Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Expedient access to saturated nitrogen heterocycles by photoredox cyclization of imino-tethered dihydropyridines†
Noah B.
Bissonnette
 ,
J. Michael
Ellis
,
J. Michael
Ellis
 ,
Lawrence G.
Hamann
and
Fedor
Romanov-Michailidis
,
Lawrence G.
Hamann
and
Fedor
Romanov-Michailidis
 *
*
Celgene Corporation, 200 Cambridge Park Drive, Suite 3000, Cambridge, MA 02140, USA. E-mail: fmichailidis@celgene.com; fedorromanov87@gmail.com
First published on 28th August 2019
Abstract
A large proportion of medicinally relevant molecules bear nitrogen and sp3-hybridized carbon functionalities. Overwhelmingly, these atoms are found as part of (hetero)cyclic structures. Despite their importance, synthetic approaches to saturated nitrogen heterocycles are limited to several established stoichiometric alkylation techniques, as well as a few methods involving C–H bond activation. The synthetic community remains interested in more general, mild, and sustainable ways to access these motifs. Here we describe a dual-catalyst system composed of an iridium photocatalyst and a lithium phosphate base that is capable of selectively homolyzing the N–H bond of 4-alkyl-1,4-dihydropyridines, presumably by proton-coupled-electron-transfer (PCET), and mediating efficient cyclization of the resultant carbon-centered radicals with tethered imines. The outcome of this transformation is access to a broad range of structurally complex nitrogen heterocycles obtainable from simple aldehyde starting materials in a highly chemoselective manner.
Therapeutic drugs display a wide range of structural diversity, dense arrays of functional groups, stereochemical complexity, and a large number of sp3-hybridized carbon centers.1 When combined, these features lead to a spectrum of specific three-dimensional molecular signatures for each compound ultimately influencing biological activity and physicochemical properties.2 Key structural elements are responsible for the “drug-likeness” of organic molecules. In particular, nitrogen heterocycles,3 as well as high-fraction sp3 (Fsp3) scaffolds4 are among the most impactful structural components of pharmaceuticals. Indeed, although these molecular components represent a small part of the entire drug molecule, they can significantly influence on-target potency, absorption–distribution–metabolism–excretion (ADME) properties, bioavailability, and pharmacokinetics.5 As a result, the synthetic community has invested considerable time and effort devising methods for the efficient assembly of sp3-rich nitrogen heterocycles such as piperidines, morpholines, thiomorpholines, and piperazines.3b,c,6
Typical access to these heterocycles relies on several established stoichiometric techniques,7 each with its own strengths and shortcomings. Various alkylations afford morpholines, piperidines, and piperazines—although substitution can be limited. In addition, catalytic C–H activation chemistry has been recently used to prepare piperidine derivatives,8 but these methods often require involved substrates and expensive transition-metal catalysts. The limitations imposed by current synthetic methods can increase the difficulty of accessing polysubstituted derivatives of these heterocycles. There remains value in more general, mild, and sustainable approaches to saturated nitrogen heterocycles.
Visible light constitutes an environmentally benign and sustainable source of energy to effect chemical reactivity.9 Photocatalysts can remove an electron from organic substrates by harnessing over 60 kcal mol−1 of energy from visible light. In addition, Brønsted bases can catalyze the photo-oxidation of weakly acidic substrates by a process known as proton-coupled-electron-transfer (PCET). PCET involves the simultaneous removal of an electron and a proton from an organic molecule in a single concerted step.10
Work by Nishibayashi and Molander demonstrated 4-alkyl-1,4-dihydropyridines (DHPs) as latent radical precursors accessed from various aldehydes.11,12 The Hantzsch ester moiety can undergo single-electron-transfer (SET) oxidation to the corresponding radical cation I. followed by homolysis of the C–C bond, releasing an alkyl radical II. and a pyridine byproduct (Scheme 1). Given the potent hydrogen-bonding ability of Hantzsch esters,13 we envisioned oxidation of a DHP moiety under mild conditions by a PCET mechanism combining an iridium photocatalyst and a Brønsted basic counterion (X−).14
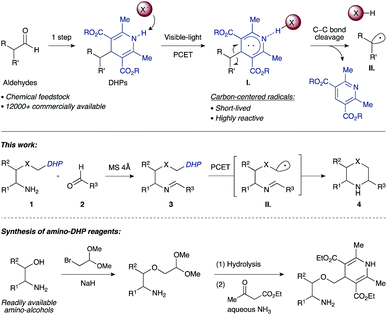 | ||
| Scheme 1 Synthesis of saturated N-heterocycles by photoredox cyclization of imino-tethered dihydropyridines. | ||
We propose that condensation of an amino-DHP reagent 1 with an aldehyde 2 would give an imino-tethered DHP intermediate 3 that could subsequently photocyclize by a radical PCET mechanism, providing entry to medicinally relevant nitrogen heterocycles such as (thio)morpholines and piperazines 4 (Scheme 1). Earlier reports with tin (SnAP)15 and silicon (SLAP)16 radical precursors require toxic reagents, tolerate a narrow substrate scope, or implement harsh reaction conditions.17 In contrast, the concerted PCET pathway would allow the use of photocatalysts and bases with redox potentials (Eox) and pKa values far removed from those of the DHP substrate being activated.18 Since radicals may be formed under mild reaction conditions, this was anticipated to afford wider functional group compatibility and overall synthetic utility. Herein, we describe our findings in this endeavour.
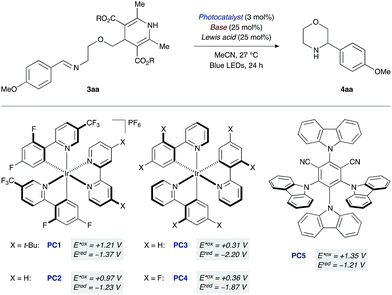
At the outset of our studies with the morpholine-forming imino-DHP substrate 3aa, we examined photoredox catalysts that provide higher oxidation potentials in their photoexcited states (E*ox) (Table 1). For example, the excited state species of the cationic iridium photocatalyst [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 (PC1, E*ox +1.21 V vs. SCE;19,20 where dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine, dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) should oxidize the DHP moiety (Eox ranging from +1.01 to +1.23 V vs. SCE).12,21 However, attempted cyclization with this catalyst led to no desired product, unless co-catalytic base was used as an additive.22 Among the various bases initially tested, tetrabutylammonium acetate (pKa of conjugate acid 12 in DMSO)23 gave the most promising results (25% yield of product 4aa, entry 1). We reasoned that the low yield was the result of insufficient ability of the reduced form of photocatalyst PC1 (Ered −1.37 V vs. SCE)19,20 to reduce the nitrogen-centered radical and complete the catalytic cycle.24 Indeed, switching to a related iridium photocatalyst lacking the t-Bu groups on the bipyridyl ligand [Ir(dF(CF3)ppy)2(bpy)]PF6 (PC2, E*ox +0.97 V, Ered −1.23 V vs. SCE; where bpy = 2,2′-bipyridine)14,20 led to an improved reaction yield (33%, entry 2). Neutral iridium photocatalysts with even lower excited-state oxidation potentials (PC3 and PC4, E*ox +0.31 and +0.36 V vs. SCE)20 were also investigated. Moderate yields of the desired morpholine 4aa were observed (18% and 20%, respectively, entries 3 and 4), indicating that strongly oxidizing photocatalysts are not required for successful cyclization given that co-catalytic tetrabutylammonium acetate is present.
| Entry | Photocat. | Base | Effective BDFEb | Yieldc (%) |
|---|---|---|---|---|
a R = Et, for entries 1–15.
b BDFE in kcal mol−1.
c Isolated yield of purified product.
d With 25 mol% of Bi(OTf)3.
e With 25 mol% of Mg(NTf2)2.
f With 25 mol% of LiNTf2.
g With CH2Cl2 as solvent.
h With CH2Cl2/TFE 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture as solvent.
i R = i-Pr. Ac, acetyl. TFE, 2,2,2-trifluoroethanol. TFA, 2,2,2-trifluoroacetate. DBP, di(n-butyl)phosphate. 1 mixture as solvent.
i R = i-Pr. Ac, acetyl. TFE, 2,2,2-trifluoroethanol. TFA, 2,2,2-trifluoroacetate. DBP, di(n-butyl)phosphate.
|
||||
| 1 | PC1 | [AcO]NBu4 | 99 | 25 |
| 2 | PC2 | [AcO]NBu4 | 94 | 33 |
| 3 | PC3 | [AcO]NBu4 | 78 | 18 |
| 4 | PC4 | [AcO]NBu4 | 80 | 20 |
| 5 | PC5 | [AcO]NBu4 | 102 | 21 |
| 6f | PC2 | [AcO]NBu4 | 94 | 39 |
| 7 | PC2 | [TFA]NBu4 | 87 | 36 |
| 8 | PC2 | [DBP]NBu4 | 84 | 41 |
| 9d | PC2 | [DBP]NBu4 | 84 | 39 |
| 10e | PC2 | [DBP]NBu4 | 84 | 45 |
| 11f | PC2 | [DBP]NBu4 | 84 | 49 |
| 12 | PC2 | [DBP]2Mg | 84 | 48 |
| 13 | PC2 | [DBP]Li | 84 | 51 |
| 14g | PC2 | [DBP]Li | 84 | 58 |
| 15h | PC2 | [DBP]Li | 84 | 67 |
| 16h,i | PC2 | [DBP]Li | 84 | 80 |
To further evaluate effective photocatalyst/base combinations for oxidative DHP cleavage, we made use of a thermodynamic formalism introduced by Mayer25 and further elaborated by Knowles14 that defines an effective bond strength (“BDFE”) for any given oxidant/base pair as a function of the redox potential and pKa value of its constituents. We tested the validity of this approach by evaluating the combinations of five photocatalysts PC1–5 and three Brønsted bases of decreasing pKa of their conjugate acids (AcO− > CF3CO2− > (n-BuO)2PO2−).22 We observed that combinations with “BDFE” values significantly lower than the strength of the N–H bond of the Hantzsch ester in substrate 3aa (ca. 90 kcal mol−1)13b are not effective catalysts for cyclization. For example, the “BDFE” values for PC3 or PC4 in combination with tetrabutylammonium acetate are 78 and 80 kcal mol−1, respectively,22 and the corresponding yields of 4aa are 18% and 20% (entries 3 and 4). Similarly, for combinations with very high “BDFE” values (>100 kcal mol−1), low yields of morpholine 4aa are obtained. An organic photocatalyst with high oxidation ability, 4CzIPN (PC5, E*ox +1.35 V vs. SCE),26 when used in conjunction with tetrabutylammonium acetate leads to only 21% yield of 4aa despite a “BDFE” value of 102 kcal mol−1 (entry 5).
However, photocatalyst/base combinations with “BDFE” values approaching or slightly exceeding the N–H BDFE of 3aa result in efficient generation of product 4aa. For example, the “BDFE” values for PC2 in conjunction with either trifluoroacetate [CF3CO2]NBu4 (pKa of conjugate acid 7 in DMSO)23 or dibutylphosphate [(BuO)2PO2]NBu4 (pKa of conjugate acid 5 in DMSO)23 are 87 and 84 kcal mol−1, respectively. This means that PCET activation of DHP substrate 3aa is thermodynamically feasible with these photocatalyst/base combinations. In accord with this, a slight increase in yield is observed experimentally (36% and 41%, entries 7 and 8). Notably, iridium complex PC2 (E*ox +0.97 V vs. SCE) and dibutylphosphate [(BuO)2PO2]NBu4 (pKa of conjugate acid 5 in DMSO) have an oxidation potential and pKa value far removed from those of the DHP substrate (Eox +1.23 V vs. SCE, pKa 14 in DMSO).21,23 These results are consistent with a PCET mechanism for the oxidation of the Hantzsch ester moiety.
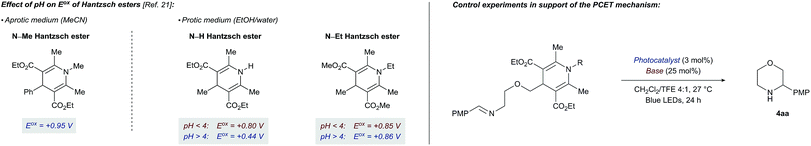
Voltammetric oxidation of 4-alkyl-1,4-dihydropyridines has been described in aprotic21a and protic21b,c media. While at pH < 4 the oxidation process is independent of the pH of the medium, at pH > 4 the process becomes markedly pH-dependent with deprotonation of the N–H bond of the Hantzsch ester now being the rate-determining step. Additionally, in the pH-dependent regime, the Hantzsch esters are more easily oxidized than at lower pH values. Conversely, a N-ethyl Hantzsch ester derivative shows a completely pH-independent oxidation behaviour. To place these observations in the context of our photocatalytic cyclization reaction and further probe the PCET mechanism for DHP cleavage, we ran additional control experiments (Table 2).
| Entry | R | Photocat. | E*ox,a | Base | pKa | Effective BDFEb | Yieldc (%) |
|---|---|---|---|---|---|---|---|
| a E*ox in V. b BDFE in kcal mol−1. c Isolated yield of purified product. Ac, acetyl. TFA, 2,2,2-trifluoroacetate. DBP, di(n-butyl)phosphate. BArF4, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate. | |||||||
| 1 | H | PC2 | +0.97 | [AcO]NBu4 | 12 | 94 | 33 |
| 2 | H | PC2 | +0.97 | [TFA]NBu4 | 7 | 87 | 36 |
| 3 | H | PC2 | +0.97 | [DBP]NBu4 | 5 | 84 | 41 |
| 4 | H | PC2 | +0.97 | [BArF4]Na | <1 | — | <5 |
| 5 | H | PC2 | +0.97 | None | — | — | <5 |
| 6 | H | PC5 | +1.35 | None | — | — | 14 |
| 7 | H | PC5 | +1.35 | [AcO]NBu4 | 12 | 102 | 21 |
| 8 | Me | PC2 | +0.97 | [AcO]NBu4 | 12 | 94 | <5 |
| 9 | Me | PC5 | +1.35 | [AcO]NBu4 | 12 | 102 | 17 |
Consistent with a PCET mechanism, moderate yields of cyclization product 4aa (33–41%, entries 1–3) are witnessed for combinations of PC2 and various bases with effective BDFE values in the range of the DHP N–H bond strength (ca. 90 kcal mol−1). In contrast, almost no product 4aa is formed when (i) the base is replaced by a non-coordinating BArF4 anion (entry 4), (ii) the base is omitted (entry 5), or (iii) a N-methyl Hantzsch ester derivative is used as substrate (entry 8). These results suggest that a base and an intact N–H bond are both required for efficient photoredox cleavage of the DHP moiety by photocatalyst PC2 (E*ox +0.97 V vs. SCE). On the other hand, a stronger photooxidant PC5 (E*ox +1.35 V vs. SCE) that does not necessarily depend on a PCET mechanism to oxidize the Hantzsch ester requires neither a base nor an intact N–H bond to promote cyclization (14% and 17% yield, entries 6 and 9). Taken together, the results of these control experiments and the published data on the pH dependency of DHP Eox potentials are most consistent with a PCET mechanism for the cleavage of the Hantzsch ester in the title reaction. Interestingly, the cyclization with PC5 is more efficient when performed with tetrabutylammonium acetate and an intact N–H bond both present (21% yield, entry 7). This result implies that hydrogen bonding between the Hantzsch ester and the base is important not only for efficient oxidation of the DHP to its corresponding radical cation but also for subsequent C–C bond cleavage (Scheme 1).
From the successful photocatalyst/base combinations tested, we elected to further study the PC2/dibutylphosphate pair. Control reactions omitting either the iridium photocatalyst or visible-light irradiation provided none of the desired cyclization product.22 Similarly, reactions run in the absence of the phosphate base resulted in <5% conversion of the starting DHP substrate 3aa. Alternatively, adding Lewis acids to the reaction mixture led to interesting results (Table 1). While strong Lewis acids such as Bi(OTf)3 did not have much effect (39% yield, entry 9), milder Lewis acids such as Mg(NTf2)2 or LiNTf2 were found to promote the cyclization (45% and 49% yield, entries 10 and 11). Most reproducible results are obtained when pre-forming the dibutylphosphate with the Lewis-acidic cation. Indeed, when using 25 mol% of the [(n-BuO)2PO2]Li additive, the desired morpholine 4aa is recovered in 51% yield (entry 13). Furthermore, we found that the reaction affords better results when (i) carried out in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of dichloromethane and 2,2,2-trifluoroethanol (67% yield, entry 15), and (ii) the substrate contains bulky iso-propyl groups on the Hantzsch ester moiety (80% yield, entry 16).
1 v/v mixture of dichloromethane and 2,2,2-trifluoroethanol (67% yield, entry 15), and (ii) the substrate contains bulky iso-propyl groups on the Hantzsch ester moiety (80% yield, entry 16).
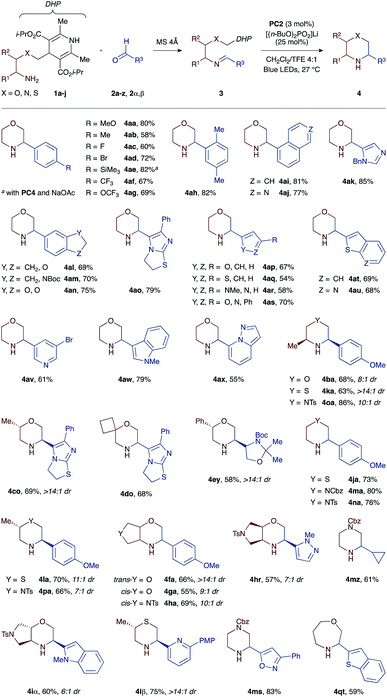
To demonstrate the versatility of our one-pot protocol, various aldehydes 2a–z, 2α, and 2β (blue colour) were condensed with either morpholine-, thiomorpholine-, or piperazine-forming amino-DHP reagents 1a–j (red colour), and then subjected to the photoredox cyclization with visible light irradiation (Scheme 2).
The cyclization tolerates a broad spectrum of substituents and functional groups. For example, benzaldehyde derivatives bearing either electron-withdrawing (CF3, OCF3, and F) or -releasing (Me, MeO, and SiMe3) substituents at the para position all cyclize well with the morpholine-forming amino-DHP reagent 1a, affording the corresponding heterocyclic products 4aa–4ag in high yields (58–82%). We were pleased to find that imines 3 derived from heteroaromatic aldehydes also work well in our reaction. These substrates are particularly interesting from a drug discovery perspective, but are challenging to incorporate into redox reactions due to competing off-target oxidation events and non-specific binding of the basic nitrogen atoms to Lewis acids. However, under our mild PCET conditions, a wide array of heteroaromatic substrates are tolerated, ranging from electron-deficient pyridines (3av and 3lβ), thienopyridines (3au), isoquinolines (3aj), imidazoles (3ak), pyrazolopyridines (3ax), oxazoles (3as and 3ms), and pyrazoles (3ar and 3hr) to electron-rich furans (3ap), thiophenes (3aq), benzothiophenes (3at and 3jt), indoles (3aw and 3iα), indolines (3am), and dihydroimidazothiazoles (3co and 3do). Lastly, imines derived from aliphatic aldehydes cyclize equally well, giving access to pharmacologically relevant products such as chiral glyceraldehyde-derived morpholine 4ey (58% yield) or cyclopropyl-substituted piperazine 4mz (61% yield). Importantly, the necessity of hydrogen-bonding between the Hantzsch ester moiety and the phosphate base enables chemoselective PCET activation even in the presence of more readily oxidizable groups such as indole (e.g., imines 3aw or 3iα with Eox +1.21 V vs. SCE).
With respect to the amino-DHP component, various branched tethers are accommodated in the cyclization reaction, including those bearing methyl (1b and 1c) or phenyl groups (1e), spirocycles (1d), and trans- (1f and 1i) or cis-fused bicycles (1g and 1h). The yields of the corresponding morpholine products are mostly high (55–69%), and excellent diastereoselectivities (up to >14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) are observed in cases where multiple stereogenic centers are created such as in products 4ba, 4co, 4fa, 4ga, 4ha, 4hr, and 4iα. Moreover, the reaction outcome is not restricted to six-membered products, and moderately sized ring systems such as 4qt can be accessed with equally high efficiency (59% yield). A more exhaustive list of obtainable heterocyclic products (>50 examples) is compiled in the ESI.†22
1 dr) are observed in cases where multiple stereogenic centers are created such as in products 4ba, 4co, 4fa, 4ga, 4ha, 4hr, and 4iα. Moreover, the reaction outcome is not restricted to six-membered products, and moderately sized ring systems such as 4qt can be accessed with equally high efficiency (59% yield). A more exhaustive list of obtainable heterocyclic products (>50 examples) is compiled in the ESI.†22
The generality of our approach was further evaluated by investigating access to saturated nitrogen heterocycles other than morpholines. To this end, we devised a set of thiomorpholine- (1j, 1k, and 1l) and piperazine-forming (1m, 1n, 1o, and 1p) amino-DHP reagents that were first condensed with aldehydes 2a, 2s, 2z, and 2β and then photocyclized with blue light (Table 1). To our great delight, all of the substrate combinations examined were found to give the expected cyclized products in high yields (63–86%) and with excellent levels of diastereocontrol (up to >14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). This is an important finding as it points to the utility of this approach versus previously described methods where the redox potentials of the tin or silicon reagents shift greatly as a function of the heteroatom present in the substrate: Eox(O) > Eox(S) > Eox(N).15–17 Conversely, because the same DHP moiety is used in all our substrate types, its oxidation potential (Eox +1.23 V vs. SCE) remains unaffected by the nature of the tethering heteroatom and permits the synthesis of morpholines, thiomorpholines, and piperazines under a single set of catalytic conditions.
1 dr). This is an important finding as it points to the utility of this approach versus previously described methods where the redox potentials of the tin or silicon reagents shift greatly as a function of the heteroatom present in the substrate: Eox(O) > Eox(S) > Eox(N).15–17 Conversely, because the same DHP moiety is used in all our substrate types, its oxidation potential (Eox +1.23 V vs. SCE) remains unaffected by the nature of the tethering heteroatom and permits the synthesis of morpholines, thiomorpholines, and piperazines under a single set of catalytic conditions.
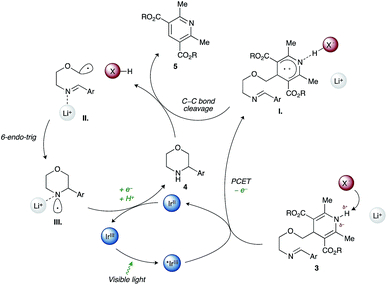
As shown in Scheme 3, a plausible redox-neutral photocatalytic cycle involves: (1) PCET quenching of the photoexcited Ir(III)* species (E*ox +0.97 V) by imino-DHP 3 (Eox +1.23 V) with help from the Brønsted-basic phosphate anion (X−, pKa of conjugate acid 5); (2) β-scission of the resultant radical-cationic intermediate I. that affords α-heteroatom stabilized C-centered radical II., conjugate acid XH, and pyridine byproduct 5; (3) Baldwin-allowed 6-endo-trig cyclization of II. that delivers N-centered radical III.; and (4) SET-reduction of III. by the Ir(II) photocatalyst (Ered −1.23 V) followed by proton transfer from acid XH that furnishes morpholine 4 and regenerates the ground-state Ir(III) photocatalyst as well as the X− counterion.
We reason that the Hantzsch ester moiety in imino-DHP 3 (pKa 14) can form a strong H-bond with dibutylphosphate (pKa of conjugate acid 5). Although H-bonding of the N–H bond is anticipated to lower the potential required for photooxidation of the Hantzsch ester, it will not fully deprotonate the resultant radical cationic intermediate I. Consequently, the nascent radical cation will only be generated when the substrate is associated with the anion. This explains the observed high chemoselectivity of our method. Indeed, the employed photocatalyst/base dual-catalyst system is reluctant to oxidizing other redox-labile functional groups present in substrates given that they do not have N–H bonds of sufficient acidity. Furthermore, we believe that the Lewis-acidic lithium cation coordinates to the N-centered radical III. leading to its stabilization and a concomitant decrease of its reduction potential relative to the uncoordinated N-centered radical.27 This in turn renders reduction of III. by the Ir(II) photocatalyst (Ered −1.23 V) more facile.
Nitrogen heterocycles are among the most abundant structural components of pharmaceuticals. For example, of the top 200 brand name commercial medicines in 2014, 116 contain a nitrogen heterocycle.3a We have developed a one-pot approach to saturated nitrogen heterocycles that harnesses energy from visible light to cleave 4-alkyl-1,4-dihydropyridines by a unique PCET mechanism. Cleavage is followed by cyclization of the resultant carbon-centered radical with a tethered imine, giving heterocyclic products. Our mild protocol is characterized by a remarkably broad substrate scope and high functional group tolerance. The concise assembly of a variety of these heterocycles should allow desirable properties such as solubility, permeability, and metabolic stability, to be built into lead series, ultimately increasing the pace of drug discovery.
Author contributions
This project was conceived and initiated by FRM. Optimization of the reaction conditions, the synthesis and characterization of starting materials and products was performed by NBB with assistance from FRM. The manuscript was drafted by FRM and proofread by FRM, NBB, LGH, and JME. The ESI was drafted by NBB and proofread by FRM, NBB, LGH, and JME.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Dr Geraint Davies and Dr Kurt Armbrust for helpful discussions.Notes and references
- M. S. Butler, A. A. Robertson and M. A. Cooper, Nat. Prod. Rep., 2014, 31, 1612 RSC
.
-
(a) P. A. Clemons, N. E. Bodycombe, H. A. Carrinski, J. A. Wilson, A. F. Shamii, B. K. Wagner, A. N. Koethler and S. L. Schreiber, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18787 CrossRef CAS PubMed
; (b) D. Morton, S. Leach, C. Cordier, S. Warriner and A. Nelson, Angew. Chem., Int. Ed., 2009, 48, 104 CrossRef CAS PubMed
.
-
(a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed
; (b) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451 CrossRef CAS PubMed
; (c) J. S. Carey, D. Laffan, C. Thomson and M. T. Wiliams, Org. Biomol. Chem., 2006, 4, 2337 RSC
.
-
(a) F. Lovering, J. Bikker and C. J. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed
; (b) F. Lovering, MedChemComm, 2013, 4, 515 RSC
.
- D. G. Brown and J. Boström, J. Med. Chem., 2016, 59, 4443 CrossRef CAS PubMed
.
- T. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal and S. W. Krska, Chem. Soc. Rev., 2016, 45, 546 RSC
.
-
(a) S. A. Worlikar, T. Kesharwani, T. Yao and R. C. Larock, J. Org. Chem., 2007, 72, 1347 CrossRef CAS PubMed
; (b) T. J. A. Graham and A. G. Doyle, Org. Lett., 2012, 14, 1616 CrossRef CAS PubMed
; (c) S.-X. Liu, C.-M. Jia, B.-Y. Yao, X.-L. Chen and Q. Zhang, Synthesis, 2016, 48, 407 CAS
; (d) C. C. Malakar, D. Schmidt, J. Conrad and U. Beifuss, Org. Lett., 2011, 13, 1972 CrossRef CAS PubMed
; (e) R. K. Orr, L.-C. Campeau, H. R. Chobanian, H. M. McCabe Dunn, B. Pio, C. W. Plummer, A. Nolting and R. T. Ruck, Synthesis, 2017, 49, 657 CrossRef CAS
; (f) T. Azuma, A. Murata, Y. Kobayashi, T. Inokuma and Y. Takemoto, Org. Lett., 2014, 16, 4256 CrossRef CAS PubMed
; (g) Y.-L. Du, Y. Hu, Y.-F. Zhu, X.-F. Tu, Z.-Y. Han and L.-Z. Gong, J. Org. Chem., 2015, 80, 4754 CrossRef CAS PubMed
; (h) M. Yan, T. Jin, Q. Chen, H. E. Ho, T. Fujita, L.-Y. Chen, M. Bao, M.-W. Chen, N. Asao and Y. Yamamoto, Org. Lett., 2013, 15, 1484 CrossRef CAS PubMed
; (i) N. Gandhamsetty, S. Park and S. Chang, Synlett, 2017, 28, 2396 CrossRef CAS
.
-
(a) F. Romanov-Michailidis, K. F. Sedillo, J. M. Neely and T. Rovis, J. Am. Chem. Soc., 2015, 137, 8892 CrossRef CAS PubMed
; (b) I. S. Hassan, A. N. Ta, M. W. Danneman, N. Semakul, M. Burns, C. H. Basch, V. N. Dippon, B. R. McNaughton and T. Rovis, J. Am. Chem. Soc., 2019, 141, 4815 CrossRef CAS PubMed
.
- J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev., 2017, 1, 1 Search PubMed
.
-
(a) J. M. Mayer, Annu. Rev. Phys. Chem., 2004, 55, 363 CrossRef CAS PubMed
; (b) D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016 CrossRef CAS PubMed
; (c) H. G. Yayla and R. R. Knowles, Synlett, 2014, 25, 2819 CrossRef CAS
.
- K. Nakajima, S. Nojima, K. Sakata and Y. Nishibayashi, ChemCatChem, 2016, 8, 1028 CrossRef CAS
.
- A. Gutiérrez-Bonet, C. Remeur, J. K. Matsui and G. A. Molander, J. Am. Chem. Soc., 2017, 139, 12251 CrossRef PubMed
.
-
(a) U. Eisner and J. Kuthan, Chem. Rev., 1972, 1, 1 CrossRef
; (b) A. I. Meyers and D. M. Stout, Chem. Rev., 1982, 82, 223 CrossRef
; (c) R. Fossheim, A. Joslyn, A. J. Solo, E. Luchowski, A. Rutledge and D. J. Triggle, J. Med. Chem., 1982, 25, 126 CrossRef CAS
; (d) S. Goldman, L. Born, S. Kazda, B. Pittel and M. Schramm, J. Med. Chem., 1990, 33, 1413 CrossRef PubMed
.
- G. J. Choi and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 9226 CrossRef CAS PubMed
.
-
(a) C.-V. T. Vo, G. Mikutis and J. W. Bode, Angew. Chem., Int. Ed., 2013, 52, 1705 CrossRef CAS
; (b) W.-Y. Siau and J. W. Bode, J. Am. Chem. Soc., 2014, 136, 17726 CrossRef CAS PubMed
.
- S.-Y. Hsieh and J. W. Bode, ACS Cent. Sci., 2017, 3, 66 CrossRef CAS PubMed
.
-
(a) M. U. Luescher, C.-V. T. Vo and J. W. Bode, Org. Lett., 2014, 16, 1236 CrossRef CAS PubMed
; (b) S.-Y. Hsieh and J. W. Bode, Org. Lett., 2016, 18, 2098 CrossRef CAS PubMed
; (c) M. K. Jackl, L. Legnani, B. Morandi and J. W. Bode, Org. Lett., 2017, 19, 4696 CrossRef CAS PubMed
; (d) C. Jindakun, S.-Y. Hsieh and J. W. Bode, Org. Lett., 2018, 20, 2071 CrossRef CAS PubMed
.
-
(a) E. C. Gentry and R. R. Knowles, Acc. Chem. Res., 2016, 49, 1546 CrossRef CAS
; (b) L. Q. Nguyen and R. R. Knowles, ACS Catal., 2016, 6, 2894 CrossRef CAS
.
-
(a) J. D. Slinker, A. A. Gorodetsky, M. S. Lowry, J. Wang, S. Parker, R. Rohl, S. Bernhard and G. G. Malliaras, J. Am. Chem. Soc., 2004, 126, 2763 CrossRef CAS PubMed
; (b) M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712 CrossRef CAS
.
- K. Teegardin, J. I. Day, J. Chan and J. Weaver, Org. Process Res. Dev., 2016, 20, 1156 CrossRef CAS PubMed
.
-
(a) J. Stradins, J. Ogle, V. Kadysh, L. Baumane, R. Gavars and G. Duburs, J. Electroanal. Chem., 1987, 226, 103 CrossRef CAS
; (b) J. Arguello, L. J. Nunez-Vergara, J. C. Sturm and J. A. Squella, Electrochim. Acta, 2004, 49, 4849 CrossRef CAS
; (c) C. Lopez-Alarcon, L. J. Nunez-Vergara and J. A. Squella, Electrochim. Acta, 2003, 48, 2505 CrossRef CAS
.
- See the ESI† for details.
- F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456 CrossRef CAS
.
-
(a) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234 CrossRef CAS PubMed
; (b) J. Luo and J. Zhang, ACS Catal., 2016, 6, 873 CrossRef CAS
.
-
(a) J. J. Warren, T. A. Tronic and J. M. Mayer, Chem. Rev., 2010, 110, 6961 CrossRef CAS PubMed
; (b) C. R. Waidmann, A. J. Miiler, C. W. A. Ng, M. L. Scheuermann, T. R. Porter, T. A. Tronic and J. M. Mayer, Energy Environ. Sci., 2012, 5, 7771 RSC
.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075 CrossRef CAS PubMed
.
-
(a) M. Jonsson, D. D. M. Wayner and J. Lusztyk, J. Phys. Chem., 1996, 100, 17539 CrossRef CAS
; (b) U. Wille, G. Heuger and C. Jargstorff, J. Org. Chem., 2008, 73, 1413 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary substrate scope. Supplementary optimizations table. BDFE calculations. Detailed experimental procedures and compound characterization. See DOI: 10.1039/c9sc03429c |
| This journal is © The Royal Society of Chemistry 2019 |