Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Genome mining and biosynthesis of kitacinnamycins as a STING activator†
Jing
Shi‡
a,
Cheng Li
Liu‡
a,
Bo
Zhang
a,
Wen Jie
Guo
a,
Jiapeng
Zhu
b,
Chin-Yuan
Chang
c,
Er Juan
Zhao
a,
Rui Hua
Jiao
a,
Ren Xiang
Tan
 *ab and
Hui Ming
Ge
*ab and
Hui Ming
Ge
 *a
*a
aState Key Laboratory of Pharmaceutical Biotechnology, Institute of Functional Biomolecules, School of Life Sciences, Nanjing University, 210023, P. R. China. E-mail: rxtan@nju.edu.cn; hmge@nju.edu.cn
bState Key Laboratory Cultivation Base for TCM Quality and Efficacy, Nanjing University of Chinese Medicine, Nanjing 210023, P. R. China
cDepartment of Biological Science and Technology, National Chiao Tung University, Hsinchu, Republic of China
First published on 2nd April 2019
Abstract
Cinnamoyl-containing nonribosomal peptides (CCNPs) are a small group of secondary metabolites with potent biological activities produced by actinobacteria. Two remarkable features in the biosynthesis of CCNPs include the nonribosomal peptide synthases (NRPSs) for assembly of the depsipeptide backbone and the type II polyketide synthases (PKSs) for N-terminal cinnamoyl moiety construction. Here, we present a genome mining approach targeting both NRPS and type II PKS for discovery of new CCNPs, which led to the identification of 51 putative CCNP gene clusters from public bacterial genome databases. After strain prioritization, a novel class of CCNP-type glycopeptides named kitacinnamycins, one of which showing potent activation ability towards the stimulator of interferon genes (STING) protein, was identified. Bioinformatic, genetic and biochemical analysis revealed the use of the NRPS assembly line to form the macrocyclic peptide backbone, followed by a P450 monooxygenase to generate terminal oxidized groups. A glycosyltransferase with relatively broad substrate specificity transfers sugars to the newly generated OH/COOH group. The protein crystallographic study further provided structural insights into this glycosylation. Our results not only demonstrated the feasibility of genome mining and strain prioritization for the discovery of new bioactive natural products but also disclosed the biosynthetic pathway for kitacinnamycins.
Introduction
Nonribosomal peptides (NRPs) are one of the most structurally diverse classes of natural products that exhibit a wide range of biological activities and include important therapeutic drugs such as vancomycin and cyclosporine A.1,2 Many NRPs possess N-terminal acylation, which is important for protecting the N-terminus from degradation or modulating specific properties. N-terminal acylation is commonly achieved through coupling with an activated fatty acid3–5 or acyl residues derived from amino acid precursors.6,7 Different to this N-terminal modification, skyllamycin, a potent inhibitor of the platelet-derived growth factor (PDGF) signaling pathway, contains a unique 2-[1-(Z)-propenyl]-cinnamoyl moiety.8 The N-terminal cinnamoyl moiety is rarely observed in NRPs and so far has only been reported in WS9326A,9 mohangamides,10 and coprisamides (Fig. S1†),11 all of which possess interesting structural features. Importantly, WS3926A is a potent agonist of the tachykinin receptor, mohangamides show strong inhibitory activity against isocitrate lyase from Candida albicans, and coprisamides display significant activity towards induction of quinone reductase.9–11 Thus, CCNPs represent a small but very unique class of NRPs, not only due to their interesting structural architectures but also due to their potent and diverse biological activities.Biosynthetic gene clusters (BGCs) for skyllamycin (sky) and WS9326A (cal) have already been identified, revealing that the backbones of both compounds were assembled through multimodule NRPSs.8,12 In addition, unusual type II polyketide synthases (PKSs) and accessory enzymes, which are conserved among the sky and cal clusters,8,12 are predicted to synthesize the cinnamoyl moiety. Briefly, the KSα–KSβ, KR, and DH in type II PKSs catalyze the repeating cycles of decarboxylative Claisen condensation, β-keto reduction and dehydration, respectively, to afford a polyene precursor. An isomerase that may reverse the 6E-polyene to 6Z-configuration is hypothesized to bring the carbon atoms close enough for the subsequent 6π-electrocyclization, followed by a desaturation step to give the required cinnamoyl residue (Fig. S2†).8 In the present work, we utilized a genome-mining strategy targeting both NRPSs and discrete enzymes of type II PKSs involved in cinnamoyl biosynthesis to mine the potential BGCs of CCNPs from bacterial genomes, leading to the identification of 51 putative CCNP BGCs. After strain prioritization and dereplication, we discovered 14 novel CCNPs, kitacinnamycins A–N (1–14), with 8 showing potent STING activation activity. The biosynthetic pathway for kitacinnamycins was revealed through a series of gene deletions, biochemical reactions, and protein crystallographic analyses.
Results and discussion
Genome mining reveals 51 CCNP biosynthetic gene clusters from the genomic database
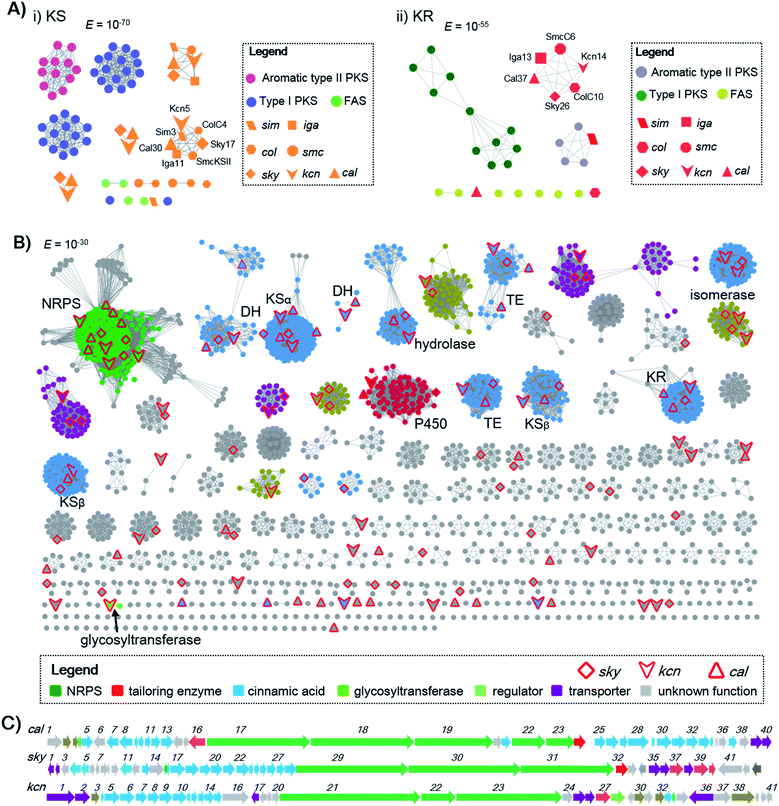
Genome mining to discover structurally specific natural products relies on the use of conserved probes that are in the targeted BGCs but not present in others. The cinnamoyl residue in CCNPs is proposed to be synthesized through a polyene precursor, which is likely formed by a type II PKS.8,13,14 To gain insight into whether the type II PKSs in polyene biosynthesis can be separated from other PKS systems, we first collected discrete enzymes of polyene type II PKSs [skyllamycin (sky),8 WS9326 (cal),12 ishigamide (iga),15 colabomycins (col)13 and simocyclinone (sim and smc)] (Fig. S1 and S3†),14,16 type I PKSs, aromatic type II PKSs, and type II FASs. Then, we generated a protein sequence similarity network (SSN) to visualize and analyze the diversity of type II PKSs using the Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST).17 A preliminary E value threshold of 1.0 × 10−10 was set to gather the related protein families together. When the E value was smaller (i.e., higher threshold value), it would allow the separation of the related enzymes and result in smaller subfamilies. At an E value of 10−70, the KSs from different PKS systems are indeed separated, but KSs from polyene type II PKSs are also distributed in several distinct clusters. However, one KSα (some polyene BGCs contain more than one pair of KSα–KSβ) from each polyene type II PKS BGC can be clustered together (Scheme 1A), indicating that this class of KSα is conserved and unique among all KSs and could be selected as a probe for genome mining. Also, KRs from polyene type II PKSs can also be separated from others at an E value threshold of 10−55, whereas DHs do not cluster at low E values (e.g., 10−15). In addition, at least one isomerase, which is expected to reverse the geometry of alkene in polyene, is present in both the cal and sky BGCs but not observed in other type II PKSs. Thus, homology searches in the bacterial genome NCBI and JGI databases utilizing the skyllamycin proteins KS (Sky17, E value ≤10−70), KR (Sky26, E value ≤10−55) and isomerase (Sky27) as queries afforded a total of 192 genomes in which all three genes were present. To further analyze if these genes are physically adjacent to NRPS genes, we subjected these genomes data to antiSMASH analysis.18 This led to the identification of 51 putative BGCs that have an intact type II PKS system (including KS, KR, DH, ACP, and isomerase) and NRPS genes clustered together (Scheme 1 and Fig. S4†). To further annotate these potential CCNP BGCs and prioritize the hits, a genome neighbourhood network (GNN) consisting of 3196 proteins from 51 putative CCNP BGCs, together with cal and sky, was generated.19 The GNN revealed that two BGCs from Streptomyces sp. Root63 and Streptomyces griseus BIG105 showed high similarities to sky and cal (identity > 90%), respectively, and may also encode skyllamycin or WS3926A (Fig. S4†). It is therefore significant that the remaining 49 BGCs are diverse and possess many functionally different enzymes, indicating that these hits represent potential novel CCNP producers. Importantly, two glycosyltransferases were observed only in Kitasatospora sp. CGMCC 16924 and Streptomyces sp. LZ35 (Scheme 1B), suggesting that glycosylation tailoring steps likely occur in the biosynthesis of their corresponding final products.Characterization of kitacinnamycins from Kitasatospora sp. CGMCC 16924 as new CCNP-type glycopeptides
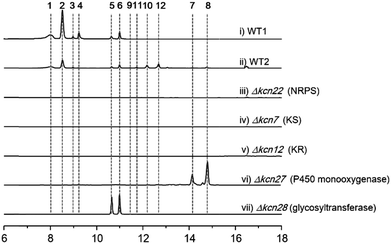
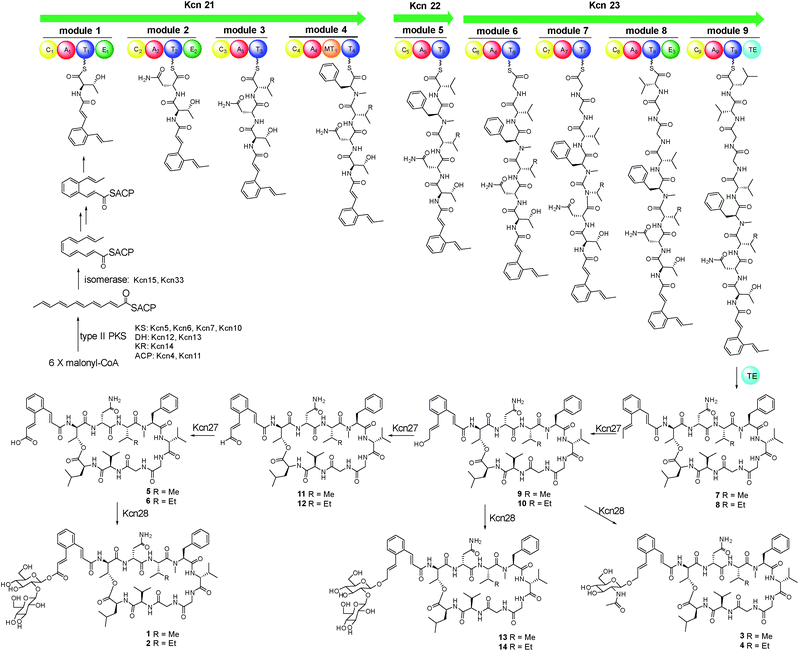
We were interested in identifying the putative CCNP-type glycopeptides in CGMCC 16924 (S. sp. LZ35 was not available). To facilitate this process, we constructed a NRPS gene deletion strain (Δkcn22). The wild-type (WT) strain was then fermented under several fermentation conditions using Δkcn22 as a control. Metabolic extracts were then analyzed by HPLC. Six peaks with UV absorbance at 260 and 300 nm from the WT strain were observed when fermented in SCAS medium. In contrast, the production in the Δkcn22 strain was completely abolished, indicating that these peaks are correlated with the kcn BGC (Fig. 1). A large scale (20 L) fermentation of the WT strain was then conducted, which resulted in the isolation of six compounds named kitacinnamycins A–F (1–6). The planar structures formula of 1–6 were determined based on the extensive analyses of spectroscopic data including high-resolution mass (HRMS) and 1D (1H and 13C) and 2D (HSQC, HMBC, 1H–1H COSY, and 1H–15N HMBC) NMR data (Tables S5–S10†). The gross structures were also supported by MS/MS fragmentation analysis (Fig. S5†). The absolute configurations of amino acid residues in 1 and 2 were assigned on the basis of the advanced Marfey's method (Fig. S6†), and the sugar moieties were determined as D-glucose through a NOESY experiment and GC/MS analysis of the trimethylsilyl derivatives of the hydrolyzed compound 1 (Fig. S7†). Structures of 1–4 consist of a 9-mer macrocyclic peptide with a cinnamoyl unit, in which a β-D-glucose-(1 → 2)-β-D-glucose disaccharide or N-acetylglucosamine moiety was attached. To our delight, structures of 1–4 indeed represent a new class of CCNP-type glycopeptides.As these structures are unusual, we set out to understand the molecular basis for the assembly of 1–6. The kcn BGC spans a ca. 55 kb DNA segment consisting of 41 open reading frames (Scheme 1C), whose putative functions were assigned according to BLAST analysis (Table S3†). Consistent with the 9-mer macrocyclic peptide backbone, three NRPS enzymes (Kcn21, Kcn22, and Kcn23) encoded in the kcn BGC comprise nine modules that were expected to incorporate nine amino acid building blocks to form a linear nonapeptide (Table S4†), with the terminal thioesterase (TE) domain in Kcn23 catalyzing macrocyclization. The cluster also encodes a set of enzymes (kcn4–kcn15 and kcn33) showing moderate homology to the type II PKS in the sky BGC, which is expected to biosynthesize the N-terminal cinnamic group. Knocking out either KS (kcn7) or KR (kcn14) gene completely eliminated the production of all metabolites (Fig. 1),8 confirming their involvement in kitacinnamycin biosynthesis (Scheme 2).
Kcn27 is a P450 monooxygenase catalyzing three successive oxidation steps
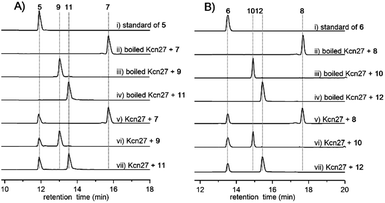
In contrast to the terminal methyl group of the cinnamoyl moiety in skyllamycin or other CCNPs,8–111–6 harbor a terminal carboxylic acid or hydroxyl group. We postulated that the original terminal methyl group is first hydroxylated, with subsequent transfer of an N-acetylglucosamine moiety. Alternatively, the hydroxyl group can be further oxidized to the carboxyl acid through an aldehyde intermediate. Cytochrome P450 enzymes that carry out successive oxidation steps on methyl carbons have been reported in several biosynthetic pathways of natural products.20–22 Indeed, only one P450 monooxygenase (Kcn27), showing 45% sequence identity to PldB, a P450 hydroxylase in pladienolide biosynthesis,23 was observed in the kcn BGC. Knocking out kcn27 abolished the production of 1–6 but clearly led to the accumulation of a pair of new products 7 and 8 with [M + Na]+ 1093.5688 and 1107.5845, respectively. The structures of 7 and 8 were determined as the expected macrocyclic peptides with an N-terminal 2-[1-(E)-propenyl]-cinnamoyl moiety, suggesting the role of Kcn27 in catalyzing successive oxidation steps at the terminal methyl group.To further verify the function of Kcn27, the recombinant P450 enzyme was overproduced and purified to homogeneity from Escherichia coli BL21(DE3) (Fig. S9†). Its cognate ferredoxin (Fdx) and ferredoxin reductase (Fdr) were also purified. Individual incubation of 7 and 8 with Kcn27 together with the required Fdx and Fdr and NADPH in 50 mM MES buffer (pH 5.8) led to the formation of a new product identical to 5 and 6 in retention time and mass, respectively (Fig. 2). In addition, the putative hydroxyl (9/10) and aldehyde (11/12) intermediates with the expected molecular weight were detected in small but reproducible amounts by LC-MS (Fig. S10†). To further verify this, we performed a fermentation time-course using the WT strain to see if these intermediates can be observed in the early time point. Gratifyingly, after 4 d of cultivation four new peaks with expected molecular ions of 9–12 can be detected (Fig. 1,ii). Compounds 9–12 were thus isolated and characterized from a large-scale fermentation. Individual incubation of 9 or 11 and 10 or 12 with Kcn27 under the same enzymatic reaction conditions led to the production of 5 and 6, respectively (Fig. 2). These in vivo and in vitro results collectively indicate that Kcn27 is a P450 oxidase responsible for three successive two-electron oxidation steps at the terminal methyl group of the cinnamoyl moiety.
 | ||
| Fig. 2 HPLC analysis (300 nm) of the Kcn27 catalyzed enzymatic reaction. Each enzymatic reaction was supplemented with Fdr, Fdx and NADPH. | ||
In vivo and in vitro characterization of Kcn28 as a glycosyltransferase
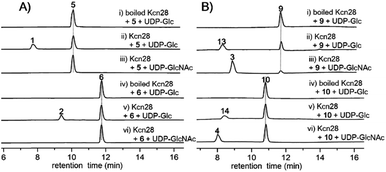
The glycosyltransferase Kcn28, which is unique among all CCNP BGCs, was expected to transfer a glycosyl group onto either the terminal COOH (5 and 6) or OH group (9 and 10). To examine its role, kcn28 was knocked out through in-frame deletion. The Δkcn28 mutant completely eliminated the production of 1–4, only leaving 5 and 6 as two dominant products (Fig. 1). This result suggested that Kcn28 could be responsible for introducing the glycosyl group at the cinnamoyl moiety.To confirm the function of Kcn28, the recombinant enzyme was overproduced from E. coli BL21(DE3) (Fig. S9†). The sugar donor UDP-D-glucose was then incubated with 5 or 6 in the presence of Kcn28, which leads to the formation of 1 or 2, respectively (Fig. 3). When the hydroxyl compound 9 or 10 was used as the sugar acceptor and incubated with UDP-D-GlcNAc and Kcn28, as expected the corresponding product 3 or 4 was detected (Fig. 3). To further generate structural diversity, 9 or 10 was assayed with UDP-D-glucose. Interestingly, the putative disaccharide product 13 (m/z 1433.7 [M + Na]+) or 14 (m/z 1447.6 [M + Na]+) was observed based on its molecular ions (Fig. 3 and S11†). In contrast, no reaction occurred when 5 or 6 was assayed with UDP-D-GlcNAc in the presence of Kcn28 (Fig. 3).
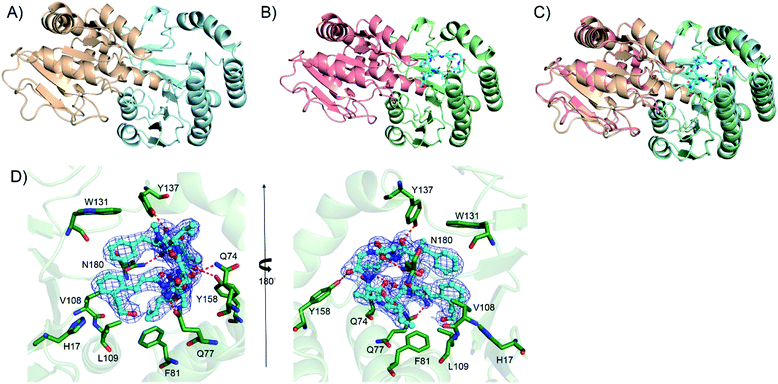
Crystal structure of Kcn28 reveals the structural basis of glycosylation
We further attempted to understand the structural basis of the Kcn28-catalyzed enzymatic reaction. Crystal structures of Kcn28 as a free enzyme and in a binary complex (with 9) were obtained at 2.50 and 2.24 Å resolution, respectively (Fig. 4A and B and Table S17†). The monomeric Kcn28 contains two Rossmann-like β/α/β domains. The N-terminal domain from residues 6–208 is a substrate binding domain consisting of seven parallel β-strands that are flanked by seven α-helices, while the C-terminal domain comprising residues 229–392 is a UDP-sugar binding domain with six parallel β-strands surrounded by seven α-helices. The overall structure of Kcn28 is similar to that of other glycosyltransferases in natural product biosynthesis, including OleD (PDB: 2IYF) (Fig. S12A†)24 and CalG3 (PDB: 3OTI) (Fig. S12B†)25 from oleandomycin and calicheamicin biosynthesis, respectively.The model of 9 was confidently built into well-defined electron densities. Superposition of free and complexed structures revealed a global conformational change (Fig. 4C). The substrate 9 is tightly encapsulated in four α-helices with an orientation directed by insertion of the macrocyclic peptide part into the cavity and exposure of the cinnamic acid portion to the UDP part. Specifically, the side chains of F81, V108, and L109 create a hydrophobic environment to accommodate the cinnamic moiety of 9. In addition, the side chain of W131 provides hydrophobic interaction with the Phe residue of the macrocyclic peptide (Fig. 4D). The side chains of Q74, Q77, Y137, Y158 and N180 provide hydrogen bond interactions with the macrocyclic peptide backbone. Site-directed mutagenesis of the above key residues revealed diminished or abolished enzyme activities (Fig. S13†), supporting their roles in substrate binding. Additionally, H17, which is conserved among structurally similar glycosyltransferase (Fig. S14†) and adjacent to the hydroxyl group of the cinnamic moiety of 9, is proposed as the catalytic base by abstracting a proton in the terminal OH/COOH group.24 Consistent with the hypothesis, the H17A mutant renders the enzyme inactive.
Kitacinnamycin H is an activator of the STING signalling pathway
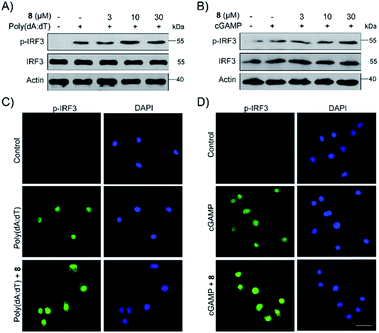
Finally, the biological activity of 1, 2 and 5–11 was tested towards the STING protein, which is a central signalling molecule of the intracellular DNA sensing pathway and is now considered as an effective target for therapeutic intervention.26–28 As shown in Fig. S15A,† compound 8 significantly promoted IFN-β production induced by poly(dA:dT), whereas 9–11 could inhibit that, and the remaining compounds 1, 2, and 5–7 have no or a weak effect on IFN-β production. The STING agonists (cyclic dinucleotides or non-dinucleotides) have demonstrated therapeutic effects in multiple mouse tumor models.29,30 Moreover, 8 also dose-dependently increased the production of IFN-β induced by poly(dA:dT) as well as cGAMP (Fig. S15B† and C†). Different from cGAMP or amidobenzimidazole STING receptor agonists, 8 itself cannot trigger IFN-β production. To further confirm the effect of 8 on the STING signaling pathway, the phosphorylation of IRF3 was examined by Western blot and immunofluorescence. Compound 8 dose-dependently enhanced the IRF3 phosphorylation induced by poly(dA:dT) as well as cGAMP (Fig. 5Aand B). Moreover, immunofluorescence showed that phosphorylation and nuclei localization of IRF3 was markedly increased after compound 8 treatment (Fig. 5C and D). Taken together, our results showed that 8 is an activator of the STING signalling pathway.Conclusions
The recent explosion in genome sequence data clearly indicated that the biosynthetic potential of microorganisms to produce new/bioactive natural products is greatly underappreciated. However, discovery of secondary metabolites has historically been a tedious and laborious process. Here, by employing a protein sequence similarity network, we systematically analyzed KS, KR and DH enzymes in type II polyene PKSs together with other PKSs and FASs. Our analysis indicated that KSs and KRs in type II polyene PKSs are distinct from those in other systems at certain E value thresholds and can be used as probes for genome mining. Using these unique KS, KR and isomerases as probes, we identified 192 gene clusters from the genomic database, 51 of which are putative CCNP BGCs. Further analysis through the GNN not only indicated the chemical diversity of these encoded CCNPs but also prioritized the strain hits.The successful identification of kitacinnamycins as a new class of CCNP-type glycopeptides validates the power of our genome mining strategy in natural product discovery. The biosynthetic machinery of kitacinnamycins was elucidated through gene inactivation studies, in vitro biochemical reaction. The X-ray crystal structure of a glycosyltransferase (Kcn28) and site-directed mutagenesis analysis provide structural insight into this glycosylation step. Notably, kitacinnamycin H (8), which is produced in a high titer from the Δkcn27 strain, exhibited promising STING activation activity. Future studies on the other hits identified here will promise the discovery of more new CCNPs and expand the chemical space of CCNP-type natural products.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank Prof. Jeffrey D. Rudolf (University of Florida) for helpful discussions and careful reading of this manuscript. This work was financially supported by the NSFC (81522042, 21572100, 81773591, 81530089, 81673333, 81803380, 21861142005, 21761142001, and 21661140001), the MOST of P.R.C. (2018YFC1706200), and the MOST of R.O.C. (107-2113-M-009-021-MY3 to C.·Y.·C.), and the Fundamental Research Funds for the Central Universities (020814380092 and 020814380103).Notes and references
- R. D. Suessmuth and A. Mainz, Angew. Chem., Int. Ed., 2017, 56, 3770–3821 CrossRef CAS PubMed.
- S. A. Sieber and M. A. Marahiel, Chem. Rev., 2005, 105, 715–738 CrossRef CAS PubMed.
- K. T. Nguyen, D. Ritz, J.-Q. Gu, D. Alexander, M. Chu, V. Miao, P. Brian and R. H. Baltz, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 17462–17467 CrossRef CAS PubMed.
- F. I. Kraas, V. Helmetag, M. Wittmann, M. Strieker and M. A. Marahiel, Chem. Biol., 2010, 17, 872–880 CrossRef CAS PubMed.
- R. A. Cacho, W. Jiang, Y.-H. Chooi, C. T. Walsh and Y. Tang, J. Am. Chem. Soc., 2012, 134, 16781–16790 CrossRef CAS PubMed.
- K. Watanabe, K. Hotta, A. P. Praseuth, K. Koketsu, A. Migita, C. N. Boddy, C. C. C. Wang, H. Oguri and H. Oikawa, Nat. Chem. Biol., 2006, 2, 423–428 CrossRef CAS PubMed.
- W. Namwat, H. Kinoshita and T. Nihira, J. Bacteriol., 2002, 184, 4811–4818 CrossRef CAS PubMed.
- S. Pohle, C. Appelt, M. Roux, H.-P. Fiedler and R. D. Suessmuth, J. Am. Chem. Soc., 2011, 133, 6194–6205 CrossRef CAS PubMed.
- K. Hayashi, M. Hashimoto, N. Shigematsu, M. Nishikawa, M. Ezaki, M. Yamashita, S. Kiyoto, M. Okuhara, M. Kohsaka and H. Imanaka, J. Antibiot., 1992, 45, 1055–1063 CrossRef CAS PubMed.
- M. Bae, H. Kim, K. Moon, S.-J. Nam, J. Shin, K.-B. Oh and D.-C. Oh, Org. Lett., 2015, 17, 712–715 CrossRef CAS PubMed.
- S. Um, S. H. Park, J. Kim, H. J. Park, K. Ko, H.-S. Bang, S. K. Lee, J. Shin and D.-C. Oh, Org. Lett., 2015, 17, 1272–1275 CrossRef CAS PubMed.
- S. Zhang, J. Zhu, D. L. Zechel, C. Jessen-Trefzer, R. T. Eastman, T. Paululat and A. Bechthold, ChemBioChem, 2018, 19, 272–279 CrossRef CAS PubMed.
- D. Du, Y. Katsuyama, K. Shin-ya and Y. Ohnishi, Angew. Chem., Int. Ed., 2018, 57, 1954–1957 CrossRef CAS PubMed.
- O. Bilyk, E. Broetz, B. Tokovenko, A. Bechthold, T. Paululat and A. Luzhetskyy, ACS Chem. Biol., 2016, 11, 241–250 CrossRef CAS PubMed.
- K. Petrickova, S. Pospisil, M. Kuzma, T. Tylova, M. Jagr, P. Tomek, A. Chronakova, E. Brabcova, L. Andera, V. Kristufek and M. Petricek, ChemBioChem, 2014, 15, 1334–1345 CrossRef CAS PubMed.
- A. Trefzer, S. Pelzer, J. Schimana, S. Stockert, C. Bihlmaier, H. P. Fiedler, K. Welzel, A. Vente and A. Bechthold, Antimicrob. Agents Chemother., 2002, 46, 1174–1182 CrossRef CAS PubMed.
- J. A. Gerlt, Biochemistry, 2017, 56, 4293–4308 CrossRef CAS PubMed.
- K. Blin, T. Wolf, M. G. Chevrette, X. Lu, C. J. Schwalen, S. A. Kautsar, H. G. S. Duran, E. L. C. d. l. Santos, H. U. Kim, M. Nave, J. S. Dickschat, D. A. Mitchell, E. Shelest, R. Breitling, E. Takano, S. Y. Lee, T. Weber and M. H. Medema, Nucleic Acids Res., 2017, 45, W36–W41 CrossRef CAS PubMed.
- J. D. Rudolf, X. Yan and B. Shen, J. Ind. Microbiol. Biotechnol., 2016, 43, 261–276 CrossRef CAS PubMed.
- J. K. Brunson, S. M. K. McKinnie, J. R. Chekan, J. P. McCrow, Z. D. Miles, E. M. Bertrand, V. A. Bielinski, H. Luhavaya, M. Obornik, G. J. Smith, D. A. Hutchins, A. E. Allen and B. S. Moore, Science, 2018, 361, 1356 CrossRef CAS PubMed.
- L. Li, M. C. Tang, S. B. Tang, S. Gao, S. Soliman, L. Hang, W. Xu, T. Ye, K. Watanabe and Y. Tang, J. Am. Chem. Soc., 2018, 140, 2067–2071 CrossRef CAS PubMed.
- J. D. Rudolf, C.-Y. Chang, M. Ma and B. Shen, Nat. Prod. Rep., 2017, 34, 1141–1172 RSC.
- K. Machida, A. Arisawa, S. Takeda, T. Tsuchida, Y. Aritoku, M. Yoshida and H. Ikeda, Biosci., Biotechnol., Biochem., 2008, 72, 2946–2952 CrossRef CAS PubMed.
- D. N. Bolam, S. Roberts, M. R. Proctor, J. P. Turkenburg, E. J. Dodson, C. Martinez-Fleites, M. Yang, B. G. Davis, G. J. Davies and H. J. Gilbert, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 5336–5341 CrossRef CAS PubMed.
- A. Chang, S. Singh, K. E. Helmich, R. D. Goff, C. A. Bingman, J. S. Thorson and G. N. Phillips, Jr., Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 17649–17654 CrossRef CAS PubMed.
- L. Deng, H. Liang, M. Xu, X. Yang, B. Burnette, A. Arina, X. D. Li, H. Mauceri, M. Beckett, T. Darga, X. Huang, T. F. Gajewski, Z. J. Chen, Y. X. Fu and R. R. Weichselbaum, Immunity, 2014, 41, 843–852 CrossRef CAS PubMed.
- S. Liu, X. Cai, J. Wu, Q. Cong, X. Chen, T. Li, F. Du, J. Ren, Y. T. Wu, N. V. Grishin and Z. J. Chen, Science, 2015, 347, aaa2630 CrossRef PubMed.
- S. M. Haag, M. F. Gulen, L. Reymond, A. Gibelin, L. Abrami, A. Decout, M. Heymann, F. G. van der Goot, G. Turcatti, R. Behrendt and A. Ablasser, Nature, 2018, 559, 269–273 CrossRef CAS PubMed.
- J. M. Ramanjulu, G. S. Pesiridis, J. Yang, N. Concha, R. Singhaus, S. Y. Zhang, J. L. Tran, P. Moore, S. Lehmann, H. C. Eberl, M. Muelbaier, J. L. Schneck, J. Clemens, M. Adam, J. Mehlmann, J. Romano, A. Morales, J. Kang, L. Leister, T. L. Graybill, A. K. Charnley, G. Ye, N. Nevins, K. Behnia, A. I. Wolf, V. Kasparcova, K. Nurse, L. Wang, Y. Li, M. Klein, C. B. Hopson, J. Guss, M. Bantscheff, G. Bergamini, M. A. Reilly, Y. Lian, K. J. Duffy, J. Adams, K. P. Foley, P. J. Gough, R. W. Marquis, J. Smothers, A. Hoos and J. Bertin, Nature, 2018, 564, 439–443 CrossRef CAS PubMed.
- L. Corrales, L. H. Glickman, S. M. McWhirter, D. B. Kanne, K. E. Sivick, G. E. Katibah, S. R. Woo, E. Lemmens, T. Banda, J. J. Leong, K. Metchette, T. W. Dubensky Jr and T. F. Gajewski, Cell Rep., 2015, 11, 1018–1030 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc00815b |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |