Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assembly of high-nuclearity Sn26, Sn34-oxo clusters: solvent strategies and inorganic Sn incorporation†
Yu
Zhu
,
Lei
Zhang
 * and
Jian
Zhang
* and
Jian
Zhang

State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: LZhang@fjirsm.ac.cn
First published on 12th August 2019
Abstract
A series of unprecedented high-nuclearity tin-oxo nanoclusters (up to Sn34) with structural diversity have been obtained. The characteristics of the applied solvents had great influence on the assembly of these Sn–O clusters. Pure alcohol environments only gave rise to small clusters of Sn6, whilst the introduction of water significantly increased the nuclearity to Sn26, which greatly exceeds those of the known tin-oxo clusters (≤14); the use of aprotic CH3CN finally produced the largest Sn34 to date. Apart from the nuclearity breakthrough, the obtained tin-oxo clusters also present new structural types that are not found in previous reports, including a layered nanorod-like structure of Sn26 and the cage-dimer structure of Sn34. The layered Sn26 clusters represent good molecular models for SnO2 materials. Moreover, an electrode derived from TOC-17 with a {Sn26} core shows better electrocatalytic CO2 reduction activity than that from TOC-18 with Sn34. This work not only provides an efficient methodology for the rational assembly of high-nuclearity Sn–O clusters, but also extends their potential applications in energy conversion.
Introduction
Tin oxide (SnO2) has attracted increasing research attention due to its application in a variety of areas, including gas sensors,1 catalysis,2,3 lithium batteries,4,5 solar cells6,7 and transparent electrodes.8 Some important factors, such as the size, composition, and structure, greatly influence the electronic and physicochemical properties of SnO2.9–11 Thus it is crucial to understand the binding mode and atomic connectivity of tin oxide materials at the molecular level, which will be beneficial for exploring the structure–property relationship and further achieving precise tuning of the physicochemical properties.As molecular models of tin oxide materials, crystalline tin-oxo clusters (TOCs) can provide precise atomic structural information by X-ray diffraction analysis. Accordingly, they are efficient molecular tools for building bridges between theoretical modeling, crystallography, and physical applications. Many research groups have made great efforts to explore the synthesis and structures of TOCs.12–22 One common way is to use organotin as precursors to react with carboxylate ligands in organic solvents, such as benzene, toluene, and alcohols.23–27 To date, the representative structural types of TOCs are characterized by cubic [n-BuSn(O)O2P(t-Bu)2]4,28 hexameric drum {R′SnO(O2CR)}6,29 ladder [(R′Sn(O)O2CR)2R′Sn(O2CR)3]2,30 and Keggin-type alkyltins.31–33 However, despite these advances, the structural diversity of tin-oxo clusters is still less developed compared to transition-metal oxo clusters. And the TOC nuclearity also remains quite low, with the highest one being Sn14.34 Therefore, it is an attractive but challenging goal to synthesize TOCs with larger core nuclearity and more structural diversity. Moreover, although most of the driving forces of the research on TOCs originate from understanding the physical attributes of tin oxide, the studies on their applications (e.g. in catalysis) still remain very rare. Thus it is rather urgent to explore the physical application of TOCs to acquire the important Sn–O connectivity–activity relationship.
The assembly of tin-oxo clusters highly depends on the reactivity of organotin precursors, which is further influenced by organic ligands, reaction temperature, solvent and so on. Therefore, to prepare a diverse range of high-nuclearity TOCs, it is necessary to develop new synthetic approaches. During our recent research in titanium-oxo clusters,35–37 high-temperature assembly reactions were widely used for the preparation of high nuclearity Ti–O clusters. Herein, we introduce this method into the field of tin-oxo clusters, and make the following indispensable modifications: (1) mixed alcohol–water solvents were applied to influence the aggregation rate of Sn atoms and the nucleation rate of tin-oxo clusters; (2) the aprotic solvent CH3CN was then used in some cases instead of the general protic solvent to affect the configuration of basic building blocks, as well as their way of connecting; (3) finally, inorganic Sn atoms without any alkyl group or phenyl group were introduced into the reaction system to supply more bridging Sn atoms to promote the formation of high nuclearity TOCs. With these synthetic strategies, a series of unprecedented high-nuclearity TOCs have been successfully obtained whose atomic structures were characterized by single crystal X-ray diffraction analysis (Table 1). They possess a much higher number of Sn atoms (26, 34) than the known TOCs (≤14). Meanwhile, they also present new structural types different from previous TOCs, including the rod-shaped Sn26 and cage-dimer Sn34. Furthermore, the application of Sn26 or Sn34 derived electrode in electrocatalytic CO2 reduction was investigated for the first time. Nuclear magnetic resonance (NMR) spectroscopy analysis indicated that formate was obtained as the only liquid reduction product. And the corresponding formate faradaic efficiency (FE) was found to be cluster dependent, with the highest value of 41.90% on the Sn26 derived electrode.
| Compound | Formula | Sn source | Solvent | Nuclearity |
|---|---|---|---|---|
| a n-Bu = butyl group; PDC = 2,6-pyridinedicarboxylate; PP = phenylphosphonate; H3BPA = 2,2-bis(hydroxymethyl)propionic acid; HTZAC = 1H-tetrazole-1-acetic acid; HIANO = isonicotinic acid-N-oxide; HPA = 2-picolinic acid. | ||||
| TOC-12 | NaH[(n-BuSn)3(PDC)3(OCH3)(OH)3]2·6CH3OH | Butyltin hydroxide oxide | Methanol–isopropanol | Sn6 |
| TOC-13 | (n-BuSn)6O2(PP)4(OCH3)6 | Butyltin hydroxide oxide | Methanol–isopropanol | Sn6 |
| TOC-14 | [(n-BuSn)12(OH)18O4(BPA)2(H2BPA)4]·6H2O | Butyltin hydroxide oxide | Methanol–water | Sn12 |
| TOC-15 | [(n-BuSn)22Sn4(OH)26O22(TZAC)6]·4Cl·2(TZAC)·2(CH3OH)·8H2O | Butyltin hydroxide oxide | Methanol–isopropanol–water | Sn26 |
| TOC-16 | [(n-BuSn)22Sn4(OH)26O22(IANO)6]·6(IANO)·6(CH3OH)·4H2O | Butyltin hydroxide oxide | Methanol–isopropanol–water | Sn26 |
| TOC-17 | [(n-BuSn)22Sn4(OH)26O22(IANO)6][(n-BuSn)2(OH)2(IANO)Cl4]2·4Cl·6CH3OH·10H2O | Butyltin hydroxide oxide and SnCl4 | Methanol–isopropanol–water | Sn26 |
| TOC-18 | [(n-BuSn)34Na2(OH)14O40(PA)8]·2(PA)·8H2O | Butyltin hydroxide oxide | Acetonitrile | Sn34 |
Results and discussion
In the initial stage of our research, mixed methanol–isopropanol was used as the solvent for reactions between butyltin hydroxide oxide and 2,6-pyridinedicarboxylic acid/NaOH or phenylphosphonic acid, resulting in the formation of two Sn6 clusters (TOC-12 and TOC-13). Structural analysis indicated that they were both composed of two O-capped {Sn3O4} units (Fig. 1a and b; Table 1). Different from the back-to-back linking mode of two O-capped {Sn3O4} units via PP ligands in TOC-13, the O-capped {Sn3O4} units in TOC-12 are linked by a Na atom in a face-to-face fashion to form a sandwich-like {Sn3NaSn3} architecture. It is notable that methanol plays a bridging role in the O-capped {Sn3O4} units of TOC-12 and TOC-13, implying that the strong coordination ability and small steric hindrance of methanol may be in favour of the formation of the O-capped {Sn3O4} unit. In order to obtain different building units and change the aggregation/nucleation rate of tin atoms, water which is crucial for the growth of oxo clusters was directly introduced into the reaction system. Consequently, a new Sn12 TOC was prepared namely [(n-BuSn)12(OH)18O4(BPA)2(H2BPA)4]·6H2O (TOC-14) (H3BPA = 2,2-bis(hydroxymethyl)propionic acid). As shown in Fig. 1c, TOC-14 is constructed from two different building blocks, the O-capped {Sn3O4} and ladder {Sn4O4} units, indicating that the introduction of water is an effective strategy to prepare different structural types of TOCs with higher nuclearity.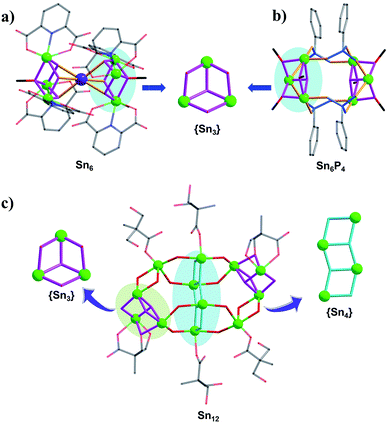 | ||
| Fig. 1 The ball-and-stick representations of TOC-12 (a), TOC-13 (b) and TOC-14 (c). Atom color code: green Sn; purple Na; red O; black C; dark blue N; light blue P. | ||
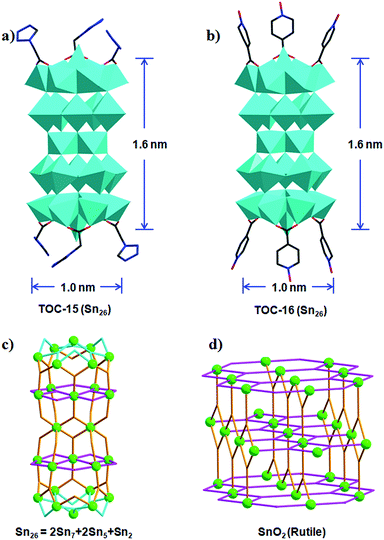
By further optimizing the reaction conditions, especially the amount of used water, TOC-15 and TOC-16 with nuclearities of Sn26 were successfully obtained in a mixed solvent system of methanol–isopropanol–water (Table 1, Fig. 2). To the best of our knowledge, the numbers of Sn atoms (26) in these TOCs far exceed the value (≤14) in reported tin-oxo clusters to date. The core size of these nanoclusters is ∼1.6 × 1.0 nm. As shown in Fig. 2, different from the usual cage structures of reported TOCs, TOC-15 presents a rod-shaped cluster core that can be considered to be made up of five tin-oxo layers, including two planar {Sn5} units, two capped {Sn7} moieties and one {Sn2} dimer. The {Sn7} and {Sn5} units are linked together by five μ3-O atoms to form a {Sn12} moiety. And two symmetry-related {Sn12} moieties are further held together through the central {Sn2} dimer to give the Sn26 core of TOC-15, which is further protected by six TZAC ligands. Such a layered structure type is found for the first time in the area of TOCs, and is reminiscent of the tin-oxo layers in SnO2 (Fig. 2c and d). By changing the functional ligands, other Sn26 clusters of TOC-16 were obtained with the six labile coordination sites decorated with IANO ligands (Fig. S6†).
Although TOC-15 and TOC-16 present interesting high-nuclearity layered structures, their yields are unfortunately quite low (2–4%), which greatly limits further applications. To obtain some mechanistic information of such synthetic shortages, we analyzed their structures in more detail. It is interesting to find that TOC-15 and TOC-16 contain pure inorganic SnO6 nodes without butyl groups. Such completely O-coordinated Sn atoms should be derived from the Sn–C bond cleavage of the applied butyltin hydroxide oxide. Considering the difficulty of Sn–C bond cleavage under the applied low-temperature conditions, it will benefit the assembly of such Sn–O cores if isolated Sn ions could be incorporated into the reactions. For this aim, the inorganic SnCl4 precursor was further introduced into the synthetic reaction of TOC-16. As expected, TOC-17 was successfully isolated in a much higher yield (∼60%). As presented in Fig. S8,†TOC-17 is composed of the same Sn26 moiety in TOC-16 and two additional {Sn2} dimers which are made up of two Sn atoms bridged by two oxygen atoms and one IANO ligand. The successful preparation of TOC-17 demonstrates that the strategy of introducing additional inorganic Sn atoms is indeed helpful for the formation of high-nuclearity TOCs.
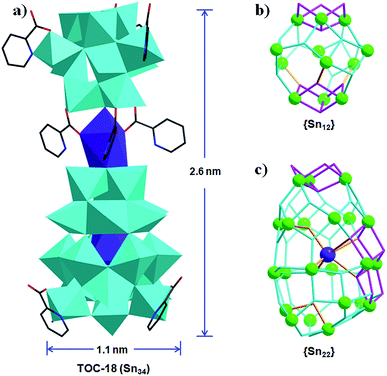
Based on the above results, we can clearly see that the assembly of Sn–O clusters is greatly influenced by the applied solvent conditions. Pure alcohol environments gave rise to small clusters of Sn6, and the introduction of water could significantly increase the nuclearities to Sn26 (Table 1).38 However, as a whole, the above used solvents are all protic ones. If an aprotic solvent, e.g. CH3CN, could be applied, the different solvent environment may change the configuration of basic Sn–O building units, as well as their way of connecting. Following this consideration, the reaction of butyltin hydroxide oxide with 2-picolinic acid and NaOH was carried out in pure CH3CN. As a consequence, TOC-18 with a nuclearity of Sn34 and a core size of ∼2.6 × 1.1 nm was successfully synthesized, which is the largest Sn–O cluster reported to date. As exhibited in Fig. 3a, TOC-18 is made up of two unprecedented {Sn12} and {Sn22} cages linked together by a Na atom and PA ligands. Compared with the typical football cage {(RSn)12O14(OH)6}2−, the {Sn12} in TOC-18 displays an asymmetric cage-like structure (Fig. 3b). The {Sn22} cage captures a central Na heteroatom via six oxygen atoms (Fig. 3c). From an architectural point of view, the {Sn22} cage can be considered to consist of four subunits with different numbers of Sn atoms, including a top Sn3 moiety, two middle Sn6 circles and a bottom Sn7 base.
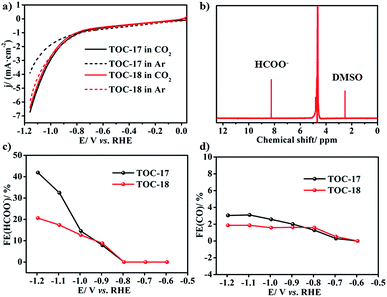
Recently, SnO2 has shown interesting energy conversion applications through electrocatalytically reducing CO2 to formate.2 Considering the similar layered characteristics of the Sn26 cluster core and rutile SnO2, the obtained Sn26 may also have potential application in the electrocatalytic CO2 reduction reaction (CO2RR). Therefore, carbon paper with TOC-17 modification was used as the working electrode to study its catalytic activity towards the CO2RR. A linear sweep voltammetry (LSV) test was conducted in Ar or CO2 saturated 0.5 M KHCO3 solution, respectively. As shown in Fig. 4a, the LSV curve of the TOC-17 derived electrode in the CO2 saturated electrolyte exhibits the onset potential at approximately −0.69 V. Beyond this onset potential, the current density continuously increases and reaches 6.73 mA cm−2 at −1.159 V, which is obviously higher than that in the Ar saturated electrolyte. This indicates that the TOC-17 derived electrode may possess high catalytic activity towards the CO2RR. In order to identify and quantify the reduction products, gas chromatography (GC) and nuclear magnetic resonance (NMR) spectroscopy were applied to analyze the gas and liquid phase products during the CO2RR process. The obtained results indicate that formate was the only liquid CO2RR product, with the highest faradaic efficiency (FE) of 41.90% at −1.196 V (Fig. 4). Meanwhile, only a small amount of CO was detected in the gas phase products. Powder X-ray diffraction analysis further confirmed that the structures of TOC-17 remained rather intact after electrolysis (Fig. S46†). For comparison, the CO2RR application of the cage-dimer structure TOC-18 was also studied. Although presenting higher nuclearity and also producing formate as the main product, the activity of the TOC-18 derived electrode was significantly lower than that modified with TOC-17. Therefore, these results indicate that the layered Sn–O structures might be beneficial for electrocatalytic CO2RR applications.
Conclusions
In summary, we have successfully synthesized a series of unprecedented high-nuclearity tin-oxo clusters by solvent dependent synthetic strategies. These obtained high-nuclearity TOCs, with core sizes ranging from 1.6 to 2.6 nm, possess a much higher number of Sn atoms (26, 34) than previously known ones (≤14). Moreover, new structural types of layered nanorods and cage-dimers were also prepared. The applied solvent environments and Sn sources have proven to play crucial roles in the assembly of these high-nuclearity tin-oxo clusters. The introduction of water into alcohol greatly increased the cluster nuclearity; the incorporation of inorganic Sn ions significantly increased the yields of layered structures, while the application of aprotic CH3CN produced the largest Sn34 to date. Moreover, electrocatalytic CO2 reduction studies confirmed that the electrodes derived from the layered Sn26 cluster presented better performance than those derived from the Sn34 cage-dimer. Therefore, these results afford effective synthetic strategies for the assembly of high-nuclearity TOCs and also extended their potential applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2018YFA0208600), NSFC (21673238), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000) and the Natural Science Foundation of Fujian Province (2017J06009).Notes and references
- J. S. Jang, S. Yu, S. J. Choi, S. J. Kim, W. T. Koo and I. D. Kim, Small, 2016, 12, 5989 CrossRef CAS PubMed.
- F. W. Li, L. Chen, G. P. Knowles, D. R. MacFarlane and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 505 CrossRef CAS PubMed.
- L. Fan, Z. Xia, M. J. Xu, Y. Y. Lu and Z. J. Li, Adv. Funct. Mater., 2018, 28, 1706289 CrossRef.
- W. J. Dong, J. J. Xu, C. Wang, Y. Lu, X. Y. Liu, X. Wang, X. T. Yuan, Z. Wang, T. Q. Lin, M. L. Sui, I. W. Chen and F. Q. Huang, Adv. Mater., 2017, 29, 1700136 CrossRef PubMed.
- G. D. Park, J. K. Lee and Y. C. Kang, Adv. Funct. Mater., 2017, 27, 1603399 CrossRef.
- J. Wei, F. W. Guo, X. Wang, K. Xu, M. Lei, Y. Q. Liang, Y. C. Zhao and D. S. Xu, Adv. Mater., 2018, 30, 1805153 CrossRef PubMed.
- X. K. Zhang, Y. C. Rui, Y. Q. Wang, J. L. Xu, H. Z. Wang, Q. H. Zhang and P. M. Buschbaum, J. Power Sources, 2018, 402, 460 CrossRef CAS.
- B. R. Koo, D. H. Oh, D. H. Riu and H. J. Ahn, ACS Appl. Mater. Interfaces, 2017, 9, 44584 CrossRef CAS PubMed.
- D. D. Zhu, J. L. Liu and S. Z. Qiao, Adv. Mater., 2016, 28, 3423 CrossRef CAS PubMed.
- B. H. Zhang, Z. H. Guo, Z. Zuo, W. Pan and J. T. Zhang, Appl. Catal., B, 2018, 239, 441 CrossRef CAS.
- P. Han, Z. J. Wang, M. Kuang, Y. F. Wang, J. N. Liu, L. F. Hu, L. P. Qian and G. F. Zheng, Adv. Energy Mater., 2018, 8, 1801230 CrossRef.
- G. L. Zheng, J. F. Ma, Z. M. Su, L. K. Yan, J. Yang, Y. Y. Li and J. F. Liu, Angew. Chem., Int. Ed., 2004, 43, 2409 CrossRef CAS PubMed.
- X. Xiao, K. Z. Shao, L. S. Yan, Z. M. Mei, D. S. Zhu and L. Xu, Dalton Trans., 2013, 42, 15387 RSC.
- G. Prabusankar, B. Jousseaume, T. Toupance and H. Allouchi, Angew. Chem., Int. Ed., 2006, 45, 1255 CrossRef CAS PubMed.
- G. Prabusankar, B. Jousseaume, T. Toupance and H. Allouchi, Dalton Trans., 2007, 3121 RSC.
- V. Chandrasekhar, V. Baskar and J. J. Vittal, J. Am. Chem. Soc., 2003, 125, 2392 CrossRef CAS PubMed.
- B. Zobel, J. Costin, B. R. Vincent, E. R. T. Tiekink and D. Dakternieks, J. Chem. Soc., Dalton Trans., 2000, 4021 RSC.
- Y. P. Xie, J. F. Ma, J. Yang and M. Z. Su, Dalton Trans., 2010, 39, 1568 RSC.
- M. Mehring, M. Schürmann, H. Reuter, D. Dakternieks and K. Jurkschat, Angew. Chem., Int. Ed., 1997, 36, 1112 CrossRef CAS.
- J. Beckmann, D. Dakternieks, A. Duthie, F. S. Kuan, K. Jurkschat, M. Schürmann and E. R. T. Tiekink, New J. Chem., 2004, 28, 1268 RSC.
- T. Zöler and K. Jurkschat, Inorg. Chem., 2013, 52, 1872 CrossRef PubMed.
- B. Glowacki, M. Lutter, D. Schollmeyer, W. Hiller and K. Jurkschat, Inorg. Chem., 2016, 55, 10218 CrossRef CAS PubMed.
- S. Kundu and V. Chandrasekhar, Cryst. Growth Des., 2015, 15, 5437 CrossRef CAS.
- K. C. K. Swamy, R. O. Day and R. R. Holmes, J. Am. Chem. Soc., 1987, 109, 5546 CrossRef CAS.
- V. Chandrasekhar, V. Baskar, K. Gopal and J. J. Vittal, Organometallics, 2005, 24, 4926 CrossRef CAS.
- S. Y. Song, J. F. Ma, J. Yang, L. L. Gao and Z. M. Su, Organometallics, 2007, 26, 2125 CrossRef CAS.
- M. C. Sharps, D. A. Marsh, L. N. Zakharov, J. E. Hutchison and D. W. Johnson, Cryst. Res. Technol., 2017, 52, 1700081 CrossRef.
- R. R. Holmes, K. C. K. Swamy, C. G. Schmid and R. O. Day, J. Am. Chem. Soc., 1988, 110, 7060 CrossRef CAS.
- K. C. K. Swamy, R. O. Day and R. R. Holmes, Inorg. Chem., 1988, 27, 958 CrossRef.
- R. R. Holmes, C. G. Schmid, V. Chandrasekhar, R. O. Day and J. M. Holmes, J. Am. Chem. Soc., 1987, 109, 1408 CrossRef CAS.
- S. Saha, D. H. Park, D. C. Hutchison, M. R. Olsen, L. N. Zakharov, D. Marsh, S. Goberna-Ferrón, R. T. Frederick, J. T. Diulus, N. Kenane, G. S. Herman, D. W. Johnson, D. A. Keszler and M. Nyman, Angew. Chem., Int. Ed., 2017, 56, 10140 CrossRef CAS PubMed.
- D. C. Hutchison, R. D. Stern, M. R. Olsen, L. N. Zakharov, K. A. Persson and M. Nyman, Dalton Trans., 2018, 47, 9804 RSC.
- H. Reuter, Angew. Chem., Int. Ed. Engl., 1991, 30, 1482 CrossRef.
- Q. F. Wang, C. L. Ma, G. F. He and Z. Li, Polyhedron, 2013, 49, 177 CrossRef CAS.
- J. X. Liu, M. Y. Gao, W. H. Fang, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 5160 CrossRef CAS PubMed.
- S. Chen, W. H. Fang, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2018, 57, 11252 CrossRef CAS PubMed.
- X. Fan, J. H. Wang, K. F. Wu, L. Zhang and J. Zhang, Angew. Chem., Int. Ed., 2019, 58, 1320 CrossRef CAS PubMed.
- X. F. Xu, W. Wang, D. L. Liu, D. D. Hu, T. Wu, X. H. Bu and P. Y. Feng, J. Am. Chem. Soc., 2018, 140, 888 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, images of the structures, IR and UV-vis spectra, PXRD patterns, TG curves and the calibration curve for formate and faradaic efficiencies of H2. CCDC 1563679, 1563680, 1563685 and 1895007–1895010. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02503k |
| This journal is © The Royal Society of Chemistry 2019 |