Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A sensor array for the discrimination of polycyclic aromatic hydrocarbons using conjugated polymers and the inner filter effect†
Joshua
Tropp
 a,
Michael H.
Ihde
a,
Michael H.
Ihde
 b,
Abagail K.
Williams
a,
Nicholas J.
White
b,
Abagail K.
Williams
a,
Nicholas J.
White
 b,
Naresh
Eedugurala
b,
Naresh
Eedugurala
 a,
Noel C.
Bell
a,
Jason D.
Azoulay
a,
Noel C.
Bell
a,
Jason D.
Azoulay
 *a and
Marco
Bonizzoni
*a and
Marco
Bonizzoni
 *b
*b
aCenter for Optoelectronic Materials and Devices, School of Polymer Science and Engineering, The University of Southern Mississippi, 118 College Drive #5050, Hattiesburg, MS 39406, USA. E-mail: jason.azoulay@usm.edu
bDepartment of Chemistry and Biochemistry, The University of Alabama, P.O. Box 870336, Tuscaloosa, AL 35487, USA. E-mail: marco.bonizzoni@ua.edu
First published on 7th October 2019
Abstract
Natural and anthropogenic activities result in the production of polycyclic aromatic hydrocarbons (PAHs), persistent pollutants that negatively impact the environment and human health. Rapid and reliable methods for the detection and discrimination of these compounds remains a technological challenge owing to their relatively featureless properties, structural similarities, and existence as complex mixtures. Here, we demonstrate that the inner filter effect (IFE), in combination with conjugated polymer (CP) array-based sensing, offers a straightforward approach for the quantitative and qualitative profiling of PAHs. The sensor array was constructed from six fluorescent fluorene-based copolymers, which incorporate side chains with peripheral 2-phenylbenzimidazole substituents that provide spectral overlap with PAHs and give rise to a pronounced IFE. Subtle structural differences in copolymer structure result in distinct spectral signatures, which provide a unique “chemical fingerprint” for each PAH. The discriminatory power of the array was evaluated using linear discriminant analysis (LDA) and principal component analysis (PCA) in order to discriminate between 16 PAH compounds identified as priority pollutants by the US Environmental Protection Agency (EPA). This array is the first multivariate system reliant on the modulation of the spectral signatures of CPs through the IFE for the detection and discrimination of closely related polynuclear aromatic species.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a ubiquitous and prominent class of organic compounds comprised of fused aromatic rings containing only carbon and hydrogen. Well over 120 years of research has intricately connected these compounds with their natural and anthropogenic origins.1 While natural sources include those such as fossil fuels, open burning, and volcanic activity; pyrogenic and petrogenic sources such as the combustion of these fossil fuels, industrial manufacturing, and dispersed sources (i.e. automotive emission, residential heating, food preparation, etc.) predominate.2 These activities result in the production of PAHs that are pervasive environmental pollutants with toxic, mutagenic, and carcinogenic properties.3 For these reasons, research efforts remain unabated toward the detection and discrimination of these compounds; however, this continues to represent a major technological hurdle owing to their uncharged and nonpolar nature, similar and relatively featureless structures, lack of heteroatoms or substituents, and low active concentrations.4Current methods for PAH detection necessitate sample collection, transport, protracted multi-stage extraction, preconcentration, and separation procedures. Subsequently, specialized analytical instrumentation sensitive to subtle chemical differences and employing multiple detectors is applied for analysis. Examples include high-performance liquid chromatography (HPLC) coupled to fluorescence or ultraviolet detection,5 gas chromatography coupled to mass spectrometry (GC-MS) or flame ionization detection (FID),6 and capillary electrophoresis coupled to fluorescence or ultraviolet detection.7 While highly sensitive, these methods require trained personnel, long analysis time, may lead to low analytical precision and analyte bias, are intended for a specific PAH type or property, and cannot discriminate between closely related compounds such as benzo[a]pyrene and dibenz[a,h]anthracene; isomers differing only in the connectivity of an aromatic ring and classified as carcinogens by numerous international agencies.3 Immunoassay-based approaches utilize antibody–antigen interactions, which demonstrate improved sensitivities in platforms that offer reduced costs, rapid detection, portability, and are amenable to high-throughput.4,8 Despite these advantages, similarities in the molecular and electronic structure of PAHs preclude the development of specific antibodies, leading to cross-reactivity and the inability to differentiate between closely related compounds.9 In a similar manner, various nanomaterial-based sensor platforms utilizing quantum dots, graphene, carbon nanotubes, and polymer composites have been advanced as alternative approaches for the detection of PAHs, but are fundamentally limited in their discriminatory power in multi-analyte detection.4c,10 As such, important questions regarding source attribution, transport and fate in the environment, bioaccumulation in the food chain, and the toxicology of these ubiquitous pollutants remain largely unresolved.11
Consequently, there remains an urgent need for the qualitative and quantitative assessment of complex mixtures of PAHs in a rapid, portable, high-throughput and cost-effective manner. Fluorescent chemosensory devices remain a leading signal transduction method owing to their high sensitivity, ease of operation, and broad applicability.12 When compared to small molecule fluorophores, significant gains in sensitivity are achieved using conjugated polymers (CPs) since their delocalized electronic structure leads to large extinction coefficients, strong fluorescence emission, efficient excited state (or exciton) migration, and collective properties that are sensitive to minor perturbations.13 In general, fluorescent sensors based on CPs operate through analyte-induced energy transfer, various aggregation phenomena, or conformational rearrangements that serve to manipulate mobile excitons and modulate the fluorescence in the form of spectral shifts, quenching, or unquenching of the emission.12,14 These mechanisms are distance-dependent and require strong CP-analyte interactions that are typically facilitated through the integration of molecular recognition elements (receptors) within or extended from the CP backbone.15 Nonspecific receptors for PAHs based on cation⋯π, relatively weak π⋯π and C–H⋯π interactions have been reported, but only show affinity toward particular PAHs.16 Supramolecular host–guest systems based on phenylene-bridged 4,4′-bipyridinium cations have demonstrated significant enhancements in binding affinities for PAHs, however, complex molecular topologies are required to form selective inclusion complexes and tailored chemistries for distinct PAHs are currently unavailable.17 Furthermore, the challenge of electronically coupling these analyte–receptor interactions into transducible optical responses further complicates the development of optical sensing platforms capable of profiling the distinct molecular signatures of diverse PAHs in complex mixtures.
More recently, array-based sensing has been used to profile combinations of structurally and chemically similar analytes through multivariate pattern recognition.18 Subtle structural differences between nonspecific CP-based sensing elements allow for differential interactions with analytes that establish unique and identifying optical response patterns.19 This creates a “chemical fingerprint” which can be used to discriminate similar compounds using linear discriminant analysis (LDA) and principal component analysis (PCA), pattern recognition algorithms which highlight and summarize distinguishing features in large data sets to provide information leading to chemical differentiation.20 Still, these methods require spatially distinct sensor units, each with its own recognition element, to build a diagnostic pattern that can be used to rapidly identify individual analytes. The inner filter effect (IFE) results from the absorption of light by a chromophore in solution, preventing photons from reaching a fluorophore, creating an observed decrease in fluorescence emission.21 Here, we demonstrate that the IFE in combination with CP-based array sensing offers a straightforward approach for the quantitative detection and qualitative discrimination of PAHs. While previous reports have demonstrated the utility of the IFE for the detection of picric acid and Sudan dyes using CPs,22 we report the use of differential quenching and pattern recognition to discriminate chemically and structurally similar PAHs, which could not be achieved using the IFE or CPs independently. To obtain the desired differential interactions required for an effective array sensor, we synthesized a series of similar but structurally distinct fluorescent CPs based on fluorene copolymer scaffolds with 2-phenylbenzimidazole optical modifiers. These CPs provide spectral overlap in regions of maximum absorption for many PAHs allowing for an IFE, with the PAH acting as optically dense absorbers. The reported system thus takes advantage of the intrinsic optical properties of individual PAHs, circumventing the need for tailored host–guest interactions. The unique response of each polymer allowed for the discrimination of 16 PAHs listed by the EPA as priority pollutants that are hazardous to human health.
Results and discussion
Polymer design, synthesis, and optical characterization
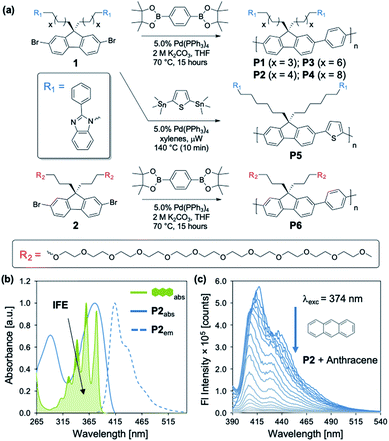
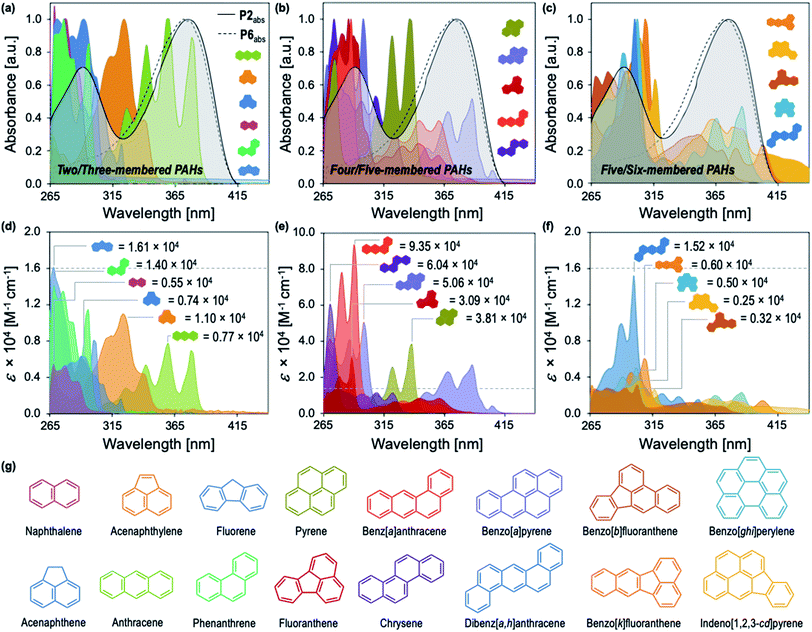
Our studies began with the synthesis of poly[2,7-(9,9-di((6′-(2-phenyl-1H-benzo[d]imidazole))hexyl)-fluorene)-alt-1,4,-phenylene] (P2) (Fig. 1a) using a Suzuki cross-coupling polymerization.23 We utilized the same approach for the synthesis of P1–P4 and P6, while P5 was synthesized using a microwave mediated Stille cross-coupling polymerization (Fig. 1a). These approaches resulted in facile access to an array of structurally distinct polymers, that were targeted to maintain solubility, high fluorescence emission, and sizes beyond the exciton diffusion length.24 General protocols regarding monomer and polymer synthesis are included in the Experimental section with full details in the ESI.†P2 exhibits an absorption maximum (λmax) centered at 374 nm, providing spectral overlap in regions of maximum absorption for many PAHs (Fig. 1 and 2). Each PAH displays a characteristic absorption profile in the 250–500 nm region (Fig. 2 and S2–S17†). The tunable nature of these CPs allows for the incorporation of optical modifiers which provide greater spectral overlap between the absorption of the polymer and each PAH, enabling efficient fluorescence quenching through the IFE. Peripheral 2-phenylbenzimidazole substituents in P2 impart an extra absorption band with λmax = 290 nm, which affords the greater spectral overlap required for the detection of PAHs through the IFE (Fig. S18†). Fig. 1b illustrates the significant spectral overlap between P2 and anthracene allowing for an IFE, with the PAH acting as a “chemical filter.” Upon excitation at 374 nm, P2 exhibits a strong emission between 400–500 nm, with maximum intensity at 415 nm. Addition of anthracene causes apparent quenching of the fluorescence through the IFE (Fig. 1c).Fig. 2 illustrates the distinct optical profiles of the 16 PAH compounds identified as priority PAH pollutants by the US Environmental Protection Agency (EPA). Each PAH demonstrates varying spectral overlap with P2 (Fig. 2a–c), and distinct wavelength dependencies of the molar absorptivity (ε), providing the basis for differential quenching through the IFE (Fig. 2d–f). To obtain the desired diversity of interactions required for an effective array sensor, a series of similar but structurally distinct CPs were synthesized, which demonstrate fluorescence quenching by PAHs through the IFE (Fig. 1a). In addition to P2, the polymer array included poly[2,7-(9,9-di((5′-(2-phenyl-1H-benzo[d]imidazole))pentyl)-fluorene)-alt-1,4,-phenylene] (P1), poly[2,7-(9,9-di((8′-(2-phenyl-1H-benzo[d]imidazole))octyl)-fluorene)-alt-1,4,-phenylene] (P3), poly[2,7-(9,9-di((10′-(2-phenyl-1H-benzo[d]imidazole))decyl)-fluorene)-alt-1,4,-phenylene] (P4), poly[2,7-(9,9-di((6′-(2-phenyl-1H-benzo[d]imidazole))hexyl)-fluorene)-alt-2,5,-thiophene] (P5), and poly[2,7-(9,9-di((undec(ethylene glycol)monomethyl ether))-fluorene)-alt-1,4,-phenylene] (P6).
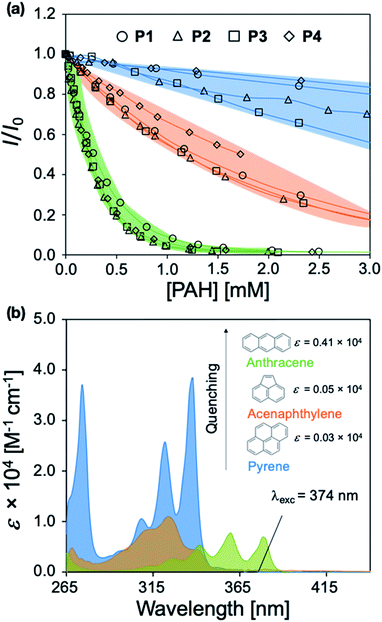
P1–P4 demonstrate a systematic lengthening of the methylene bridge (–CH2–)x between the 2-phenylbenzimidazole optical modifier and the CP backbone, with x = 3, 4, 6, and 8 units, respectively (Fig. 1a). The structural changes in the polymer series cause subtle but distinct differences in their optical spectra and molar absorptivity (Fig. S1†). To establish that these slight modifications could manifest into discernible responses, several PAHs were titrated into solutions of P1–P4 to study their effect on the fluorescence emission of each polymer. Three PAHs, anthracene, acenaphthylene, and pyrene were chosen to represent a range of ring fusion and optical properties (e.g. intrinsic absorption) in the PAH family. The resulting titration profiles for P1–P4 are summarized in Fig. 3a, in which quenching of polymer emission is caused by the selected PAHs through the IFE. Small but noticeable differences in quenching were observed between P1–P4 and each PAH, demonstrating that even subtle structural modifications affected the spectral response. More dramatic differential responses were shown between the PAHs, which can be explained by the distinct dependence of molar absorptivity for each PAH at a given wavelength (Fig. 3b). At an excitation wavelength (λexc) of 374 nm, anthracene has the greatest molar extinction coefficient (ε = 0.41 × 104 M−1 cm−1) and was the most efficient fluorescence quencher of each polymer through the IFE. As a representative example, the detection limit of anthracene using P2 was calculated to be 2.4 μM, demonstrating the low limit of detection (LOD) for the array (Fig. S19†). A fluorene copolymer with a thiophene structural unit in the backbone (P5) was incorporated into the array to provide distinctive quenching behavior from the other copolymers. P5 shows a red-shifted absorption (λmax = 420 nm) and allows for differential quenching through the IFE when compared to the other copolymers of the array. A fluorene-co-phenylene copolymer without 2-phenylbenzimidazole optical modifiers (P6) and incorporated oligo(ethylene glycol) side chains was synthesized. P6 lacks the extra band in the absorption spectrum (λmax = 290 nm) seen in P1–P5, providing another source of differential interaction through the IFE. Minor structural variations between each polymer, in combination with the unique relationship of molar absorptivity and wavelength for each PAH, provide a library of differential responses which can be used for discrimination.
Conjugated polymer-based sensor array for polycyclic aromatic hydrocarbons via pattern recognition
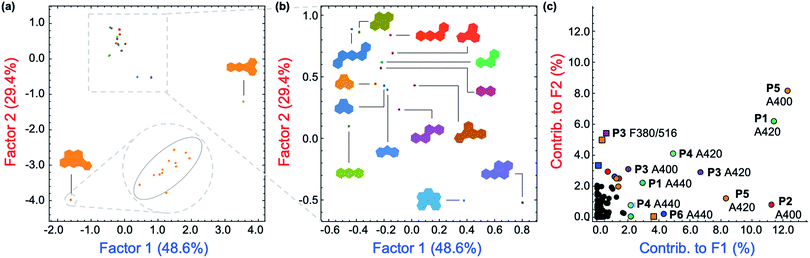
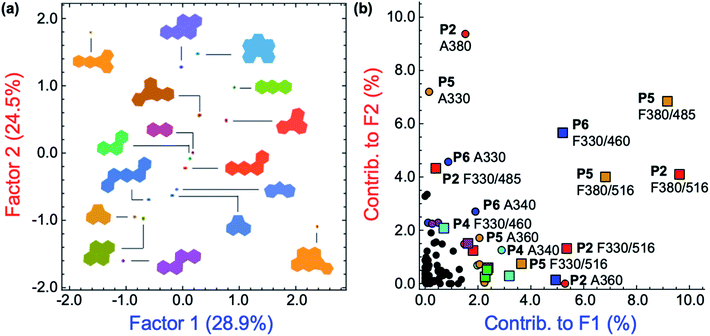
Solutions of P1–P6 in DMF (15 mg L−1) were arranged on a 384-well plate and exposed to 500 μM solutions of each PAH (Fig. 2g).25 Each PAH-polymer combination was prepared in 12 replicates and multiple spectroscopic measurements were collected on a microwell plate reader, corresponding to the regions of spectral overlap between the polymers and each PAH, including 21 absorbance measurements from 280–700 nm, and fluorescence measurements using the following filter combinations (λexc/λem): 330/460 nm, 330/485 nm, 330/516 nm, 380/460 nm, 380/485 nm, and 380/516 nm. The raw instrumental response pattern for each measurement is tabulated and visually summarized as a heat map in the ESI (Fig. S20–S40†).Superfluous instrumental variables introducing experimental noise were removed from the original data set whose contribution to PAH discrimination was negligible; these included absorbance measurements above 500 nm as PAHs lack absorption in this region. After removal of these absorbance measurements, the optical responses of P1–P6 to each PAH were analyzed through linear discriminant analysis (LDA), a well-established multivariate patterning algorithm.26 Complete differentiation of 16 PAHs was observed using the first two factors obtained from LDA analysis, while retaining 78.0% of the total information content that was present in the raw dataset. Fig. 4a displays the corresponding two-dimensional LDA scores plot. Replicates of the same PAH sample were found to cluster tightly, whereas clusters of replicates from different samples were well-separated. Tight intra-cluster spacing indicates excellent reproducibility, while large inter-cluster spacing indicates strong discriminatory power of the polymer-based array.
The most important contributors to the differentiation were absorption signals in the range of 420–480 nm, as shown in the corresponding LDA loadings plot (Fig. 4c), which arise from absorption bands displayed only by benzo[k]fluoranthene and indeno[1,2,3-cd]pyrene. The unique features of these two PAHs between 420–480 nm are overrepresented in the discrimination displayed in Fig. 4a and are therefore assigned a disproportionally high weighting in the LDA analysis, thus differentiating these two very well from the other 14 PAHs, but providing very little discriminatory power for the other 14, thus drastically reducing the array's effectiveness for analytical applications. An overview of the quality of the information conveyed by each instrumental measurement is presented visually in the ESI (Fig. S42†). The absorbance measurements for each polymer between 420–480 nm contain little coherent signal and are dominated by noise, relative to the absorption at lower wavelengths (<420 nm) and were therefore removed from the dataset. The LDA analysis was then repeated on this reduced dataset.
After removal of the information-poor absorbance measurements between 420–480 nm, subsequent LDA analysis and data reduction provided a better performing differentiation, representing a significant improvement in the overall discriminatory power of the array (Fig. 5a). The corresponding LDA loadings plot reflects a reduced importance of absorption signals to the differentiation, with a more diverse group of variables such as the fluorescence measurements acting as the most important contributors to the discrimination (Fig. 5b). Increased reliance on fluorescence signals is desirable as the high photoluminescence quantum efficiencies of fluorene-based copolymers should offer considerably lower limits of differentiation for PAHs compared to an array relying primarily on the absorption properties of PAHs. Despite the improvement in separation of all 16 PAHs, only half of the total information was retained in the first two factors (53.4%). By including a third factor, the data could be displayed as a three-dimensional plot, while preserving a larger portion of the total information. As shown in the ESI (Fig. S43†), the three-dimensional scores plot now retains 74.7% of the total information contained in the original dataset.
The dataset was also analyzed by a similar multivariate technique, principal component analysis (PCA), as PCA provides an unsupervised representation of the variances in a given dataset. The two-dimensional PCA plot is presented in Fig. S44 in the ESI.† PCA analysis also gives complete separation of all 16 PAHs with very small intra-cluster distances, while retaining 70.8% of the total information from the original dataset within the first two components.
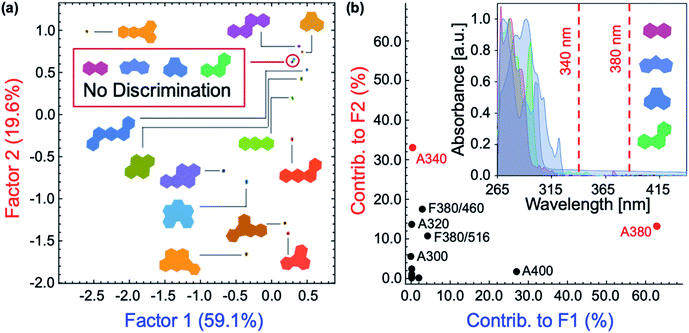
Role of the inner filter effect in polycyclic aromatic hydrocarbon discrimination
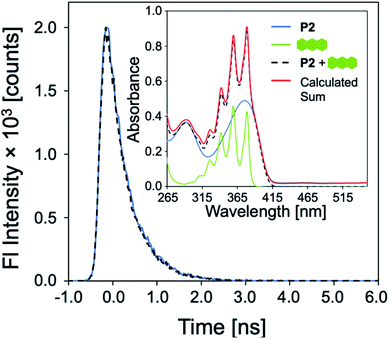
The role of P1–P6 in the discrimination was investigated by analyzing a dataset containing optical measurements from only the PAHs, in the absence of polymers. LDA analysis of this dataset showed that complete separation of all 16 PAHs was not achieved (Fig. 6a). Moreover, the corresponding loadings for the first two factors (Fig. 6b) are, once again, mostly reliant upon the absorbance measurements of the PAHs: not only is this detrimental for the sensitivity of the assay, it also prevents PAHs with similar absorption features such as naphthalene, fluorene, acenaphthene, and phenanthrene from being differentiated (Fig. 6b, inset). The modulation of P1–P6 fluorescence was therefore found to be critical in the discrimination of all 16 PAHs, as demonstrated by the large contribution of fluorescence measurements in the factor loadings (Fig. 5b).To elucidate the mechanism of fluorescence quenching, steady-state absorption, fluorescence lifetime, and fluorescence anisotropy measurements were performed. As illustrated in Fig. 2, the PAH absorption spectra span 250–480 nm, overlapping with the absorption and emission spectra of P1–P6. The spectral overlap indicates the possibility of fluorescence resonance energy transfer (FRET),27 which may take place in the presence of overlapped emission spectrum of a fluorophore (CP) with the absorption spectrum of a quencher (PAH), or the IFE. The fluorescence lifetime was measured in the absence and presence of PAH, where P2 and anthracene were chosen as a representative example. It is evident from Fig. 7 that the fluorescence lifetime of P2 does not display any significant change upon the addition of anthracene, which rules out a dynamic quenching mechanism.22 Furthermore, the fact that the absorption spectrum of a mixture between polymer and PAH is completely additive rules out static quenching due to aggregation and formation of a ground–state complex between P2 and anthracene (Fig. 7, inset). Fluorescence anisotropy experiments are commonly used to investigate molecular interactions between a small fluorophore and a macromolecule, where a strong increase in anisotropy indicates a slower rotational diffusion rate of the fluorophore due to binding.27,28 For these experiments, P5 was titrated into a solution of anthracene ([anthracene] = 100 μM in DMF). Polymer P5 was chosen because its emission spectrum is well-separated from that of anthracene, allowing the emission of anthracene and changes in its tumbling rate in solution to be directly observed. However, no increase in fluorescence anisotropy was observed (Fig. S45†), suggesting that no binding occurred between anthracene and P5. When all these results are considered, the IFE is the most probable mechanism responsible for quenching of the polymers.
Conclusions
In summary, we have demonstrated the detection of 16 priority PAHs through a six-membered sensor array. A new set of fluorescent CPs was readily prepared, incorporating side chain optical modifiers, which were strongly and yet non-selectively affected by the presence of diverse PAHs through an IFE. Multivariate pattern recognition strategies were used to generate two-dimensional score plots, which can operate as effective calibration plots for the differentiation of unknown PAHs using simple, common, and cost-effective instrumentation (UV-vis absorption and fluorescence spectroscopies). The reported platform is highly tunable, owing to facile modification of the polymer structure and ease with which optical modifiers can be designed and integrated. The discrimination of similar analytes that lack well-defined recognition elements was achieved, widening the scope of the IFE in CP-based sensors. These results lay the groundwork for an extension into qualitative and quantitative detection and discrimination of molecular species with high optical densities that are otherwise inaccessible through energy transfer and aggregation-based mechanisms traditionally utilized in CP-based array sensors.Experimental section
Materials
Reagents were purchased from Sigma-Aldrich and used without further purification, unless otherwise specified. Spectral grade N,N-dimethylformamide (DMF) was purchased from EMD Chemicals Inc. and used as received. Xylenes and tetrahydrofuran (THF) were degassed and dried over 4 Å molecular sieves. Chloroform-d (CDCl3) was purchased from Cambridge Isotope Labs and used as received. Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) was purchased from Strem Chemicals and used without further purification. 2,7-Dibromo-9,9-bis(5-bromopentyl)-9H-fluorene,29 2,7-dibromo-9,9-bis(8-bromooctyl)-9H-fluorene,30 and 1,1′-((2,7-dibromo-9H-fluorene-9,9-diyl)bis(hexane-6,1-diyl))bis(2-phenyl-1H-benzo[d]imidazole)23 were prepared according to literature procedures.General procedure for the synthesis of functionalized monomers
2,7-Dibromo-9,9-bis(6-bromoalkyl)-9H-fluorene (1.02 mmol, 1.00 equiv.), 2-phenylbenzimidazole (2.24 mmol, 2.20 equiv.) and NaH (4.08 mmol, 4.00 equiv.) were dissolved in DMF (10 mL) under nitrogen. The mixture was stirred at 70 °C for 12 h. The reaction mixture was then cooled to room temperature, quenched with deionized water, and extracted with ethyl acetate (3 × 50 mL). The organic layer was then washed with water (3 × 50 mL) and dried over anhydrous MgSO4. Volatiles were removed in vacuo, and purification was accomplished by flash chromatography using a hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate gradient.
ethyl acetate gradient.
General procedure for the synthesis of functionalized conjugated polymers
A microwave tube was loaded with bifunctional aromatic bromide (SI-1–SI-3) (0.100 mmol, 1.00 equiv.), 1,4-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene (0.105 mmol, 1.05 equiv.), 2 M K2CO3 (1.0 mL, 20 equiv.) in water, and THF (1.0 mL). The mixture was sparged with nitrogen, and 0.70 mL of a Pd(PPh3)4/THF stock solution (5 mol%) was added via syringe. The mixture was stirred vigorously and heated at 70 °C for 15 h. After this time, the reaction was allowed to cool leaving a solid gelled material. The mixture was precipitated into methanol and collected via filtration. The residual solid was loaded into an extraction thimble and washed with methanol (8 h), acetone (4 h), and hexanes (4 h). The polymer was dried in vacuo.Spectroscopic methods
All spectra were recorded at ambient temperature, unless otherwise stated. UV-vis absorbance measurements were performed on a Hewlett-Packard 8452a diode array UV-vis spectrophotometer. Benchtop steady-state fluorescence measurements were carried out with an ISS PC1 spectrofluorimeter. Excitation was carried out using a broad-spectrum high-pressure xenon lamp (CERMAX, 300W). Excitation correction was performed through a rhodamine B quantum counter with a dedicated detector. Detection was through a Hamamatsu red-sensitive PMT. High-aperture Glan-Thompson calcite polarizers were used in the excitation and emission channels to measure steady-state fluorescence anisotropy. Experimental temperature (25 °C) was controlled by an external circulating water bath.All optical spectroscopy and binding experiments were performed in DMF. Polymer and PAH stock solutions were prepared separately, then (2 mL) of the polymer solutions were inserted into a quartz cuvette, and absorption and fluorescence spectra were collected for P1–P6. Fluorescence emission spectra were collected by exciting the polymer at their most red-shifted spectral maximum. Binding titrations were performed by adding aliquots (1–100 μL per addition) of PAH solution to the polymers. Fluorescence decay profiles were recorded using a Horiba PPD850 time-correlated single-photon counting (TCSPC) detector with a Fianium WhiteLase SC-400 laser excitation source at a repetition rate of 2 MHz.
Multivariate data was acquired on a BioTek Synergy II multimode microwell plate reader, capable of measuring absorption spectra through a monochromator and steady-state fluorescence intensity measurements through a set of bandpass filters. The sample compartment in this instrument was electrically thermostatted to 25 °C. Experiments were laid out by hand using Eppendorf Research multichannel pipettors and disposable plastic tips into Aurora microwell plates with clear bottoms for UV absorption and fluorescence spectroscopy in a 384-well configuration. The plates were made of non-treated cyclo-olefin polymer (COP) with clear flat bottoms. Each well was filled with (100 μL) of the sample solution. Plates were read on a multimode plate reader immediately after preparation. Further details regarding multivariate data analysis are available in the ESI.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation (OIA 1632825). J. T. acknowledges traineeship support from the NSF NRT program “Interface” (DGE 1449999) through the University of Southern Mississippi. M. B. acknowledges support from the Alabama Water Institute.Notes and references
- (a) R. G. Harvey, Polycyclic Aromatic Hydrocarbons, Wiley-VCH, New York, 1997 Search PubMed; (b) H. I. Abdel-Shafy and M. S. M. Mansour, Egypt. J. Pet., 2016, 25, 107 CrossRef.
- (a) K. H. Kim, S. A. Jahan, E. Kabir and R. J. C. Brown, Environ. Int., 2013, 60, 71 CrossRef CAS PubMed; (b) A. M. Mastral and M. S. Callén, Environ. Sci. Technol., 2000, 34, 3051 CrossRef CAS.
- (a) World Health Organization, WHO guidelines for indoor air quality: selected pollutants, WHO Regional Office for Europe, Copenhagen, 2010 Search PubMed; (b) C. A. Menzie, B. B. Potocki and J. Santodonato, Environ. Sci. Technol., 1992, 26, 1278 CrossRef CAS.
- (a) H. M. Dixon, R. P. Scott, D. Holmes, L. Calero, L. D. Kincl, K. M. Waters, D. E. Camann, A. M. Calafat, J. B. Herbstman and K. A. Anderson, Anal. Bioanal. Chem., 2018, 410, 3059 CrossRef CAS PubMed; (b) L. B. Paulik, C. E. Donald, B. W. Smith, L. G. Tidwell, K. A. Hobbie, L. Kincl, E. N. Haynes and K. A. Anderson, Environ. Sci. Technol., 2016, 50, 7921 CrossRef CAS PubMed; (c) S. Kumar, S. Negi and P. Maiti, Environ. Sci. Pollut. Res., 2017, 24, 25810 CrossRef CAS PubMed.
- (a) S. A. Wise, L. C. Sander and W. E. May, J. Chromatogr., 1993, 642, 329 CrossRef CAS; (b) S. C. C. Lung and C. H. Liu, Sci. Rep., 2015, 5, 12992 CrossRef CAS PubMed; (c) G. Purcaro, S. Moret, M. B. Miklavcic and L. S. Conte, J. Sep. Sci., 2012, 35, 922 CrossRef CAS PubMed; (d) K. Dost and C. Ideli, Food Chem., 2012, 133, 193 CrossRef CAS; (e) A. Toriba, Y. Kuramae, T. Chetiyanukornkul, R. Kizu, T. Makino, H. Nakazawa and K. Hayakawa, Biomed. Chromatogr., 2003, 17, 126 CrossRef CAS PubMed.
- (a) L.-b. Liu, Y. Liu, J.-m. Lin, N. Tang, K. Hayakawa and T. Maeda, J. Environ. Sci., 2007, 19, 1 CrossRef CAS; (b) C.-D. Dong, C.-F. Chen and C.-W. Chen, Int. J. Environ. Res. Public Health, 2012, 9, 2175 CrossRef CAS PubMed; (c) H. K. Bojes and P. G. Pope, Regul. Toxicol. Pharmacol., 2007, 47, 288 CrossRef CAS PubMed.
- (a) J. Wu, J. Sun, H. Cheng, J. Liu and Y. Wang, Sep. Sci. plus, 2018, 1, 446 CrossRef CAS; (b) T. Nolte and J. T. Andersson, Polycyclic Aromat. Compd., 2011, 31, 287 CrossRef CAS; (c) L. Ferey and N. Delaunay, Anal. Bioanal. Chem., 2015, 407, 2727 CrossRef CAS PubMed.
- (a) D. Knopp, M. Seifert, V. Vaananen and R. Niesser, Environ. Sci. Technol., 2000, 34, 2035 CrossRef CAS; (b) C. R. Spier, G. G. Vadas, S. L. Kaattari and M. A. Unger, Environ. Toxicol. Chem., 2011, 30, 1557 CrossRef CAS PubMed; (c) K. A. Fahnrich, M. Pravda and G. G. Guilbault, Anal. Lett., 2002, 35, 1269 CrossRef CAS.
- (a) X. Li, S. L. Kaattari, M. A. Vogelbein, G. G. Vadas and M. A. Unger, Sens. Biosensing Res., 2016, 7, 115 CrossRef PubMed; (b) B. K. Beheera, A. Das, D. J. Sarkar, P. Weerathunge, P. K. Parida, B. K. Das, P. Thavamani, R. Ramanathan and V. Bansal, Environ. Pollut., 2018, 241, 212 CrossRef PubMed.
- S. A. Nsibande, H. Montaseri and P. B. C. Forbes, Trends Anal. Chem., 2019, 115, 52 CrossRef CAS.
- P. E. T. Douben, PAHs: an ecotoxicological perspective, Wiley-VCH, New York, 2003 Search PubMed.
- (a) T. L. Mako, J. M. Racicot and M. Levine, Chem. Rev., 2019, 119, 322 CrossRef CAS PubMed; (b) C. McDonagh, C. S. Burke and B. D. MacCraith, Chem. Rev., 2008, 108, 400 CrossRef CAS PubMed.
- (a) S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef CAS PubMed; (b) S. Rochat and T. M. Swager, ACS Appl. Mater. Interfaces, 2013, 5, 4488 CrossRef CAS PubMed; (c) H. Jiang, P. Taranekar, J. R. Reynolds and K. S. Schanze, Angew. Chem., Int. Ed., 2009, 48, 4300 CrossRef CAS PubMed; (d) A. K. Williams, J. Tropp, E. R. Crater, N. Eedugurala and J. D. Azoulay, ACS Appl. Polym. Mater., 2019, 1, 309 CrossRef CAS.
- W. Wu, G. C. Bazan and B. Liu, Chem, 2017, 2, 760 CAS.
- A. P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed.
- (a) A. Zengin, U. Tamer and T. Caykara, J. Raman Spectrosc., 2018, 49, 452 CrossRef CAS; (b) P. Leyton, S. Sanchez-Cortes, J. V. Garcia-Ramos, C. Domingo, M. Campos-Vallette, C. Saitz and R. E. Clavijo, J. Phys. Chem. B, 2004, 108, 17484 CrossRef CAS; (c) I.-S. Tamgho, S. Chaudhuri, M. Verderame, D. J. DiScenza and M. Levine, RSC Adv., 2017, 7, 28489 RSC.
- (a) E. J. Dale, N. A. Vermeulen, M. Juríček, J. C. Barnes, R. M. Young, M. R. Wasielewski and J. F. Stoddart, Acc. Chem. Res., 2016, 49, 262 CrossRef CAS PubMed; (b) J. C. Barnes, M. Juríček, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 183 CrossRef CAS PubMed.
- (a) Z. Li, J. R. Askim and K. S. Suslick, Chem. Rev., 2019, 119, 231 CrossRef CAS PubMed; (b) A. T. Wright and E. V. Anslyn, Chem. Soc. Rev., 2006, 35, 14 RSC; (c) Y. Geng, W. J. Peveler and V. M. Rotello, Angew. Chem., Int. Ed., 2019, 58, 5190 CrossRef CAS PubMed.
- J. Freudenberg, F. Hinkel, D. Jänsch and U. H. F. Bunz, Top. Curr. Chem., 2017, 375, 67 CrossRef PubMed.
- S. Stewart, M. A. Ivy and E. V. Anslyn, Chem. Soc. Rev., 2014, 43, 70 RSC.
- S. Chen, Y.-L. Yu and J.-H. Wang, Anal. Chim. Acta, 2018, 999, 13 CrossRef CAS PubMed.
- (a) A. S. Tanwar, S. Patidar, S. Ahirwar, S. Dehingia and P. K. Iyer, Analyst, 2019, 144, 669 RSC; (b) A. S. Tanwar, L. R. Adil, M. A. Afroz and P. K. Iyer, ACS Sens., 2018, 3, 1451 CrossRef CAS PubMed; (c) A. S. Tanwar, S. Hussain, A. H. Malik, M. A. Afroz and P. K. Iyer, ACS Sens., 2016, 1, 1070 CrossRef CAS; (d) M. Wu, L. Sun, K. Miao, Y. Wu and L. J. Fan, ACS Appl. Mater. Interfaces, 2018, 10, 8287 CrossRef CAS PubMed.
- G. Saikia and P. K. Iyer, Macromolecules, 2011, 44, 3753 CrossRef CAS.
- (a) G. Klaerner and R. D. Miller, Macromolecules, 1998, 31, 2007 CrossRef CAS; (b) M. Grell, D. D. C. Bradley, G. Ungar, J. Hill and K. S. Whitehead, Macromolecules, 1999, 32, 5810 CrossRef CAS.
- (a) L. H. Keith, Polycyclic Aromat. Compd., 2015, 35, 147 CrossRef CAS; (b) D. Lerda, P. López, S. Szilagyi, T. Wenzl and G. Buti, Report on the Inter-Laboratory Comparison Organised by the European Union Reference Laboratory for Polycyclic Aromatic Hydrocarbons for the Validation of a Method for Quantifying the Four EU Marker PAHs in Food, Geel, Belgium, 2011 Search PubMed.
- (a) K. J. Albert, N. S. Lewis, C. L. Schauer, G. A. Sotzing, S. E. Stitzel, T. P. Vaid and D. R. Walt, Chem. Rev., 2000, 100, 2595 CrossRef CAS PubMed; (b) F. Rock, N. Barsan and U. Weimar, Chem. Rev., 2008, 108, 705 CrossRef PubMed; (c) Y. Liu and M. Bonizzoni, J. Am. Chem. Soc., 2014, 136, 14223 CrossRef CAS PubMed.
- J. R. Lakowicz, in Principles of Fluorescence Spectroscopy, Springer, Singapore, 3rd edn, 2010 Search PubMed.
- B. Liu, Y. Bao, H. Wang, F. Du, J. Tian, Q. Li, T. Wang and R. Bai, J. Mater. Chem., 2012, 22, 3555 RSC.
- C.-S. Wu, C.-Y. Chou and Y. Chen, J. Mater. Chem. C, 2014, 2, 6665 RSC.
- K. Kim, M. Suh, J. Choi, D. Lee, Y. Kim, S. H. Cheong, D. Kim and D. Y. Jeon, Adv. Funct. Mater., 2015, 25, 7450 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc03405f |
| This journal is © The Royal Society of Chemistry 2019 |