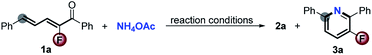Open Access Article
Open Access ArticleControlled chemoselective defluorination and non-defluorination for [5 + 1] aromatic annulation via Meisenheimer-type nitrogen anion and radical intermediates†
Jinlong
Qian
a,
Jinlong
Zhang
a,
Huameng
Yang
a,
Lei
Kang
ab and
Gaoxi
Jiang
 *a
*a
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Center for Excellence in Molecular Synthesis, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou 730000, P. R. China. E-mail: gxjiang@licp.cas.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 7th August 2019
Abstract
Reported is a unique chemoselectivity approach to base-promoted defluorinative and Cu(I)-catalyzed aerobic oxidative non-defluorinative [5 + 1] condensation aromatizations of simple unsaturated ketones with ammonium salts via Meisenheimer-type nitrogen anion and radical intermediates. The CuBr/O2 catalysis provides a straightforward approach to diverse 3-fluoropyridines in high yields. The synthetic utility of the strategy is highlighted by the concise synthesis of several F-modified bioactive compounds.
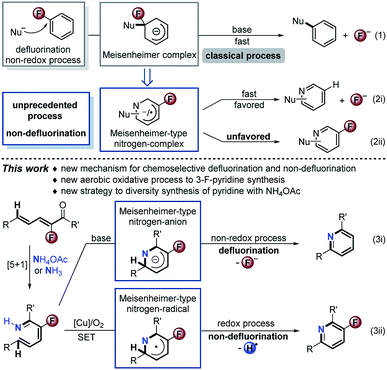
Organofluorine chemistry has played a privileged role in all aspects of pure and applied chemistry.1 It is well known that introduction of a fluorine atom into a molecule could significantly modify its biological properties.2 Exploitation of highly chemoselective synthetic strategies to obtain easily accessible organofluorine compounds of biological importance is an attractive method. Theoretically, effective control of intermediate conversion is one of the origins of chemoselective synthesis. Nucleophilic aromatic substitution (SNAr) of fluorinated arenes is a versatile synthetic tactic. Nevertheless, the exhaustive defluorination is rather inevitable via Meisenheimer intermediates (Scheme 1, eqn (1)).3 3-Fluoro-2H-pyridin-1-ide should be more reactive due to the electronegativity of the nitrogen atom and fluorine substitution. Such a Meisenheimer-type nitrogen complex might follow two reaction pathways: defluorination (Scheme 1, eqn (2i)) and non-defluorination (eqn (2ii)), the latter has not been realized so far. Herein, we report a unique chemoselectivity approach to base-promoted defluorinative and Cu-catalyzed aerobic oxidative non-defluorinative [5 + 1] condensation aromatizations of simple unsaturated ketones with ammonium salts (eqn (3)). The strategy features a formal Csp2–H activation, good atom-/step-economy, and wide substrate scope, providing a new practical approach to diverse pyridines. The usefulness of this method is highlighted in the concise total synthesis of a blood lipid regulator, its fluorine-modified analogues and fluorine-modified AMPK receptor inhibitor. Preliminary mechanism investigation reveals that the [Cu]/O2 oxidative catalysis involves a single electron transfer (SET) process and unprecedentedly interrupts the traditional preponderant defluorination of Meisenheimer-type intermediates.
 | ||
| Scheme 1 Traditional SNAr defluorination and current chemoselective defluorination/non-defluorination via [5 + 1] aromatic annulation. | ||
The pyridine skeleton is a fundamental moiety in bio-, chemical-, and pharmaceutical molecules.4 Among these, 3-fluoropyridine as a core fragment is widespread in the design of pharmaceuticals.5 Currently available methods toward 3-fluoropyridines involve the Balz–Schiemann reaction, electrophilic/nucleophilic fluorination, deoxofluorination, and Rh-catalyzed [4 + 2] cyclization, which always suffer from narrow scope, poor chemoselectivity, and requirement of expensive raw materials.5 In this regard, development of a new practical access is still in great demand. In principle, the straightforward [5 + 1] cyclization of 2-fluoro-2,4-dien-1-ones with simple ammonium salts or free ammonia represents the state-of-the-art tactic from the standpoint of atom and step economy,6 but still is undisclosed reasonably because of the overwhelmingly competitive defluorination. By an oxidative SET/radical strategy, we anticipate that efficiently non-defluorinative aromatization of the aforementioned Meisenheimer-type nitrogen intermediate could be a potential solution to the issue.
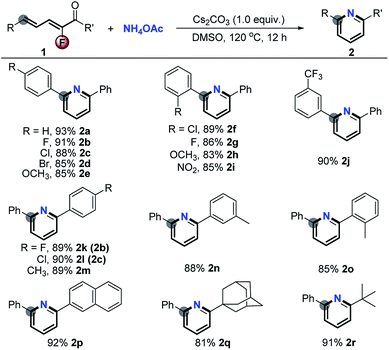
Initially, in order to verify the feasibility of the designed [5 + 1] aromatic annulation, we treated a wide range of 2-fluoro-2,4-dien-1-ones 1 with a simple ammonium salt, NH4OAc (5.0 equiv.) under basic conditions (Cs2CO3) in DMSO at 120 °C for 12 h. As shown in Scheme 2, the reactions (Scheme 1, eqn (3i)) took place smoothly and gave a series of pyridines 2a–r in excellent yields via complete defluorination regardless of the steric hindrance and electronic properties of substituents (for optimization of the reaction conditions, see the ESI†).
Encouraged by the above results and the recent surge of interest to develop copper-catalyzed aerobic oxidation reactions of amines,7 we anticipate that Cu/O2-catalysis might have the potential to interdict fluoride displacement of Meisenheimer-type intermediates to realize the first access to 3-fluoropyridines through a straightforward [5 + 1] cyclization with simple ammonium salts and free ammonia as the sole nitrogen source. As shown in Table 1, the reaction of 2-fluoro-1,5-diphenylpenta-2,4-dien-1-one 1a with NH4OAc (5.0 equiv.) in the presence of CuBr (10 mol%) in a mixed solvent (DMSO/toluene, 5% v/v) at 140 °C in a sealed O2 atmosphere for 12 h afforded 3a in 17% yield. Surprisingly, as a sharp contrast to the defluorinative aromatic annulation (Scheme 2), only a trace of the defluorinated product 2a was detected in the Cu-catalyzed aerobic oxidation process (entry 1). Stimulated by the excellent ratio of the non-defluorinative and defluorinative product (chemoselectivity) and promising yield, further optimization of the reaction conditions was carried out to improve the yield. To our delight, just addition of 10 mol% trifluoroacetic acid (TFA) dramatically increased the yield from 17% up to 89% with only 2% loss of fluorine in the product (entry 2), reasonably due to the facilitative formation of imine which is the real intermediate in this Cu-catalyzed aerobic oxidation reaction. Lowering the loading of CuBr to 5.0 mol% decreased the yield to 59% (entry 3). Other catalysts including CuCl, CuI, Cu(MeCN)4PF6, CuBr2, FeBr2 and MnBr2 had adverse effects on the reaction (entries 4–9). Other acids such as HOTf and HOAc had a detrimental effect on the yield but still without release of fluorine (entries 10 and 11). NH4Cl and free NH3 (in MeOH) as nitrogen donors also showed acceptable performance (entries 12 and 13). The competitive dehalogenative aromatization was dominant if the reaction took place in air (entry 14). As expected, only dehalogenative aromatization occurred in the absence of O2 or CuBr catalyst (entries 15 and 16).
| Entry | Reaction conditions (mol%) | Yield (%) |
|---|---|---|
| a 1a (0.1 mmol), NH4OAc (5.0 equiv.), O2 (1 atm, closed), 12 h, at 140 °C, 5% (v/v) DMSO/toluene (1.0 mL), yields given by GC-MS using n-dodecane as an internal standard. b Yield of isolated 3a was given in the parentheses. c Using NH4Cl instead of NH4OAc. d Using NH3 (in MeOH) instead of NH4OAc. | ||
| 1 | CuBr (10), O2 | 2a/3a, trace/17 |
| 2 | CuBr (10), TFA (10), O2 | 2a/3a, 2/89 (80)b |
| 3 | CuBr (5.0), TFA (10), O2 | 3a, 59 |
| 4 | CuCl (10), TFA (10), O2 | 2a/3a, 17/61 |
| 5 | CuI (10), TFA (10), O2 | 2a/3a, 15/52 |
| 6 | Cu(MeCN)4PF6 (10), TFA (10), O2 | 2a/3a, 21/30 |
| 7 | CuBr2 (10), TFA (10), O2 | 2a/3a, 30/10 |
| 8 | FeBr2 (10), TFA (10), O2 | 2a/3a, 28/60 |
| 9 | MnBr2 (10), TFA (10), O2 | 2a/3a, 10/80 |
| 10 | CuBr (10), HOTf (10), O2 | 3a, 6 |
| 11 | CuBr (10), HOAc (10), O2 | 3a, 18 |
| 12c | CuBr (10), TFA (10), O2 | 2a/3a, 3/61 |
| 13d | CuBr (10), TFA (10), O2 | 2a/3a, 5/80 |
| 14 | CuBr (10), TFA (10), air | 2a/3a, 68/20 |
| 15 | CuBr (10), TFA (10), Ar | 2a, 85 |
| 16 | TFA (10), O2 | 2a, 10 |
As illustrated in Scheme 3, the CuBr/TFA oxidative catalysis effectively converted a series of 2-fluoro-2,4-dien-1-ones with NH4OAc into diverse polysubstituted 3-fluoropyridine derivatives in good yields with excellent chemoselectivity. Different groups including halide, methoxyl and nitro in the 5-aromatic ring were well tolerated, giving the corresponding products 3a–j in good yields (71–82%) and excellent chemoselectivity of more than 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Substrates with F, Cl and methyl at the carbonyl aromatic ring were also applicable, delivering the products 3k–o in 59–75% yields without negative effects on fluorine retention. Reasonably, steric hindrance had a strong effect on the reactivity. Surprisingly, the more bulky substrate 1p, especially adamantyl (1q) and tert-butyl 2-fluoro-2,4-dien-1-one (1r) were also compatible with the direct [5 + 1] aromatic annulation without any defluorination although the yields of the desired adducts 3p–r were decreased from 85% to 31% on account of the increase of steric hindrance. The 4-methyl substituted starting material 1s was readily converted into the multifunctional 3-fluoropyridine 3s with moderate result.
5. Substrates with F, Cl and methyl at the carbonyl aromatic ring were also applicable, delivering the products 3k–o in 59–75% yields without negative effects on fluorine retention. Reasonably, steric hindrance had a strong effect on the reactivity. Surprisingly, the more bulky substrate 1p, especially adamantyl (1q) and tert-butyl 2-fluoro-2,4-dien-1-one (1r) were also compatible with the direct [5 + 1] aromatic annulation without any defluorination although the yields of the desired adducts 3p–r were decreased from 85% to 31% on account of the increase of steric hindrance. The 4-methyl substituted starting material 1s was readily converted into the multifunctional 3-fluoropyridine 3s with moderate result.
Stimulated by the satisfactory performance and to further evaluate the generality of the valuable approach, we next extended the scope of substrates to diverse types of α-fluoro-α,β-unsaturated inert aromatic ketones. As demonstrated in Scheme 4, several hetero-polycyclic fused 3-fluoropyridines 5a–d were easily obtained in good yields with >97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 chemoselectivity via a formal Csp2–H activation. This reaction provides a practical access to such useful kinds of compounds which are generally difficult to synthesize by traditional methods.5 Significantly, with (Z)-3-(benzofuran-3-yl)-2-fluoro-1-phenylprop-2-en-1-one 4e and (Z)-3-(benzo[b]thiophen-3-yl)-2-fluoro-1-phenylprop-2-en-1-one 4f as the reactants, the concomitant cleavage of C–O and C–S bonds happened during the aromatic cyclization to exclusively furnish the corresponding 2-(5-fluoro-6-phenylpyridin-3-yl)phenol 5e and 2-(5-fluoro-6-phenylpyridin-3-yl)benzenethiol 5f in 68% and 63% yields, respectively.
3 chemoselectivity via a formal Csp2–H activation. This reaction provides a practical access to such useful kinds of compounds which are generally difficult to synthesize by traditional methods.5 Significantly, with (Z)-3-(benzofuran-3-yl)-2-fluoro-1-phenylprop-2-en-1-one 4e and (Z)-3-(benzo[b]thiophen-3-yl)-2-fluoro-1-phenylprop-2-en-1-one 4f as the reactants, the concomitant cleavage of C–O and C–S bonds happened during the aromatic cyclization to exclusively furnish the corresponding 2-(5-fluoro-6-phenylpyridin-3-yl)phenol 5e and 2-(5-fluoro-6-phenylpyridin-3-yl)benzenethiol 5f in 68% and 63% yields, respectively.
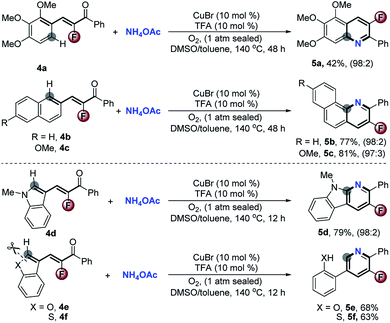 | ||
| Scheme 4 Scope of typical α-fluoro-α,β-unsaturated ketones for the CuBr-catalyzed aerobic oxidative reaction. | ||
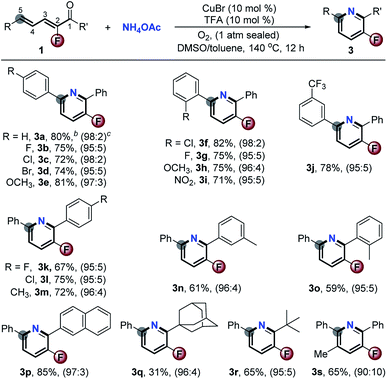
Finally, the synthetic utility of this [5 + 1] aromatic annulation was highlighted by the concise synthesis of several bioactive compounds (Scheme 5). Accordingly, treatment of 1t under basic (Scheme 2) and Cu-catalyzed aerobic oxidation conditions (Scheme 3) could exclusively afford 2t and its F-substituted derivative 3t in 90% and 78% yields, respectively. Demethylation of 2t/3t by BBr3 gave 6a/b in good yields. After etherification with ClCCH2CO2Et and hydrolysis, acids 8a/b could be isolated in good yields. Blood lipid regulator 9a8 and its F-modified analogue 9b were easily obtained via direct amidation with (tetrahydro-2H-pyran-4-yl)methanamine. Valuably, this method avoided the use of noble transition-metal catalysts which are indispensable in conventional approaches. Another interesting utilization is to prepare the key intermediate 13 of the F-modified AMPK receptor inhibitor 14.9Via [5 + 1] aromatic cyclization and concomitant ring-opening of furan, the Cu-catalyzed aerobic oxidation could facilitate the synthesis of 2-(6-(4-bromophenyl)-5-fluoropyridin-3-yl)phenol 10 with high step-economy by employing 4g as the starting substrate. Upon CuI-catalyzed amination with NH4OH followed by acetylation, 10 was smoothly converted into 12 in acceptable GC-yield. Compound 13 was formed in 47% yield by reacting 12 with ICH2CO2Et in the presence of a Pd catalyst. The 4-step reaction provided 13 in total 15% yield. Otherwise, the traditional process for the preparation of its defluorinated complex requires 8 steps in about 10% yield. Obviously, the current approach offers great advantages in terms of efficiency and low cost.
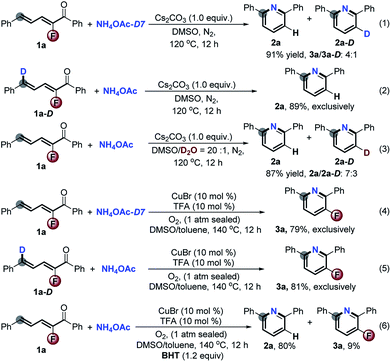
To gain insight into the mechanism, a series of deuterium labeled control reactions were executed (Scheme 6). Under the basic reaction conditions (Scheme 2), the treatment of NH4OAc-D7 instead of NH4OAc with 1a gave 2a and 2a-D in 91% yield with a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (eqn (1)). No deuterium labeled product was detected when 1a-D reacted with NH4OAc under the same reaction conditions (eqn (2)). Using D2O/DMSO (5%, v/v) as the solvent, the yield of 2a and 2a-D was 87% with a ratio of 7
1 (eqn (1)). No deuterium labeled product was detected when 1a-D reacted with NH4OAc under the same reaction conditions (eqn (2)). Using D2O/DMSO (5%, v/v) as the solvent, the yield of 2a and 2a-D was 87% with a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (eqn (3)), which reveals the hydrogen exchange between the substrates and H2O during the reaction process. In dramatic contrast, the CuBr/O2 catalytic system provided 3a in about 80% yield exclusively for both the above deuterium labeled reactions (eqn (4) and (5)). The addition of butylated hydroxytoluene (BHT) in the CuBr-catalyzed aerobic reaction strictly exacerbated the defluorination process (eqn (6)), which suggests that this reaction might involve radical intermediates. In order to verify the radical-mediated pathway, we next detected the presence of radicals by means of electron paramagnetic resonance (EPR) with the addition of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) for the reaction 2a and NH4OAc under CuBr-catalyzed aerobic conditions. Fortunately, the sextet signal for ˙O2– was captured, while no signal was observed during the Cs2CO3-promoted reaction (see the ESI†). All of the results reasonably confirmed our initial hypothesis (Scheme 1, eqn (3)). The 3-fluoro-2H-pyridin-1-ide Meisenheimer-type anion intermediate underwent a non-redox defluorination process under basic conditions, and the CuBr catalytic aerobic conditions promoted it to an unfavored non-defluorination via a SET/radical pathway.7
3 (eqn (3)), which reveals the hydrogen exchange between the substrates and H2O during the reaction process. In dramatic contrast, the CuBr/O2 catalytic system provided 3a in about 80% yield exclusively for both the above deuterium labeled reactions (eqn (4) and (5)). The addition of butylated hydroxytoluene (BHT) in the CuBr-catalyzed aerobic reaction strictly exacerbated the defluorination process (eqn (6)), which suggests that this reaction might involve radical intermediates. In order to verify the radical-mediated pathway, we next detected the presence of radicals by means of electron paramagnetic resonance (EPR) with the addition of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) for the reaction 2a and NH4OAc under CuBr-catalyzed aerobic conditions. Fortunately, the sextet signal for ˙O2– was captured, while no signal was observed during the Cs2CO3-promoted reaction (see the ESI†). All of the results reasonably confirmed our initial hypothesis (Scheme 1, eqn (3)). The 3-fluoro-2H-pyridin-1-ide Meisenheimer-type anion intermediate underwent a non-redox defluorination process under basic conditions, and the CuBr catalytic aerobic conditions promoted it to an unfavored non-defluorination via a SET/radical pathway.7
Conclusions
In summary, we developed a versatile strategy towards chemoselective synthesis of diverse pyridines via the straightforward [5 + 1] aromatic cyclization of 2-fluoro-2,4-dien-1-ones with ammonium salts. By controlling the oriented conversion of Meisenheimer-type nitrogen anion and radical intermediates under basic and CuBr-catalyzed aerobic reaction conditions, unique chemoselectivity in defluorination and non-defluorination was realized. The synthetic utility of this strategy was highlighted by the concise synthesis of several F-modified bioactive compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Hundred Talent Program of Chinese Academy of Sciences (CAS), the Natural Science Foundation of Jiangsu (Grant No. BK20160396) and CAS “Light of West China” Program is gratefully acknowledged. We are grateful to Professor Xiaobing Wan (Chemical Engineering and Materials Science, Soochow University) for his help on EPR experiment.Notes and references
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Application, ed. V. A. Petrov, John Wiley and Sons, Inc, Hoboken, 2009 Search PubMed; (c) T. Ahrens, J. Kohlmann, M. Ahrens and T. Braun, Chem. Rev., 2015, 115, 931 CrossRef CAS; (d) V. G. Nenajdenko, V. M. Muzalevskiy and A. V. Shastin, Chem. Rev., 2015, 115, 973 CrossRef CAS PubMed; (e) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed; (f) C. Ni and J. Hu, Chem. Soc. Rev., 2016, 45, 5441 RSC.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (b) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed; (c) H.-J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander and M. Stahl, ChemBioChem, 2004, 5, 637 CrossRef PubMed; (d) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed.
- (a) J. March, Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley, New York, 1992, pp. 641–676 Search PubMed; (b) M. Bella, S. Kobbelgaard and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 3670 CrossRef CAS PubMed; (c) I.-H. Um, S.-W. Min and J. M. Dust, J. Org. Chem., 2007, 72, 8797 CrossRef CAS PubMed; (d) I.-H. Um, L.-R. Im, J.-S. Kang, S. S. Bursey and J. M. Dust, J. Org. Chem., 2012, 77, 9738 CrossRef CAS PubMed; (e) S. Shirakawa, K. Koga, T. Tokuda, K. Yamamoto and K. Maruoka, Angew. Chem., Int. Ed., 2014, 53, 6220 CrossRef CAS PubMed; (f) D. Sadowsky, K. McNeill and C. J. Cramer, Environ. Sci. Technol., 2014, 48, 10904 CrossRef CAS PubMed; (g) S. Senaweera and J. D. Weaver, Chem. Commun., 2017, 53, 7545 RSC; (h) A. J. J. Lennox, Angew. Chem., Int. Ed., 2018, 57, 14686 CrossRef CAS PubMed.
- (a) Y.-J. Wu, in Progress in Heterocyclic Chemistry, ed. G. W. Gribble and J. A. Joule, Elsevier, Amsterdam, 2012 Search PubMed; (b) V. A. F. F. M. Santos, L. O. Regasini, C. R. Nogueira, G. D. Passerini, I. Martinez, V. S. Bolzani, M. A. S. Graminha, R. M. B. Cicarelli and M. Furlan, J. Nat. Prod., 2012, 75, 991 CrossRef CAS PubMed; (c) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265 CrossRef PubMed; (d) C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed; (e) T. L. S. Kishbaugh, Curr. Top. Med. Chem., 2016, 16, 3274 CrossRef CAS PubMed; (f) L.-G. Xie, S. Shaaban, X. Chen and N. Maulide, Angew. Chem., Int. Ed., 2016, 55, 12864 CrossRef CAS PubMed; (g) Z. Zhao, H. Wei, K. Xiao, B. Cheng, H. Zhai and Y. Li, Angew. Chem., Int. Ed., 2019, 58, 1148 CrossRef CAS PubMed.
- (a) A. M. Shestopalov, A. A. Shestopalov, L. A. Rodinovskaya and A. V. Gromova, in Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, Part I, ed. V. A. Petrov, Wiley, Hoboken, 2009, vol. 1, pp 243−272 Search PubMed; (b) S. Chen, R. G. Bergman and J. A. Ellman, Org. Lett., 2015, 17, 2567 CrossRef CAS PubMed; (c) S. I. Scherbinina, O. V. Fedorov, V. V. Levin, V. A. Kokorekin, M. I. Struchkova and A. D. Dilman, J. Org. Chem., 2017, 82, 12967 CrossRef CAS PubMed; (d) M. Soethoudt, S. C. Stolze, M. V. Westphal, L. van Stralen, A. Martella, E. J. van Rooden, W. Guba, Z. V. Varga, H. Deng, S. I. van Kasteren, U. Grether, A. P. IJzerman, P. Pacher, E. M. Carreira, H. S. Overkleeft, A. Ioan-Facsinay, L. H. Heitman and M. van der Stelt, J. Am. Chem. Soc., 2018, 140, 6067 CrossRef CAS PubMed.
- (a) X. Wu, K. Li, S. Wang, C. Liu and A. Lei, Org. Lett., 2016, 18, 56–59 CrossRef CAS PubMed; (b) Z. Song, X. Huang, W. Yi and W. Zhang, Org. Lett., 2016, 18, 5640–5643 CrossRef CAS PubMed.
- (a) X. Lia and N. Jiao, Chin. J. Chem., 2017, 35, 1349 CrossRef; (b) C. E. Elwell, N. L. Gagnon, B. D. Neisen, D. Dhar, A. D. Spaeth, G. M. Yee and W. B. Tolman, Chem. Rev., 2017, 117, 2059 CrossRef CAS PubMed.
- P. Harikishore, M. Pankaj, P. Vrajesh and K. V. V. M. Sairam, WO Patent 2013132509, 2013.
- L. Chen, L. Feng, M. Huang, J. Li, F. Nan, T. Pang, L. Yu and M. Zhang, WO Patent 2011033099, 2011.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc03216a |
| This journal is © The Royal Society of Chemistry 2019 |