Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient development of stable and highly functionalised peptides targeting the CK2α/CK2β protein–protein interaction†
Jessica
Iegre
 a,
Paul
Brear
a,
Paul
Brear
 b,
David J.
Baker
c,
Yaw Sing
Tan
d,
Eleanor L.
Atkinson
a,
Hannah F.
Sore
a,
Daniel H.
O' Donovan
b,
David J.
Baker
c,
Yaw Sing
Tan
d,
Eleanor L.
Atkinson
a,
Hannah F.
Sore
a,
Daniel H.
O' Donovan
 e,
Chandra S.
Verma
e,
Chandra S.
Verma
 dfg,
Marko
Hyvönen
dfg,
Marko
Hyvönen
 *b and
David R.
Spring
*b and
David R.
Spring
 *a
*a
aDepartment of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW, Cambridge, UK. E-mail: spring@ch.cam.ac.uk
bDepartment of Biochemistry, University of Cambridge, Tennis Court Road, CB2 1GA, Cambridge, UK. E-mail: mh256@cam.ac.uk
cDiscovery Sciences, IMED Biotech Unit, AstraZeneca, Cambridge, UK
dBioinformatics Institute, Agency for Science, Technology and Research (A*STAR), 30 Biopolis Street, #07-01 Matrix, Singapore 138671
eOncology, IMED Biotech Unit, AstraZeneca, Cambridge, UK
fDepartment of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore 117543
gSchool of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551
First published on 12th April 2019
Abstract
The discovery of new Protein–Protein Interaction (PPI) modulators is currently limited by the difficulties associated with the design and synthesis of selective small molecule inhibitors. Peptides are a potential solution for disrupting PPIs; however, they typically suffer from poor stability in vivo and limited tissue penetration hampering their wide spread use as new chemical biology tools and potential therapeutics. In this work, a combination of CuAAC chemistry, molecular modelling, X-ray crystallography, and biological validation allowed us to develop highly functionalised peptide PPI inhibitors of the protein CK2. The lead peptide, CAM7117, prevents the formation of the holoenzyme assembly in vitro, slows down proliferation, induces apoptosis in cancer cells and is stable in human serum. CAM7117 could aid the development of novel CK2 inhibitors acting at the interface and help to fully understand the intracellular pathways involving CK2. Importantly, the approach adopted herein could be applied to many PPI targets and has the potential to ease the study of PPIs by efficiently providing access to functionalised peptides.
Introduction
Proteins are an essential component of the cell. They exert physio-pathological functions in response to external and internal messengers in a highly regulated and selective manner. Many of these functions are made possible by a complicated network of protein–protein interactions (PPIs). Targeting of PPIs is seen as an attractive therapeutic strategy considering the large number of PPIs involved in pathological mechanisms. In addition, targeting of PPIs provides orthogonality with respect to conventional therapeutics, which are typically targeted against existing binding sites for small molecules. Therefore, inhibition of PPIs may result in the development of safer drugs.1 PPIs are characterised by shallow surfaces which make targeting them with conventional small molecules (<500 Da), whilst possible,2–7 a non-trivial and lengthy process. Synthetic peptides, on the other hand, provide a valuable alternative to small molecules: their structural properties make them amenable to mimic portions of the native proteins resulting in favorable interactions with the large and shallow interfaces of PPIs.8 In addition, when structural information pertaining to the PPI of interest is known, potent and selective peptides can be rapidly designed based upon the sequence of targeted motifs of the native proteins.9However, the poor pharmacokinetic (PK) properties of synthetic peptides in the body limit their use and interest to the pharmaceutical industry.10 Many strategies have been adopted to overcome the PK limitations of peptides; yet, very few of them are set up to provide peptides that are highly functionalised and cell-permeable in an efficient manner.11–15 Taking into account the potency and selectivity of peptides, methodologies that introduce functionalities to make them simultaneously stable and cell-permeable would yield chemical probes of invaluable importance.16 To this end, we have developed a two-component (2C) copper catalyzed azido alkyne (CuAAC) peptide stapling (PS) methodology that allows the development of highly functionalised, potent, and selective peptides targeting intracellular PPIs.17 In 2C-CuAAC-PS only a limited number of peptides are synthesised and their design is based upon the structural information available for the PPI of interest.18 Subsequently, peptide stapling is carried out by using amino acids with azido side chains and a di-alkyne linker as a constraint. One of the advantages of this methodology is that the linker can be modified and functionalised to improve the in vitro pharmacological properties independently from the peptide sequence: the result is a more efficient optimisation of the peptides. Biophysical and cellular assays are successively used to assess the peptides synthesised. Importantly, the 2C-CuAAC-PS chemistry proved to be compatible with all the natural amino acids, led to enhanced binding affinities for both helical and non-helical peptides and improved the overall in vitro pharmacological properties of the peptides, including stability to proteases.19–21
Herein, we have applied this robust approach to efficiently develop the first stable and highly functionalised conformationally-constrained peptide acting on the PPI of CK2.
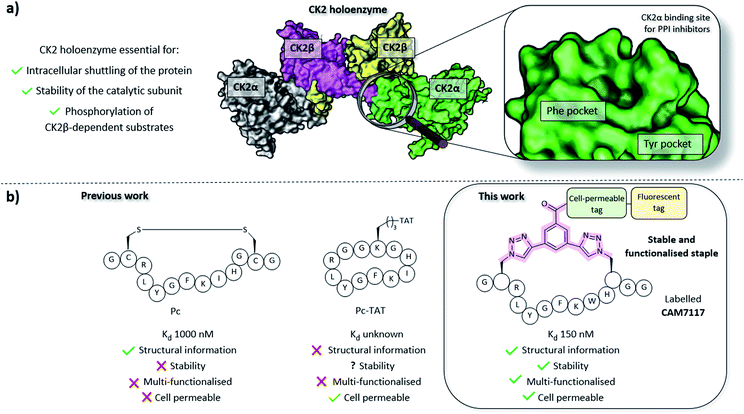
CK2 is a protein kinase overexpressed in cancer cells and a validated oncology target; CX4945, a traditional small molecule ATP-binding site inhibitor of CK2, is currently undergoing clinical studies.22 However, CX4945 targets the ATP-binding site, which is well conserved among the kinome. More recently, there have been increasing efforts to develop non-ATP competitive inhibitors of CK2 to reduce the off-target effects of competitive ligands.23–25 Among the strategies designed to target CK2 outside its orthosteric binding site, is the inhibition of the PPI between the α and the β subunits.26–29 Disruption of the holoenzyme assembly affects the function of CK2 by preventing phosphorylation of β-dependent substrates, the shuttling of the protein between different intracellular compartments, and by reducing the stability of the catalytic α subunit (Fig. 1).30–33 With the exception of the Phe pocket, the CK2α/β interface is a shallow and hydrophobic surface; consequently, peptides are an ideal class of molecule to target this PPI. To this end, two cyclic peptides have been developed. However, one of these, Pc,26,28 is a disulfide-linked cyclic peptide that lacks cell permeability and stability in the reducing intracellular environment, and the other, TAT-Pc,34 has not been assessed structurally or for stability in physiologic fluids (Fig. 1). Therefore, a stable chemical probe that could be used in vitro and in vivo to study the interface of the important protein CK2 is still required.
 | ||
| Fig. 1 (a) Importance of the holoenzyme to the functions of CK2 (PDB: 1JWH). The catalytic α subunits are shown in grey and green, the regulatory β subunits in yellow and pink. The binding site on CK2α for inhibitors of the PPI is shown on the right. (b) A comparison of the peptides developed prior to this work (Pc and TAT-Pc)26,28,34 and the lead peptide CAM7117 developed in this work. | ||
Starting from the sequences of CK2β and Pc, we investigated alternative ways of constraining the peptide into its bioactive conformation using a stable linkage compatible with the 2C-CuAAC-PS chemistry. At a later stage, X-ray crystallography guided our investigation on sequence variation to increase the binding affinity of the peptide for CK2α. The most promising peptide was easily modified into a fluorescent, cell-permeable probe via a novel highly functionalised constraint that allowed us to study the peptide's activity in cancer cells.
The peptide developed in this work is the first stable, cell permeable macrocyclic peptide that disrupts the CK2α/β PPI in vitro and leads to cancer cell death and arrest of the cell cycle; as such, it will serve as a useful chemical probe in oncology. Furthermore, the structure of the peptide in complex with CK2α will act as a valuable starting point to develop novel CK2 inhibitors.
Results and discussion
In order to design stable peptides targeting the CK2α/β interaction, we used a rational-design approach based on the valuable crystal structures of CK2β and the disulfide bridged Pc peptide.34Disulfide bridges are unstable under reducing environments; therefore, our aim was to replace the labile disulfide group with a stable constraint. To this end, the 2C-CuAAC macrocyclisation technique was chosen for its validated ability to constrain peptides in their binding conformation, simultaneously enhance the stability against proteolytic cleavage, introduce functionalities (cell-penetrating peptide (CPP), fluorescent dyes, biotin, and PEG and other tags), and improve the poor stability in physiological fluids and cell-penetration in a combinatorial manner.19,20,35–38
Rational design of conformationally constrained peptides mimicking CK2β
Molecular modelling identified Cys2 and Gly11 of Pc,34 corresponding to P185 and P194 of CK2β, as suitable residues to staple: they make negligible contributions to the binding and are positioned at a suitable distance from each other to accommodate a 2C-CuAAC staple (ESI, Fig. S1†). To cyclise the peptide, azido amino acids bearing one-carbon-atom side chains (Fmoc-Aza-OH) were used in combination with aliphatic linkers of different lengths as proposed by molecular modelling (Fig. 2 and ESI Table S1†). The ability of the synthesised macrocycles to disrupt the α/β PPI of CK2 was then tested in a preliminary fluorescent polarisation assay (FP) (Fig. 2).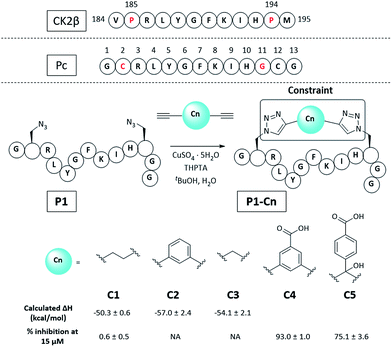 | ||
| Fig. 2 Sequence of the portion of CK2β binding to CK2α, Pc peptide and structure of the constrained peptides reported in this work with calculated enthalpic values of binding and percentage of CK2β-like probe displacement. NA = not measured. A detailed table with the structures of all the peptides presented in this work is provided in the ESI (Table S2†). Pc, P1, and P1-Cn (where n = 1–5) peptides feature an amide at the C-terminus and an acetyl cap at the N-terminus. All the amino acids are the L isomers. | ||
Peptides constrained with linkers C2 and C3 proved to be insoluble under the assay conditions. To overcome this limitation, it was decided to incorporate a functional handle on the constraint to simultaneously improve the solubility and allow for future functionalisation of the peptides. Peptide P1-C4 was the most effective macrocyclised peptide at displacing the FP probe from the CK2α subunit (93 ± 1% inhibition at 15 μM). This suggested that constraint C4 was optimal at constraining the peptide in its binding conformation. The binding affinity of P1-C4 and P1-C5 for the CK2α subunit was measured via isothermal titration calorimetry (ITC). P1-C4 showed the highest affinity with a Kd of 460 nM, showing a modest increase compared to Pc (1000 nM, ESI Table S3†).
To understand how P1-C4 was able to bind CK2α, we determined its crystal structure in complex with CK2α (PDB: 6Q38). The crystal structure showed the peptide binding in a conformation that overlays well with CK2β and Pc (Fig. 3a–c). The backbone residues of P1-C4 are all slightly shifted compared to Pc; however, these differences are greatest closer to the constraint that holds the two ends of the peptide further apart than the disulfide linker does; consequently, the terminal residues adopt entirely different conformations (Fig. 3b and c).
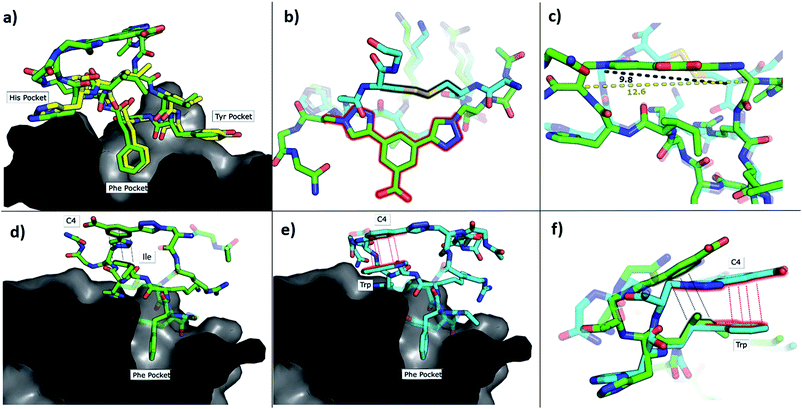 | ||
| Fig. 3 Crystallographic structures of conformationally constrained peptides. (a) P1-C4 (green, PDB: 6Q38) and residues 186-193 of CK2β (yellow, PDB: 4NH1 (ref. 44)) in complex with CK2α. The image is shown as a cross-section of the interface site. (b) A comparison of the binding mode of the two different linkers in Pc (cyan, PDB: 4IB5 (ref. 34)) and P1-C4 (green). (c) Difference in the distance between the α-carbons of the amino acids involved in the constraint in Pc (cyan) and P1-C4 (green). (d) Crystallographic structure of P1-C4 (green) binding at the interface site. The image is shown as a cross-section of the interface site. (e) Crystallographic structure of P2-C4 (cyan, PDB: 6Q4Q) binding at the interface site. Stacking of the Trp residue with the constraint is highlighted in red. The image is shown as a cross-section of the interface site. (f) Comparison of the binding mode of P1-C4 (green) and P2-C4 (cyan) bound to the interface site. | ||
The X-ray crystal structure of P1-C4 (Fig. 3d) shows Ile9 oriented in the right direction to be replaced by an amino acid capable of π–π stacking with the benzene ring of the C4 constraint. Similarly, chlorobenzene probes in ligand-mapping MD simulations39,40 of P1-C4 indicated a region of high occupancy by the aromatic carbon atoms of chlorobenzene around Ile9 of the peptide (ESI Table S1†). Therefore, the sequence of P1 was modified to replace Ile9 with a larger, non-polar Trp to create peptide P2 (Ac-GXRLYGFKWHXGG-NH2 where X = Fmoc-Aza-OH). The resulting peptide was macrocyclised using C4 as a linker to afford P2-C4.
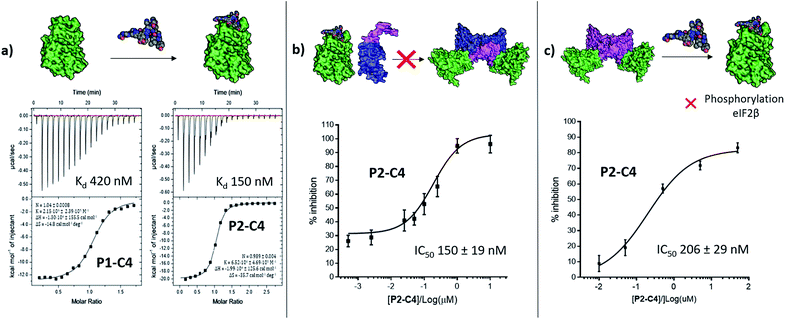
The binding affinity of the P2-C4 peptide was found to be enhanced (Kd 150 nM) compared to both P1-C4 (Kd 460 nM) and Pc peptide (Kd 1000 nM) (Fig. 4a and ESI Table S3†). The constrained P2-C4 peptide showed also a 300-fold improvement compared to the linear variant P2 (Kd 44 μM). The crystal structure of P2-C4 bound to CK2α (PDB: 6Q4Q, Fig. 3e and f) shows the π–π stacking between the Trp and the phenyl ring of the constraint. It is likely that this π–π stacking is the main factor leading to the higher affinity of P2-C4 as no other significant interactions were observed (ESI Fig. S2†). Therefore, the increased binding affinity of P2-C4 for CK2α may be explained with a reduced entropic penalty upon binding due to the rigidifying interaction occurring between the constraint and the Trp residue.41–43
Inhibition of the CK2α/β protein–protein interaction
We then wanted to investigate whether the peptides would be able to inhibit the binding of the β subunit to the α subunit. To this end, the binding affinity of the regulatory β subunit for the catalytic domain was determined via ITC (Kd 9 nM). This was then repeated in the presence of 100 μM P1-C4 and P2-C4 (ESI, Table S4†). This preliminary assay showed that no binding of CK2β to CK2α was detected in the presence of either of the peptides: this result confirms that the peptides bind at the interface and prevent CK2β binding despite its high binding affinity for CK2α. As an orthogonal proof of inhibition, we used competition Bio-Layer Interferometry (BLI) experiments to demonstrate that P2-C4 was able to prevent the formation of the CK2α/CKβ-RAD‡ complex with an IC50 of 150 ± 19 nM (Fig. 4b). As one of the key roles of CK2β is to recruit substrates to the kinase, the effect of PPI inhibition on substrate phosphorylation was studied using CK2β dependent and independent substrates. It was shown that P2-C4 was able to inhibit the phosphorylation of a CK2β-dependent substrate – the transcription factor eIF2β – with an IC50 of 206 ± 29 nM (Fig. 4c). It should be noted that the kinase assay was performed using the pre-formed CK2α/β complex as the ability of CK2α to phosphorylate eIF2β requires the presence of the CK2β subunit. The assay indicates that P2-C4 can disrupt, in a dose-dependent manner, the CK2 holoenzyme thereby reducing the ability of CK2α to phosphorylate CK2β-dependent substrates. As expected, P2-C4 did not affect the phosphorylation of a β-independent substrate peptide (RRRADDSDDDD) meaning that binding at the interface site does not displace the ATP or significantly alter the kinase activity allosterically (ESI Fig. S3†).Development of a multi-functional constraint
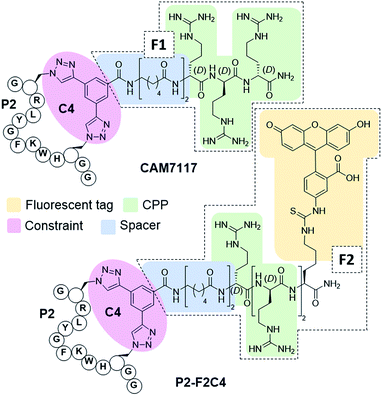
To overcome the limitations of Pc, the main goal of this work was to efficiently develop chemical probes for the CK2 PPI that could be used in cells and ultimately in vivo. Therefore, cell-permeability and stability in serum were required. Peptide macrocyclisation has been described on several occasions as a powerful technique to enhance the stability of the peptides to proteases.20,21,45 Although the ability of peptide macrocyclisation alone to enhance cell-permeability is highly debated, cell-penetrating peptides (CPPs) are well-established and are often added to the cyclised peptides to gain cytosolic entry.46,47 Preliminary cellular uptake experiments carried out with FITC-labelled peptide showed that the peptide was not able to permeate the cell membrane of osteosarcoma cancer cells (U2OS) and thus, functionalisation was needed. Considering that both cell-penetrating motifs and fluorescent tags were necessary for the cellular assays, we decided to develop a novel multi-functional constraint that would simultaneously: constrain the peptide in its binding conformation, enhance the stability to proteases, provide cell-permeability to the CK2 peptide, and act as a fluorophore (F2C4, Fig. 5). In addition, we wanted to develop functionalised constraints that could be synthesised in an automated manner using Fmoc-SPPS so to be accessible by the wider scientific community. Exploiting one of the advantages of 2C-PS, linker C4 was elaborated independently from the CK2 peptide (ESI Scheme S1†). The benzoic acid derivative linker C4 (pink in Fig. 5) was attached to a CPP via a spacer (blue in Fig. 5) to avoid steric clashes with the CK2 peptide and the CK2α domain, generating the full multi-functional linker F1C4. Previously, we have reported the use of an (L)-arginine tripeptide as an effective CPP to carry peptide cargos into cells.20,36 We decided to use (D)-arginine instead to confer cell-permeability to the CK2 peptide and simultaneously provide a proteolysis resistant alternative. The CPP was in turn attached to the fluorescent tag FITC via an orthogonally protected Lys to monitor the peptide entrance into the cells (full multi-functional linker F2C4, Fig. 5). A fluorescent constraint without the CPP motif (F3C4) was also synthesised. The functionalised linker F1C4 was then reacted with P2 to obtain CAM7117 (Fig. 5). Importantly, CAM7117 displayed significant stability in human serum (47% intact peptide after 24 hours incubation, Fig. S4†) highlighting how the covalent constraint F1C4 provided a peptide that is stable under physiological fluids.Activity of CAM7117 in cancer cells
CAM7117 was successfully internalised by the cancer cells as observed by confocal microscopy of its FITC-labelled analogue, P2-F2C4 (Fig. 6a). Once internalised, the peptide was able to inhibit human osteosarcoma (U2OS) cell growth with a GI50 of 32 ± 2 μM after 4 day incubation, and induced apoptosis after just 4 hours (ESI Fig. S5–S7†). A marginally reduced biological effect was observed for both CAM7117 and the clinical candidate CX4945 when human colorectal cancer cells (HCT116) were used (Fig. 6b). No biological effects were observed when the functionalised constraint only (F1C4) or the negative control peptide (P3-F1C4) were used. The negative control P3-F1C4 features an F7W mutation in the sequence and did not show binding to CK2α by ITC (ESI Table S3, Fig. S5–S7†).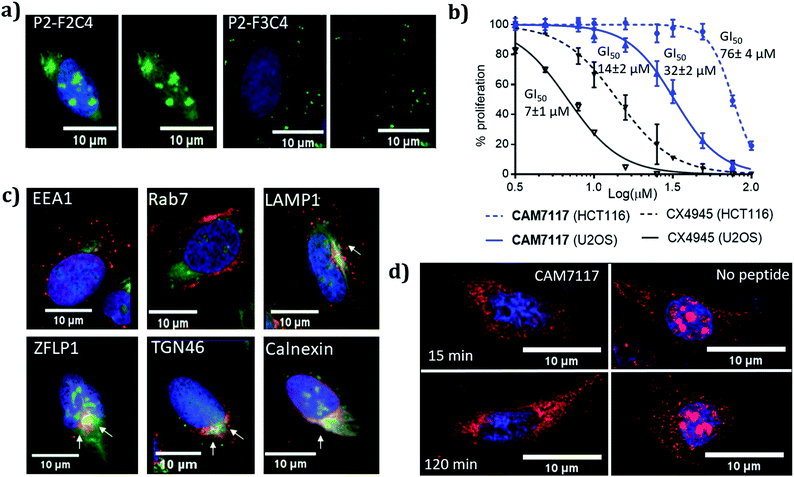 | ||
| Fig. 6 Biological evaluation of CAM7117 and its analogues in cancer cells. (a) Cellular uptake in U2OS cell line for P2-F2C4 and P2-F3C4. Cell nuclei are stained with Hoechst 33342 stain (blue). (b) GI50 curves of the antiproliferative activity of CAM7117 (solid and dashed blue lines) and CX4945 (solid and dashed black line) in U2OS and HCT116 cells. (c) Immunofluorescence experiments in the presence of FITC-labelled peptide P2-F2C4 (green), stained with antibodies against markers of different organelles (all in red): EEA1 (early endosome), Rab7 (late endosome), LAMP1 (lysosomes), ZFLP1 (cis Golgi), TGN46 (trans Golgi) and calnexin (endoplasmic reticulum). Details of antibodies are found in Table S11.† Nuclei are stained with Hoechst 33342 (blue). (d) Change in intracellular localisation of CK2β (red) following treatment with CAM7117 (30 μM) for 15 and 120 minutes. Nuclei are stained with Hoechst 33342 (blue). | ||
Although specific biological activity was observed in cancer cells, the drop-off between the enzymatic and cellular assays was higher than expected. Therefore, we investigated the effect of intracellular localisation of the FITC-labelled CAM7117, and imaging experiments were carried out to look at co-localisation with several organelle markers. We were unable to detect any co-localisation with endosomal markers (Fig. 6c, ESI Fig. S8 and S9†), but partial co-localisation was detected with lysosomal marker, and a significant amount of the peptide was found to localise to the same place as the Golgi and endoplasmic reticulum (ER) markers (Fig. 6c, ESI Fig. S8 and S9†). A significant proportion of the peptide was able to diffuse to the cytosol and localised mainly in the nucleus (Fig. 6c).
Therefore, the drop-off in cellular activity may be attributed to trapping of the peptide in the Golgi/ER. Moreover, the activity drop-off in cells could also be due to the mechanism of inhibition: unlike the inhibition of the α catalytic domain (such as CX4945), displacing the β subunit does not inhibit the phosphorylation of all CK2 substrates.
Further imaging experiments using U2OS cells were carried out to evaluate the effect of CAM7117 in subcellular localisation of the CK2 subunits.
Already after 15 minute incubation, little or no fluorescence associated with the CK2β antibody was found in the nucleus; on the contrary, untreated cells showed significant punctate staining nuclear accumulation of the CK2β (Fig. 6d). This evidence suggests that CAM7117 could engage with CK2α and compete with CK2β in a cellular context.
Conclusions
In the present study, we have showcased an approach to efficiently develop peptides that are highly functionalised, cell-permeable and stable in serum. In particular, using the 2C-CuAAC-PS methodology, we have developed conformationally constrained peptides that act as inhibitors of the CK2 α/β PPI in vitro and in cancer cells. The lead peptide, CAM7117, presents an enhanced binding affinity for CK2α with respect to the previously developed Pc and, most importantly, is stable under conditions mimicking physiological fluids. Owing to the lack of intrinsic cell-permeability of the peptides, we developed an easily-synthesised multi-functional constraint that allowed us to investigate the intracellular activity of CAM7117, which arrests cancer cell proliferation and induces apoptosis in a dose-dependent manner. To the best of our knowledge, this study describes the development of the first inhibitory peptide of the CK2α/β PPI that is: stable in serum, cell permeable, active in cells, able to engage the target and structurally characterised. Such a peptide would act as a chemical probe that enables the study of the CK2 PPI using endogenous levels of proteins and could, therefore, be used to elucidate CK2 dependent mechanisms leading to cancer progression.Remarkably, the multi-functional constraint developed in this work could be used to lock other peptides into their binding conformation and simultaneously functionalise them. Therefore, the strategy adopted herein could, provide a more universal approach to develop modulators of PPIs for many targets where linear sequence epitope provides the majority of the binding energy. Moreover, considering that the peptide addition to either cells or organisms can be done with rigorous temporal and quantitative control, these probes are extremely powerful tools for validating PPIs in drug discovery and dissecting biological processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC and the Wellcome Trust Strategic (090340/Z/09/Z) Award (to DRS and MH). In addition, the group research was supported by grants from the Engineering and Physical Sciences Research Council, Biotechnology and Biological Sciences Research Council, Medical Research Council and Royal Society. JI would like to thank Trinity College, University of Cambridge for funding. YST and CSV would like to thank A*STAR (IAF-PP H17/01/a0/010) for support. We would like to thank Diamond Light Source for access to and support at beamline iO4 (proposal 18548) and the Department of Biochemistry X-ray crystallographic and Biophysics facilities for access to instrumentation.References
- L.-G. Milroy, T. N. Grossmann, S. Hennig, L. Brunsveld and C. Ottmann, Chem. Rev., 2014, 114, 4695–4748 CrossRef CAS PubMed.
- L. D. Fader, E. Malenfant, M. Parisien, R. Carson, F. Bilodeau, S. Landry, M. Pesant, C. Brochu, S. Morin, C. Chabot, T. Halmos, Y. Bousquet, M. D. Bailey, S. H. Kawai, R. Coulombe, S. LaPlante, A. Jakalian, P. K. Bhardwaj, D. Wernic, P. Schroeder, M. Amad, P. Edwards, M. Garneau, J. Duan, M. Cordingley, R. Bethell, S. W. Mason, M. Bös, P. Bonneau, M. A. Poupart, A. M. Faucher, B. Simoneau, C. Fenwick, C. Yoakim and Y. Tsantrisos, ACS Med. Chem. Lett., 2014, 5, 422–427 CrossRef CAS PubMed.
- K. G. McLure, E. M. Gesner, L. Tsujikawa, O. A. Kharenko, S. Attwell, E. Campeau, S. Wasiak, A. Stein, A. White, E. Fontano, R. K. Suto, N. C. Wong, G. S. Wagner, H. C. Hansen and P. R. Young, PLoS One, 2013, 8, e83190 CrossRef PubMed.
- B. B. Lao, I. Grishagin, H. Mesallati, T. F. Brewer, B. Z. Olenyuk and P. S. Arora, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7531–7536 CrossRef CAS PubMed.
- K. Busschots, L. A. Lopez-Garcia, C. Lammi, A. Stroba, S. Zeuzem, A. Piiper, P. M. Alzari, S. Neimanis, J. M. Arencibia, M. Engel, J. O. Schulze and R. M. Biondi, Chem. Biol., 2012, 19, 1152–1163 CrossRef CAS PubMed.
- O. Mirguet, R. Gosmini, J. Toum, C. A. Clément, M. Barnathan, J. M. Brusq, J. E. Mordaunt, R. M. Grimes, M. Crowe, O. Pineau, M. Ajakane, A. Daugan, P. Jeffrey, L. Cutler, A. C. Haynes, N. N. Smithers, C. W. Chung, P. Bamborough, I. J. Uings, A. Lewis, J. Witherington, N. Parr, R. K. Prinjha and E. Nicodème, J. Med. Chem., 2013, 56, 7501–7515 CrossRef CAS PubMed.
- S. He, T. J. Senter, J. Pollock, C. Han, S. K. Upadhyay, T. Purohit, R. D. Gogliotti, C. W. Lindsley, T. Cierpicki, S. R. Stauffer and J. Grembeka, J. Med. Chem., 2014, 57, 1543–1556 CrossRef CAS PubMed.
- T. A. F. Cardote and A. Ciulli, ChemMedChem, 2016, 11, 787–794 CrossRef CAS PubMed.
- M. Pelay-Gimeno, A. Glas, O. Koch and T. N. Grossmann, Angew. Chem., Int. Ed., 2015, 54, 8896–8927 CrossRef CAS PubMed.
- K. Fosgerau and T. Hoffmann, Drug Discovery Today, 2015, 20, 122–128 CrossRef CAS PubMed.
- J. Iegre, J. S. Gaynord, N. S. Robertson, H. F. Sore, M. Hyvonen and D. R. Spring, Adv. Ther., 2018, 1, 1800052 CrossRef.
- Y. Wang, B. J. Bruno, S. Cornillie, J. M. Nogieira, D. Chen, T. E. Cheatham, C. S. Lim and D. H. C. Chou, Chem.–Eur. J., 2017, 23, 7087–7092 CrossRef CAS PubMed.
- C. M. Grison, G. M. Burslem, J. A. Miles, L. K. A. Pilsl, D. J. Yeo, Z. Imani, S. L. Warriner, M. E. Webb and A. J. Wilson, Chem. Sci., 2017, 8, 5166–5171 RSC.
- N. Assem, D. J. Ferreira, D. W. Wolan and P. E. Dawson, Angew. Chem., Int. Ed., 2015, 54, 8665–8668 CrossRef CAS PubMed.
- G. K. Dewkar, P. B. Carneiro and M. C. T. Hartman, Org. Lett., 2009, 11, 4708–4711 CrossRef CAS PubMed.
- C. J. O'Connor, L. Laraia and D. R. Spring, Chem. Soc. Rev., 2011, 40, 4332–4345 RSC.
- Y. H. Lau, Y. Wu, P. de Andrade, W. R. J. D. Galloway and D. R. Spring, Nat. Protoc., 2015, 10, 585–594 CrossRef CAS PubMed.
- Y. S. Tan, D. P. Lane and C. S. Verma, Drug Discovery Today, 2016, 21, 1642–1653 CrossRef CAS PubMed.
- W. Xu, Y. H. Lau, G. Fischer, Y. S. Tan, A. Chattopadhyay, M. De La Roche, M. Hyvönen, C. Verma, D. R. Spring and L. S. Itzhaki, J. Am. Chem. Soc., 2017, 139, 2245–2256 CrossRef CAS PubMed.
- J. Iegre, N. S. Ahmed, J. S. Gaynord, Y. Wu, K. M. Herlihy, Y. S. Tan, M. E. Lopes-Pires, R. Jha, Y. H. Lau, H. F. Sore, C. Verma, D. H. O'Donovan, N. Pugh and D. R. Spring, Chem. Sci., 2018, 20, 4638–4643 RSC.
- L. Zhang, T. Navaratna and G. M. Thurber, Bioconjugate Chem., 2016, 27, 1663–1672 CrossRef CAS PubMed.
- A. Siddiqui-Jain, D. Drygin, N. Streiner, P. Chua, F. Pierre, S. E. Oeapos Brien, J. Bliesath, M. Omori, N. Huser, C. Ho, C. Proffitt, M. K. Schwaebe, D. M. Ryckman, W. G. Rice and K. Anderes, Cancer Res., 2010, 70, 10288–10298 CrossRef CAS PubMed.
- R. Prudent and C. Cochet, Chem. Biol., 2009, 16, 112–120 CrossRef CAS PubMed.
- P. Brear, C. De Fusco, K. Hadje Georgiou, N. J. Francis-Newton, C. J. Stubbs, H. Sore, A. Venkitaraman, C. Abell, D. R. Spring and M. Hyvönen, Chem. Sci., 2016, 7, 6839–6845 RSC.
- J. Iegre, P. Brear, C. De Fusco, M. Yoshida, S. L. Mitchell, M. Rossmann, L. Carro, H. F. Sore, M. Hyvönen and D. R. Spring, Chem. Sci., 2018, 11, 3041–3049 RSC.
- B. Laudet, C. Barette, V. Dulery, O. Renaudet, P. Dumy, A. Metz, R. Prudent, A. Deshiere, O. Dideberg, O. Filhol and C. Cochet, Biochem. J., 2007, 408, 363–373 CrossRef CAS PubMed.
- B. Laudet, V. Moucadel, R. Prudent, O. Filhol, Y. S. Wong, D. Royer and C. Cochet, Mol. Cell. Biochem., 2008, 316, 63–69 CrossRef CAS PubMed.
- B. Bestgen, Z. Belaid-Choucair, T. Lomberget, M. Le Borgne, O. Filhol and C. Cochet, Pharmaceuticals, 2017, 10, e16 CrossRef PubMed.
- P. Brear, A. North, J. Iegre, K. Hadje Georgiou, A. Lubin, L. Carro, W. Green, H. F. Sore, M. Hyvönen and D. R. Spring, Bioorg. Med. Chem., 2018, 26, 3016–3020 CrossRef CAS PubMed.
- O. Filhol, A. Nueda, D. Gerber-scokaert, M. Jose, C. Souchier, Y. Saoudi and C. Cochet, Mol. Cell. Biol., 2003, 23, 975–987 CrossRef CAS PubMed.
- G. Poletto, J. Vilardell, O. Marin, M. A. Pagano, G. Cozza, S. Sarno, A. Falqués, E. Itarte, L. A. Pinna and F. Meggio, Biochemistry, 2008, 47, 8317–8325 CrossRef CAS PubMed.
- O. Marin, S. Sarno, M. Boschetti, M. A. Pagano, F. Meggio, V. Ciminale, D. M. D'Agostino and L. A. Pinna, FEBS Lett., 2000, 481, 63–67 CrossRef CAS PubMed.
- A. C. Bibby and D. W. Litchfield, Int. J. Biol. Sci., 2005, 1, 67–79 CrossRef CAS PubMed.
- J. Raaf, B. Guerra, I. Neundorf, B. Bopp, O. G. Issinger, J. Jose, M. Pietsch and K. Niefind, ACS Chem. Biol., 2013, 901–907 CrossRef CAS PubMed.
- M. M. Wiedmann, Y. S. Tan, Y. Wu, S. Aibara, W. Xu, H. F. Sore, C. S. Verma, L. Itzhaki, M. Stewart, J. D. Brenton and D. R. Spring, Angew. Chem., Int. Ed., 2017, 56, 524–529 CrossRef CAS PubMed.
- Y. H. Lau, P. de Andrade, S.-T. Quah, M. Rossmann, L. Laraia, N. Sköld, T. J. Sum, P. J. E. Rowling, T. L. Joseph, C. Verma and D. R. Spring, Chem. Sci., 2014, 5, 1804 RSC.
- P. T. Tran, C. Ø. Larsen, T. Røndbjerg, M. De Foresta, M. B. A. Kunze, A. Marek, J. H. Løper, L. E. Boyhus, A. Knuhtsen, K. Lindorff-Larsen and D. S. Pedersen, Chem.–Eur. J., 2017, 23, 3490–3495 CrossRef CAS PubMed.
- L. Zhang, T. Navaratna, J. Liao and G. M. Thurber, Bioconjugate Chem., 2015, 26, 329–337 CrossRef CAS PubMed.
- Y. S. Tan, D. R. Spring, C. Abell and C. Verma, J. Chem. Inf. Model., 2014, 54, 1821–1827 CrossRef CAS PubMed.
- Y. S. Tan, D. R. Spring, C. Abell and C. S. Verma, J. Chem. Theory Comput., 2015, 11, 3199–3219 CrossRef CAS PubMed.
- M. S. Searle and D. H. Williams, J. Am. Chem. Soc., 1992, 114, 10690–10697 CrossRef CAS.
- S. Vajda, Z. Wheng, R. Rosenfeld and C. DeLisi, Biochemistry, 1994, 33, 13977–13988 CrossRef CAS PubMed.
- A. R. Khan, J. C. Parrish, M. E. Fraser, W. W. Smith, P. A. Barlett and M. N. James, Biochemistry, 1998, 37, 16839–16845 CrossRef CAS PubMed.
- A. Schnitzler, B. B. Olsen, O. G. Issinger and K. Niefind, J. Mol. Biol., 2014, 426, 1871–1882 CrossRef CAS PubMed.
- G. H. Bird, N. Madani, A. F. Perry, A. M. Princiotto, J. G. Supko, X. He, E. Gavathiotis, J. G. Sodroski and L. D. Walensky, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14093–14098 CrossRef CAS PubMed.
- M. Kristensen, D. Birch and H. M. Nielsen, Int. J. Mol. Sci., 2016, 17, e185 CrossRef PubMed.
- C. Bechara and S. Sagan, FEBS Lett., 2013, 587, 1693–1702 CrossRef CAS PubMed.
- M. Rossmann, S. J. Greive, T. Moschetti, M. Dinan and M. Hyvönen, Protein Eng., Des. Sel., 2017, 30, 419–430 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc00798a |
| ‡ CK2α-interacting loop of CK2β (GGLKRLYGFKIHPMAYQLQLKGG) displayed on the biotinylated globular ATPase domain of recombinase RadA.48 |
| This journal is © The Royal Society of Chemistry 2019 |