Open Access Article
Open Access ArticleSymmetry-guided syntheses of mixed-linker Zr metal–organic frameworks with precise linker locations†
Hyehyun
Kim
a,
Dongwook
Kim
a,
Dohyun
Moon
 b,
Yong Nam
Choi
c,
Seung Bin
Baek
b,
Yong Nam
Choi
c,
Seung Bin
Baek
 *a and
Myoung Soo
Lah
*a and
Myoung Soo
Lah
 *a
*a
aDepartment of Chemistry, Ulsan National Institute of Science and Technology, Ulsan 44919, Korea. E-mail: mslah@unist.ac.kr; sbbaek@unist.ac.kr
bPohang Acceleratory Laboratory, Pohang 37673, Korea
cKorea Atomic Energy Research Institute, Daejeon 34057, Korea
First published on 7th May 2019
Abstract
While the one-pot reaction of zirconium metal ions with a mixture of two dicarboxylate heterolinkers yielded a 12-c fcu Zr MOF with randomly distributed linkers, the symmetry-guided stepwise reaction produced the same MOF with both linkers precisely located in the framework. In the latter method, linear terephthalic acid (H2BDC) derivatives with mmm symmetry were inserted into the mmm-symmetry sites of the flexible Zr MOF with 8-c bcu topology (ZRN-bcu), which is composed of zigzag 2,6-naphthalenedicarboxylic acid with 2/m symmetry. Although the length of the symmetry-matching BDC2− derivatives was much shorter than the distance between the unlinked nearest-neighbor Zr clusters in ZRN-bcu, induced fitting of the derivatives into the framework was possible, resulting in well-defined locations for the two different dicarboxylate linkers. Thus, controlled synthesis of MOFs with the desired topology and functionality can be achieved using a symmetry-guided approach.
Introduction
Metal–organic frameworks (MOFs) are a class of highly porous crystalline materials consisting of metal ions (or clusters) as nodes and organic ligands as linkers.1–3 To modulate framework properties, such as pore size, shape, and surface properties, organic ligands with various functional groups are often introduced via either direct solvothermal reactions4–7 or stepwise post-synthetic modifications.6–11Recently, carboxylate-based Zr MOFs have received much attention owing to their improved thermal and chemical stabilities compared with those of most reported MOFs.12 It is well known that the Zr4+ ion has a strong tendency to form hexanuclear [Zr6O4(OH)4(COO)12] clusters when [Zr6O4(OH)4]12+ is fully coordinated by linear dicarboxylate ligands,12–23 and this usually produces Zr MOFs with 12-c fcu topology.12 Several UiO-66-type Zr MOFs containing two different kinds of linkers with the same length have been prepared via either one-pot synthesis using a mixture of heterolinkers24,25 or post-synthetic linker exchange.25,26 However, the linkers in these Zr MOFs are randomly distributed, and it remains a challenge to place them at specific positions in the framework.25
This drawback of current approaches can be overcome by performing post-synthetic insertion of secondary linkers into predetermined positions in the framework. A metal cluster with a connectivity lower than its maximum connectivity in a MOF can be further utilized as post-synthetic insertion sites for another MOF with a different net topology. From a topological point of view, the 8-c bcu and 12-c fcu topologies are similar except for the four missing equatorial linkages in the former, which generates the 8-c node.
Post-synthetic insertion of different carboxylate linkers into 8-c bcu Zr MOFs can give rise to 10-c, 11-c, and/or 12-c Zr MOFs with linkers positioned at specific sites in the framework.27–29 For example, Zhou et al. synthesized a new Zr MOF with a 12-c hexanuclear [Zr6O4(OH)4(COO)12] cluster via post-synthetic linker insertion into 8-c bcu PCN-700.27 The 8-c bcu topology is due to steric hindrance by the methyl substituents of the 2,2′-dimethylbiphenyl-4,4′-dicarboxylate (Me2-BPDC) linker, which forces the two carboxylate groups in a twisted conformation (Scheme 1). Because the Zr clusters alternate in two different orientations, a 12-c Zr MOF can only be obtained via stepwise post-synthetic insertion of both symmetry- and length-matching linear dicarboxylate linkers into PCN-700 (Scheme 1).28 The resulting Zr MOF is an unprecedented 12-c network with the {324·428·513·6} point symbol.
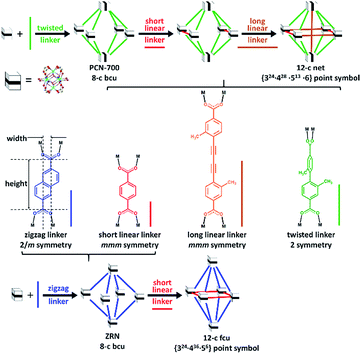 | ||
| Scheme 1 Stepwise linker insertion of different types of dicarboxylate linkers to produce 12-c Zr MOFs. | ||
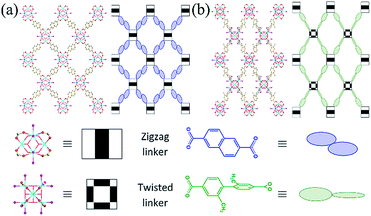
An 8-c bcu Zr MOF can also be obtained by using a zigzag dicarboxylate linker with 2/m symmetry (Scheme 1).30 The reduced connectivity of the Zr clusters in this Zr MOF is due to the transversal parameter of the linker, which reflects its width-to-height ratio. This Zr MOF has two sets of intercluster sites between two neighboring Zr clusters. The neighboring Zr clusters associated with the first set of intercluster sites with 2/m symmetry are interlinked by a zigzag linker of the same symmetry. On the other hand, the missing linkage between the other neighboring Zr clusters associated with the second set of intercluster sites is believed to be due to the mismatch between the intercluster distance and linker length. The extent of length mismatch depends on the transversal parameter of the zigzag linker, i.e., the width-to-height ratio. Interestingly, a zigzag linker with a small width-to-height ratio, 2,6-naphthalenedicarboxylic acid (H2NDC), also yielded an 8-c bcu Zr MOF (hereafter referred to as ZRN-bcu) even though the second type of intercluster distance (∼8.0 Å) was only ∼10% longer than the first type linked by NDC2− (∼7.4 Å).29 Considering the flexibility of a framework with bcu topology, the missing linkage at the second type of intercluster site is probably due to symmetry mismatch and not length mismatch.
Herein, we report the symmetry-guided synthesis of such Zr MOFs using linear and zigzag dicarboxylate linkers with different symmetries and lengths whose precise locations in the framework were crystallographically determined. Post-synthetic insertion of symmetry-matching but length-mismatching linear terephthalate (H2BDC) derivatives into the flexible ZRN-bcu produced a series of 12-c fcu Zr MOFs with two different types of ditopic heterolinkers (Scheme 1). To the best of our knowledge, this is the first report on 12-c fcu Zr MOFs containing two crystallographically well-defined linkers with different linker symmetries and lengths.
Results and discussion
Mixed-linker Zr-MOFs with random linker locations
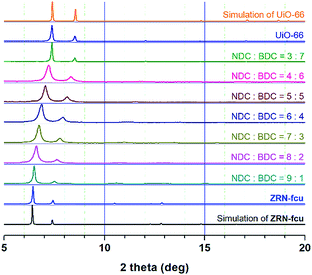
Interestingly, Zr MOFs containing two dicarboxylates with different symmetries and lengths, zigzag NDC2− and linear BDC2−, can be prepared via one-pot solvothermal reactions. However, all attempts to synthesize 12-c fcu Zr MOFs using various ratios of the linkers produced only hybrid products (Fig. 1). The powder X-ray diffraction (PXRD) patterns of the Zr MOFs can be fitted to the same face-centered cubic lattice type, and the lattice parameters gradually decrease as the amount of the short BDC2− linker increases. The linkers are not located at specific positions but randomly distributed in the framework, which accounts for the observed lattice type.Preparation and structure of ZRN-bcu
For the synthesis of a 12-c fcu Zr-MOF containing two different linkers at well-defined locations via post-synthetic linker insertion, it is necessary to prepare an 8-c bcu Zr-MOF with its Zr-clusters in proper relative orientations. Because both ZRN-bcu and 12-c fcu Zr MOFs containing a zigzag NDC2− linker can be prepared under similar synthetic reaction conditions, the reaction condition was modified to exclusively produce the former. The use of excess acetic acid (HAc) as the modulator produced the corresponding 12-c fcu Zr MOF (hereafter referred to as ZRN-fcu), which is also known as DUT-52.21By employing trifluoroacetic acid (HTFA) as the modulator, ZRN-bcu crystals can be obtained under a wide range of reaction conditions (Fig. S1†). On the other hand, ZRN-fcu can only be obtained under very narrow reaction conditions at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal
1 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
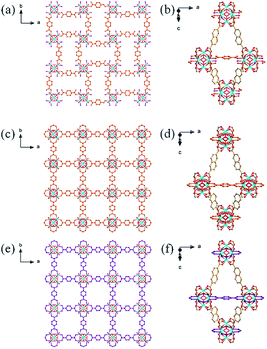
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio and in the presence of only a small amount of HTFA. Crystallographic analysis confirmed that ZRN-bcu is a 3D MOF with 8-c bcu topology, where the cationic [Zr6O4(OH)4(COO)8(H2O)8]4+ cluster on the 4/mmm (D4d) site serves as the 8-c secondary building unit and NDC2− is located on the 2/m (C2h) site (Fig. 2a and S2†). Statistically disordered TFA counter anions are found in the solvent pore near the Zr clusters. The framework of ZRN-bcu is the same as that of the reported structure, [Zr6O4(OH)4(NDC)4(H2O)8]4+.29 Although ZRN-bcu and PCN-700 have the same topology, the arrangement of the Zr clusters in their respective frameworks is different (Fig. 2). Zr clusters in PCN-700 alternate in two different orientations since the two carboxylates of Me2-BPDC are perpendicular to each other (Fig. 2b). On the other hand, all Zr clusters in ZRN-bcu are in the same orientation owing to the coplanar conformations of the two carboxylates of NDC2− (Fig. 2a). Despite the coplanarity of all potential linkage sites of the Zr clusters in ZRN-bcu, only a network with 8-c bcu topology was obtained. This preference over the 12-c fcu topology can be attributed to the inherent symmetry mismatch with NDC2−.
ligand ratio and in the presence of only a small amount of HTFA. Crystallographic analysis confirmed that ZRN-bcu is a 3D MOF with 8-c bcu topology, where the cationic [Zr6O4(OH)4(COO)8(H2O)8]4+ cluster on the 4/mmm (D4d) site serves as the 8-c secondary building unit and NDC2− is located on the 2/m (C2h) site (Fig. 2a and S2†). Statistically disordered TFA counter anions are found in the solvent pore near the Zr clusters. The framework of ZRN-bcu is the same as that of the reported structure, [Zr6O4(OH)4(NDC)4(H2O)8]4+.29 Although ZRN-bcu and PCN-700 have the same topology, the arrangement of the Zr clusters in their respective frameworks is different (Fig. 2). Zr clusters in PCN-700 alternate in two different orientations since the two carboxylates of Me2-BPDC are perpendicular to each other (Fig. 2b). On the other hand, all Zr clusters in ZRN-bcu are in the same orientation owing to the coplanar conformations of the two carboxylates of NDC2− (Fig. 2a). Despite the coplanarity of all potential linkage sites of the Zr clusters in ZRN-bcu, only a network with 8-c bcu topology was obtained. This preference over the 12-c fcu topology can be attributed to the inherent symmetry mismatch with NDC2−.
 | ||
| Fig. 2 Ball-and-stick models and schematic drawings of the frameworks of (a) ZRN-bcu and (b) PCN-700. | ||
Mixed-linker Zr-MOFs with precise linker locations
Although the maximum site symmetry of a dicarboxylate linker in a 12-c fcu Zr MOF is mmm, it is still possible to have only the zigzag NDC2− linker of 2/m symmetry by using the appropriate one-pot solvothermal reaction established in this work or the previously reported one.21 Interestingly, NDC2− is statistically disordered on the mmm-symmetry site of the ZRN-fcu prepared via one-pot solvothermal reaction.21 In contrast, it should be noted that the ZRN-bcu framework does not allow the post-synthetic incorporation of the symmetry-mismatching NDC2− into the potential linkage site of mmm symmetry, as confirmed by all our unsuccessful attempts.With this in mind, two different symmetries of dicarboxylate linkers were used to prepare new 12-c fcu Zr MOFs through post-synthetic insertion into ZRN-bcu (Fig. 3). To examine the effects of symmetry and length on this process, a series of symmetry-matching but length-mismatching H2BDC derivatives (H2XBDC) were chosen. The linker length between the carboxylic carbons (∼5.4 Å) is much shorter than the ideal linker length (∼8.0 Å) required to form a 12-c fcu Zr MOF without significant tetragonal distortion of ZRN-bcu.
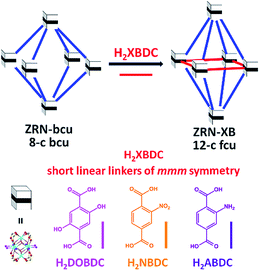 | ||
| Fig. 3 Preparation of 12-c fcu ZRN-XB (X = DO, N, and A for the –(OH)2, –NO2, and –NH2 substituents, respectively) via post-synthetic insertion of the symmetry-matching linear linker into ZRN-bcu. | ||
The new Zr MOFs were obtained by soaking ZRN-bcu single crystals in a methanol (MeOH) solution of linear H2XBDC (X = DO, N, and A for the –(OH)2, –NO2, and –NH2 substituents, respectively) of mmm symmetry (ignoring the substituents). The replacement of the TFA counter anions in ZRN-bcu by XBDC2− was confirmed by 1H nuclear magnetic resonance (NMR) (Fig. S3 and S4†). The ratio of NDC2− to XBDC2− in ZRN-NB and ZRN-AB MOFs is the same as the value (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) expected from fully inserted 12-c fcu topology Zr MOF structures regardless of the substituent. In the case of ZRN-DOB, the ratio of the NDC2− to DOBDC linker is about 15% smaller than the expected value, suggesting the presence of ligand-vacancy defect sites in the ZRN-DOB framework. The formation of ligand-vacancy defect sites is understandable given the post-synthetic ligand insertion processes as demonstrated in sequential ligands insertions into 8-c bcu PCN-700 MOF.28
1) expected from fully inserted 12-c fcu topology Zr MOF structures regardless of the substituent. In the case of ZRN-DOB, the ratio of the NDC2− to DOBDC linker is about 15% smaller than the expected value, suggesting the presence of ligand-vacancy defect sites in the ZRN-DOB framework. The formation of ligand-vacancy defect sites is understandable given the post-synthetic ligand insertion processes as demonstrated in sequential ligands insertions into 8-c bcu PCN-700 MOF.28
Despite the incorporation of the linear 2,5-dihydroxy-1,4-benzenedicarboxylate (DOBDC2−) linker into the framework (as verified by the 1H NMR spectrum), the PXRD pattern of as-synthesized ZRN-DOB is similar to that of as-synthesized ZRN-bcu (Fig. 4). Under ambient conditions, ZRN-DOB quickly transforms to a new crystalline phase, ZRN-DOB-amb, which is different from ZRN-w obtained from ZRN-bcu. On the other hand, the PXRD patterns of ZRN-AB and ZRN-NB, containing ABDC2− and NBDC2−, respectively, are different from that of ZRN-bcu but very similar to that of ZRN-DOB-amb regardless of the sample state (i.e., as-synthesized crystal and crystal exposed to ambient conditions) (Fig. 4).
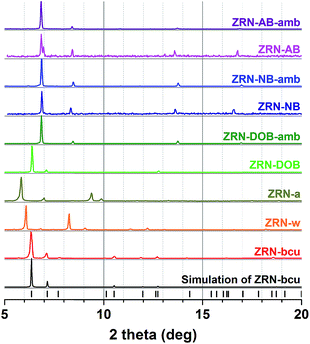 | ||
| Fig. 4 PXRD patterns of ZRN-bcu, ZRN-w, ZRN-a, as-synthesized ZRN-XB, and the corresponding crystals exposed to ambient conditions. | ||
Because of the flexibility of the ZRN-bcu crystals (ESI, Fig. S5–S14†), single crystallinity during post-synthetic insertion of H2XBDC was retained, which allowed single-crystal structure analysis. The incorporation of DOBDC2− into the ZRN-bcu framework changed the lattice type from body-centered tetragonal to primitive tetragonal. However, the unit cell parameters of ZRN-DOB and ZRN-bcu were very similar, suggesting that the insertion did not cause any significant framework distortion. Structural analysis revealed that additional DOBDC2− ligands are present in the framework but not inserted between the Zr clusters of the potential linkage site to form the 12-c fcu Zr MOF (Fig. 5 and S15†).
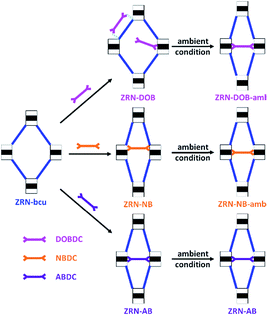 | ||
| Fig. 5 Schematic drawing of the post-synthetic insertion of short linear linkers with mmm symmetry into the ZRN-bcu framework to form stable 12-c fcu ZRN-XB and ZRN-XB-amb under ambient conditions. | ||
Two different interaction modes of the statistically disordered DOBDC2− ligands are therefore observed: one ligated to a Zr center in monodentate coordination mode and the other located between Zr clusters that are interconnected by an NDC2− ligand. DOBDC2− interacts with the framework through the combination of two different weak interactions, hydrogen bonding and π–π stacking (Fig. S15†). The carboxylate oxygen atoms of DOBDC2− are hydrogen bonded to both the ligated water molecule and μ3-hydroxo oxygen atom of the Zr cluster. DOBDC2− is further stabilized by π–π stacking interaction with NDC2−.
As mentioned earlier, under ambient conditions, as-synthesized ZRN-DOB quickly transforms to ZRN-DOB-amb, which has a PXRD pattern similar to those of ZRN-NB and ZRN-AB (Fig. 4). Unfortunately, ZRN-DOB-amb single crystals were unsuitable for single-crystal structure analysis.
ZRN-NB can be prepared using 2-nitro-1,4-benzenedicarboxylic acid (H2NBDC) instead of 2,5-dihydroxy-1,4-benzenedicarboxylic acid (H2DOBDC). Induced fitting of the short NBDC2− linker into the potential linkage sites in ZRN-bcu to form 12-c fcu ZRN-NB resulted in significant tetragonal distortion of the unit cell parameters. Lattice parameters a and b shrank from 17.445 to 14.955 Å, while lattice parameter c elongated from 22.934 to 26.077 Å. Crystallographic analysis confirmed that ZRN-NB has 12-c fcu topology. However, the NBDC2− linkers at the linkage sites between the Zr clusters are ligated in a statistically disordered monodentate coordination mode rather than a bridging bidentate coordination mode (Fig. 5, 6a and b). During the insertion of NBDC2− into the ZRN-bcu framework, the conformation of the NDC2− linkers in ZRN-NB also rotate by ∼180° to relieve the steric repulsion between them, as observed in ZRN-w (Fig. S9†).
As-synthesized ZRN-NB is unstable under ambient conditions and quickly transforms to a new crystalline phase, ZRN-NB-amb. The two crystals have similar unit cell parameters (ESI†), as expected from the similarity of their PXRD patterns (Fig. 4). ZRN-NB-amb is also a 12-c fcu Zr MOF with two different types of dicarboxylate linkers (Fig. 5, 6c and d). However, the bridging mode of the NBDC2− linker with mmm symmetry in ZRN-NB-amb is different from that in ZRN-NB. NBDC2− is on an mmm-symmetry site in bridging bidentate coordination mode. It also induces significant tetragonal distortion of the framework, contraction of the framework in the ab plane, and elongation along the c axis. The similarity of the PXRD patterns of ZRN-DOB-amb and ZRN-NB-amb indicates that the former is also likely a 12-c fcu Zr MOF with DOBDC2− on the mmm-symmetry site. ZRN-AB single crystals containing both NDC2− and ABDC2− linkers were also synthesized using 2-amino-1,4-benzenedicarboxylic acid (H2ABDC) instead of H2DOBDC via the same procedure to prepare ZRN-DOB. Structural analysis of ZRN-AB revealed that it is isostructural to 12-c fcu ZRN-NB-amb. The potential linkage sites between the Zr clusters in ZRN-AB are also interlinked by the ABDC2− linker with mmm symmetry in a bridging bidentate coordination mode as in 12-c fcu ZRN-NB-amb (Fig. 5, 6e and f). As expected, 12-c fcu ZRN-AB with symmetry-matching linkers on the mmm-symmetry sites is stable under ambient conditions.
The permanent porosities of the 12-c fcu Zr-MOFs, ZRN-DOB, ZRN-NB and ZRN-AB, were confirmed by using the N2 sorption behaviours of their activated samples, ZRN-DOB-a, ZRN-NB-a and ZRN-AB-a. All the Zr-MOFs showed type-I N2 adsorption isotherms with the Brunauer–Emmett–Teller (BET) surface areas, 1378 m2 g−1, 1339 m2 g−1, and 1448 m2 g−1, for ZRN-DOB-a, ZRN-NB-a, and ZRN-AB-a, respectively (Fig. S14†). The slight variation of N2 uptake amounts reflects the mass and size variations of the functional residues of the XBDC2− linkers. The N2 adsorption amounts of the series of the 12-c fcu MOFs are much larger than that of the activated ZRN-bcu (ZRN-a) with short TFA linker. The BET surface area of ZRN-a is determined to be 861 m2 g−1.
Conclusions
We demonstrated that linker symmetry can play a pivotal role in not only determining the connectivity of a metal cluster node, but also precisely locating the linkers in the framework. A Zr MOF with 12-c fcu topology is not a framework where a zigzag NDC2− linker with 2/m symmetry can be inserted without causing disorder at the linker site. Careful adjustment of the reaction conditions produced ZRN-bcu with 8-c node containing this linker. ZRN-bcu has two sets of intercluster sites between two nearest-neighbor Zr clusters with different site symmetries. While the first intercluster site with 2/m symmetry is interconnected by the NDC2− linker with the same symmetry, the second one with mmm symmetry remains unlinked owing to symmetry mismatch. A 12-c fcu Zr MOF containing both the NDC2− and linear XBDC2− linkers at specific sites in the same framework can only be achieved by post-synthetic linker insertion into ZRN-bcu. Symmetry-guided insertion of XBDC2− with (pseudo)mmm point symmetry into the mmm-symmetry sites of ZRN-bcu produced a series of stable 12-c fcu ZRN-XB MOFs. Although the length of XBDC2− is much shorter than the distance between the unlinked neighboring Zr clusters in ZRN-bcu, the structural flexibility of the framework allowed induced fitting of the length-mismatching linker. Single-crystal structure analyses clearly showed that structural change occurred during post-synthetic linker insertion. The exact locations of the linkers in the framework were successfully defined. Symmetry-guided post-synthetic linker insertion is anticipated to become a general tool for the bottom-up synthesis of new MOFs with the desired topology and multiple functionalities in a controlled and well-defined manner.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NRF (2016R1A5A1009405) through the National Research Foundation of Korea. The authors acknowledge PAL for beam line use (2018-1st-2D-029 and 2018-2nd-2d-033).Notes and references
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed
.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed
.
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed
.
- D. N. Bunck and W. R. Dichtel, Chem.–Eur. J., 2013, 19, 818–827 CrossRef CAS PubMed
.
- J.-S. Qin, S. Yuan, Q. Wang, A. Alsalme and H.-C. Zhou, J. Mater. Chem. A, 2017, 5, 4280–4291 RSC
.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS PubMed
.
- K. Koh, J. D. Van Oosterhout, S. Toy, A. G. Wong-Foy and A. J. Matzger, Chem. Sci., 2012, 3, 2429–2432 RSC
.
- S. M. Cohen, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS PubMed
.
- Y. Han, J.-T. Li, Y. Xie and G. Guo, Chem. Soc. Rev., 2014, 43, 5896–5912 RSC
.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 43, 5896–5912 RSC
.
- O. Karagiaridi, W. Bury, J. E. Mondloch, J. E. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2014, 53, 4530–4540 CrossRef CAS PubMed
.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC
.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed
.
- S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700–7702 RSC
.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed
.
- H.-L. Jiang, D. Feng, T.-F. Liu, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2012, 134, 14690–14693 CrossRef CAS PubMed
.
- L. Li, S. Tang, C. Wang, X. Lv, M. Jiang, H. Wu and X. Zhao, Chem. Commun., 2014, 50, 2304–2307 RSC
.
- C. Wang, O. Volotskova, K. Lu, M. Ahmad, C. Sun, L. Xing and W. Lin, J. Am. Chem. Soc., 2014, 136, 6171–6174 CrossRef CAS PubMed
.
- R. J. Marshall, S. L. Griffin, C. Wilson and R. S. Forgan, J. Am. Chem. Soc., 2015, 137, 9527–9530 CrossRef CAS PubMed
.
- G. Wißmann, A. Schaate, S. Lilienthal, I. Bremer, A. M. Schneider and P. Behrens, Microporous Mesoporous Mater., 2012, 152, 64–70 CrossRef
.
- V. Bon, I. Senkovska, M. S. Weiss and S. Kaskel, CrystEngComm, 2013, 15, 9572–9577 RSC
.
- B. Wang, H. Huang, X.-L. Lv, Y. Xie, M. Li and J. R. Li, Inorg. Chem., 2014, 53, 9254–9259 CrossRef CAS PubMed
.
- K. Wang, H. Huang, W. Xue, D. Liu, X. Zhao, Y. Xiao, Z. Li, Q. Yang, L. Wang and C. Zhong, CrystEngComm, 2015, 17, 3586–3590 RSC
.
- M. Kim, J. F. Cahill, K. A. Prather and S. M. Cohen, Chem. Commun., 2011, 47, 7629–7631 RSC
.
- W. Schrimpf, J. Jiang, Z. Ji, P. Hirschle, D. C. Lamb, O. M. Yaghi and S. Wuttke, Nat. Commun., 2018, 9, 1647 CrossRef PubMed
.
- M. Kim, J. F. Cahill, Y. Su, K. A. Prather and S. M. Cohen, Chem. Sci., 2012, 3, 126–130 RSC
.
- S. Yuan, W. Lu, Y.-P. Chen, Q. Zhang, T.-F. Liu, D. Feng, X. Wang, J. Qin and H.-C. Zhou, J. Am. Chem. Soc., 2015, 137, 3177–3180 CrossRef CAS PubMed
.
- S. Yuan, Y.-P. Chen, J.-S. Qin, W. Lu, L. Zou, Q. Zhang, X. Wang, X. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 8912–8919 CrossRef CAS PubMed
.
- C.-X. Chen, Z. Wei, J.-J. Jiang, Y.-Z. Fan, S.-P. Zheng, C.-C. Cao, Y.-H. Li, D. Fenske and C.-Y. Su, Angew. Chem., Int. Ed., 2016, 55, 9932–9936 CrossRef CAS PubMed
.
- V. Guillerm, T. Grancha, I. Imaz, J. Juanhuix and D. Maspoch, J. Am. Chem. Soc., 2018, 140, 10153–10157 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of MOFs, PXRD, 1H NMR, and crystal data. CCDC 1896107–1896112. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc01301f |
| This journal is © The Royal Society of Chemistry 2019 |