, 2013, 51, 143–147 CrossRef CAS; (b) M. Ricco, M. Belli, M. Mazzani, D. Pontiroli, D. Quintavalle, A. Jánossy and G. Csányi, Phys. Rev. Lett., 2009, 102, 145901 CrossRef CAS PubMed.
- For endohedral fullerenes see: (a) S. Yang, T. Wei and F. Jin, Chem. Soc. Rev., 2017, 46, 5005–5048 RSC; (b) A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed; (c) H. Cong, B. Yu, T. Akasaka and X. Lu, Chem. Rev., 2013, 257, 2880–2898 CAS; (d) X. Lu, L. Feng, T. Akasaka and S. Nagase, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- (a) D. Soto and R. Salcedo, Molecules, 2012, 17, 7151–7168 CrossRef CAS; (b) A. L. Balch and M. M. Olmstead, Chem. Rev., 1998, 98, 2123–2165 CrossRef CAS.
- A. V. Zabula and M. A. Petrukhina, Adv. Organomet. Chem., 2013, 61, 375–462 CrossRef CAS.
- For examples of soluble higher fulleride complexes of alkali metals see: (a) M. B. Boeddinghaus, W. Klein, B. Wahl, P. Jakes, R. A. Eichel and T. F. Fässler, Z. Anorg. Allg. Chem., 2014, 640, 701–712 CrossRef CAS; (b) M. B. Boeddinghaus, B. Wahl, T. F. Fässler, P. Jakes and R. A. Eichel, Z. Anorg. Allg. Chem., 2012, 638, 2205–2212 CAS; (c) M. B. Boeddinghaus, M. Salzinger and T. F. Fässler, Chem.–Eur. J., 2009, 15, 3261–3267 CrossRef CAS PubMed; (d) S. D. Hoffmann and T. F. Fässler, Z. Naturforschung B, 2004, 59b, 1579–1584 Search PubMed; (e) T. F. Fässler, R. Hoffmann, S. Hoffmann and M. Wörle, Angew. Chem., Int. Ed., 2000, 39, 2091–2094 CrossRef; (f) T. F. Fässler, A. Spiekermann, M. E. Spahr and R. Nesper, Angew. Chem., Int. Ed., 1997, 36, 486–488 CrossRef; (g) Y. Su and C. A. Reed, Chem. Commun., 1997, 747–748 Search PubMed; (h) C. Janiak, S. Mühle, H. Hemling and K. Köhler, Polyhedron, 1996, 15, 1559–1563 CrossRef CAS.
- For structurally characterised fulleride materials/complexes with ammonia-coordinated alkaline earth metals cations see: (a) M. Panthöfer, U. Wedig, H. Brumm and M. Jansen, Solid State Sci., 2004, 6, 619–624 CrossRef; (b) U. Wedig, H. Brumm and M. Jansen, Chem.–Eur. J., 2002, 8, 2769–2774 CrossRef CAS; (c) H. Brumm, E. Peters and M. Jansen, Angew. Chem., Int. Ed., 2001, 40, 2069–2071 CrossRef CAS; (d) K. Himmel and M. Jansen, Inorg. Chem., 1998, 37, 3437–3439 CrossRef CAS.
- (a) C. A. Howard, J. C. Wasse, N. T. Skipper, H. Thompson and A. K. Soper, J. Phys. Chem. C, 2007, 111, 5640–5647 CrossRef CAS; (b) C. A. Howard, PhD thesis, University College London, 2005; (c) P. Durand, Y. Dubitsky, M. J. Rosseinsky and A. Zaopo, Dalton Trans., 2004, 3137–3143 RSC.
- (a) J. W. Bausch, G. K. S. Prakash, G. A. Olah, D. S. Tse, D. C. Lorents, Y. K. Bae and R. Malhotra, J. Am. Chem. Soc., 1991, 113, 3205–3206 CrossRef CAS; (b) T. Sternfeld, R. E. Hoffman, M. Saunders, R. J. Cross, M. S. Syamala and M. Rabinovitz, J. Am. Chem. Soc., 2002, 124, 8786–8787 CrossRef CAS PubMed; (c) T. Sternfeld, C. Thilgen, R. E. Hoffman, M. d. R. C. Heras, F. Diederich, F. Wudl, L. T. Scott, J. Mack and M. Rabinovitz, J. Am. Chem. Soc., 2002, 124, 5734–5738 CrossRef CAS PubMed; (d) E. Shabtai, A. Weitz, R. C. Haddon, R. E. Hoffman, M. Rabinovitz, A. Khong, R. J. Cross, M. Saunders, P.-C. Cheng and L. T. Scott, J. Am. Chem. Soc., 1998, 120, 6389–6393 CrossRef CAS.
- S. P. Green, C. Jones and A. Stasch, Science, 2007, 318, 1754–1757 CrossRef CAS.
- R. Lalrempuia, C. E. Kefalidis, S. J. Bonyhady, B. Schwarze, L. Maron, A. Stasch and C. Jones, J. Am. Chem. Soc., 2015, 137, 8944–8947 CrossRef CAS PubMed.
- S. J. Bonyhady, C. Jones, S. Nembenna, A. Stasch, A. J. Edwards and G. J. McIntyre, Chem.–Eur. J., 2010, 16, 938–955 CrossRef CAS PubMed.
- R. S. Ruoff, D. S. Tse, R. Malhotra and D. C. Lorents, J. Phys. Chem., 1993, 97, 3379–3383 CrossRef CAS.
- (a) A. Friedrich, J. Pahl, H. Elsen and S. Harder, Dalton Trans., 2019, 48, 5560–5568 RSC; (b) L. Garcia, M. D. Anker, M. F. Mahon, L. Maron and M. S. Hill, Dalton Trans., 2018, 47, 12684–12693 RSC; (c) J. Pahl, S. Brand, H. Elsen and S. Harder, Chem. Commun., 2018, 54, 8685–8688 RSC; (d) J. Pahl, S. Friedrich, H. Elsen and S. Harder, Organometallics, 2018, 37, 2901–2909 CrossRef CAS.
- A. P. Dove, V. C. Gibson, P. Hormnirun, E. L. Marshall, J. A. Segal, A. J. P. White and D. J. Williams, Dalton Trans., 2003, 3088–3097 RSC.
- See for example: (a) K. Y. Amsharov, Y. Krämer and M. Jansen, Angew. Chem., Int. Ed., 2011, 50, 11640–11643 CrossRef CAS PubMed; (b) D. V. Konarev, A. V. Kuzmin, S. V. Simonov, S. S. Khasanov, E. I. Yudanova and R. N. Lyubovskaya, Dalton Trans., 2011, 40, 4453–4458 RSC; (c) N. V. Kozhemyakina, J. Nuss and M. Jansen, Eur. J. Inorg. Chem., 2009, 3900–3903 CrossRef CAS; (d) A. Wachowiak, R. Yamachika, K. H. Koo, Y. Wang, M. Grobis, D.-H. Lee, S. G. Louie and M. F. Crommie, Science, 2005, 310, 468–470 CrossRef CAS PubMed; (e) W. C. Wan, X. Liu, G. M. Sweeney and W. E. Broderick, J. Am. Chem. Soc., 1995, 117, 9580–9581 CrossRef CAS; (f) P. Paul, Z. Xie, R. Bau, P. D. W. Boyd and C. A. Reed, J. Am. Chem. Soc., 1994, 116, 4145–4146 CrossRef CAS.
- I. Haiduc, Coord. Chem. Rev., 2017, 338, 1–26 CrossRef CAS.
- (a) D. A. Tomalia and S. N. Khanna, Chem. Rev., 2016, 116, 2705–2774 CrossRef CAS PubMed; (b) G. J. Dutton, D. B. Dougherty, W. Jin, J. E. Reutt-Robey and S. W. Robey, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 195435 CrossRef; (c) M. Feng, J. Zhao and H. Petek, Science, 2008, 320, 359–362 CrossRef CAS PubMed.
- (a) M. H. Chisholm, C. E. Hammond and J. C. Huffman, J. Chem. Soc., Chem. Commun., 1987, 1423–1424 RSC; (b) D. C. Bradley, M. H. Chisholm, C. E. Heath and M. B. Hursthouse, J. Chem. Soc., Chem. Commun., 1969, 1261 RSC.
- L. Hajji, F. Rachdi, C. Goze, M. Mehring and J. E. Fischer, Solid State Commun., 1996, 100, 493–496 CrossRef CAS.
- (a) E. M. Schubert, J. Chem. Educ., 1992, 69, 62–63 CrossRef CAS; (b) D. F. Evans, J. Chem. Soc., 1959, 2003–2005 RSC.
- (a) P. D. W. Boyd, P. Bhyrappa, P. Paul, J. Stinchcombe, R. D. Bolskar, Y. Sun and C. A. Reed, J. Am. Chem. Soc., 1995, 117, 2907–2914 CrossRef CAS; (b) P. Bhyrappa, P. Paul, J. Stinchcombe, P. D. W. Boyd and C. A. Reed, J. Am. Chem. Soc., 1993, 115, 11004–11005 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, 87th edn, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic, crystallographic and computational details (PDF); xyz coordinates. CCDC 1914895–1914903. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc03857d |
| This journal is © The Royal Society of Chemistry 2019 |