Open Access Article
Open Access ArticleGyroid structured aqua-sheets with sub-nanometer thickness enabling 3D fast proton relay conduction†
Tsubasa
Kobayashi
 a,
Ya-xin
Li
b,
Ayaka
Ono
a,
Xiang-bing
Zeng
a,
Ya-xin
Li
b,
Ayaka
Ono
a,
Xiang-bing
Zeng
 b and
Takahiro
Ichikawa
b and
Takahiro
Ichikawa
 *ac
*ac
aDepartment of Biotechnology, Tokyo University of Agriculture and Technology, Naka-cho, Koganei, Tokyo, 184-8588, Japan. E-mail: t-ichi@cc.tuat.ac.jp
bDepartment of Materials Science and Engineering, University of Sheffield, Sheffield S1 3JD, UK
cJST, PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012, Japan
First published on 17th June 2019
Abstract
A polymerizable amphiphile having two zwitterionic head-groups has been designed. This compound co-organizes with an acid, bis(trifluoromethanesulfonyl)imide (HTf2N), into a gyroid bicontinuous cubic liquid-crystalline phase. In situ polymerization of this phase has been successfully achieved by UV irradiation in the presence of a photoinitiator, yielding a self-standing gyroid-nanostructured polymer film. When the polymer film is placed under different relative humidity conditions or in water, it absorbs water owing to the strong hydration ability of the zwitterionic parts. It has been found that the polymer film preserves the gyroid nanostructure after the water absorption. Based on reconstructed electron density maps, it is assumed that the absorbed water molecules form a 3D continuous network along the gyroid minimal surface, which satisfies several key conditions for inducing fast proton conduction. As expected, such hydrated films show high ionic conductivities in the order of 10−1 S cm−1 when the water content of the film reaches 15.6 wt% at RH = 90%. The high conductivity is attributed to the induction of the Grotthuss mechanism, that is, proton conduction via the hydrogen-bonding network of the incorporated water molecules.
Introduction
Proton conductive membranes are key materials that play a crucial role in developing a sustainable world because of their versatile utilities in fuel cells, bio-devices, sensors, catalysis, soft robotics, and so on. Following the discovery of proton-conductive fluoropolymers, e.g., Nafion,1,2 a variety of proton-conductive membranes have been designed using aromatic polymers,3–6 block-copolymers,7 gels,8 liquid crystals,9–14 metal–organic frameworks,15 and some hybrid materials.16 It is considered that an ideal proton-conductive membrane should have properties such as high ionic conductivity, high mechanical strength, extremely low fuel (H2 gas or MeOH) permeability, and surface morphology forming effective interfaces with catalysts. For the realization of such ideal membranes, there have been intensive demands for the development of an innovative membrane design strategy. We expect that the construction of polymer membranes with 3D-continuous proton conductive pathways should be a promising approach and therefore focus on the matrix design based on a gyroid surface,17 a type of infinite periodic minimal surface.A gyroid bicontinuous cubic (Cubbi) phase, consisting of two interpenetrating networks separated by a 3D infinite periodic minimal surface with the space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d, is typically found in liquid crystals with a lattice parameter of several nanometers.18–24 Despite its 3D continuous and periodic structure and hence potential for application, the number of studies on the application of Cubbi liquid crystals is still limited.25–39 There has been recent progress on our original molecular design principle for creating Cubbi liquid crystals. In a representative system, we have employed amphiphilic zwitterions consisting of an organic cation and a sulfonate anion.12,40–43 Owing to the ability of zwitterions to form adducts with suitably selected acids, it is possible to tune their self-organization behavior in order to form Ia
d, is typically found in liquid crystals with a lattice parameter of several nanometers.18–24 Despite its 3D continuous and periodic structure and hence potential for application, the number of studies on the application of Cubbi liquid crystals is still limited.25–39 There has been recent progress on our original molecular design principle for creating Cubbi liquid crystals. In a representative system, we have employed amphiphilic zwitterions consisting of an organic cation and a sulfonate anion.12,40–43 Owing to the ability of zwitterions to form adducts with suitably selected acids, it is possible to tune their self-organization behavior in order to form Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d-type (gyroid-type) Cubbi liquid-crystalline (LC) assemblies by controlling the addition of acids. Since the Cubbi structures formed by the combination of amphiphilic zwitterions and acids possess a hydrophilic gyroid minimal surface where their sulfonic acid groups align densely, they have been expected to be a potential matrix for proton conductive materials.
d-type (gyroid-type) Cubbi liquid-crystalline (LC) assemblies by controlling the addition of acids. Since the Cubbi structures formed by the combination of amphiphilic zwitterions and acids possess a hydrophilic gyroid minimal surface where their sulfonic acid groups align densely, they have been expected to be a potential matrix for proton conductive materials.
For the realization of fast proton conduction, one of the most general but advantageous approaches is to create hydrogen-bonding networks of water molecules in the matrices because water molecule networks enable fast transport of protons based on a bucket brigade conduction mechanism. This approach is effective for our Cubbi LC systems to some extent.12 However, since the Cubbi LC materials have fluidity, it is impossible to introduce a sufficient amount of water molecules into them while maintaining the LC nanostructures.12,40,41 In general, conversion of fluidic LC materials to solid ones can be achieved by introducing polymerizable groups into LC molecules and subsequent in situ polymerization.44–54 In the present study, we report the development of self-standing polymer films with a hydrophilic gyroid minimal surface via in situ polymerization of amphiphilic zwitterions. These polymer films can incorporate water molecules onto the gyroid minimal surface, forming a 3D continuous water network, which leads to the induction of high ionic conductivities in the order of 10−1 S cm−1.
Results and discussion
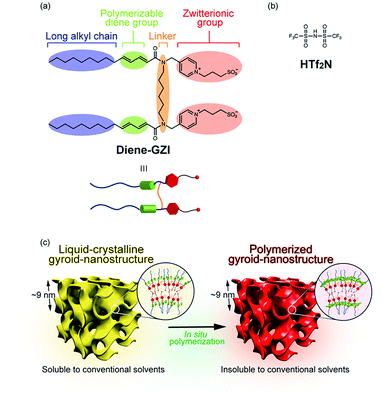
We have designed a new gemini-type polymerizable amphiphile, Diene-GZI (Fig. 1a). Several unique features are incorporated into the molecular design which was inspired by several previous studies on amphiphilic liquid crystals.12,40,47,55–59 One is that the new gemini monomer has zwitterionic head groups consisting of pyridinium cations and sulfonate anions.12,40 A second is that two single-head/single-tail halves of this monomer are connected via a linker to create a gemini-type amphiphile.55–59 A third is that polymerizable diene groups are introduced in between the zwitterionic head groups and the long alkyl chains.47 The detailed synthesis steps are shown in the ESI (Scheme S1†). The thermal behavior of pristine Diene-GZI was examined by polarized optical microscopy (POM), differential scanning calorimetry, and X-ray diffraction (XRD) measurements (see the ESI†). It has been found that pristine Diene-GZI shows thermotropic LC behavior. Although it is difficult to identify the mesophase behavior because thermal polymerization of Diene-GZI starts before isotropization on heating, it is assumed that Diene-GZI forms a layered smectic (Sm) phase through nanosegregation of the zwitterionic and ionophobic parts.It is of importance that the isotropization temperature and mesophase behavior of Diene-GZI can be tuned by adding some acid and water. By examining the amphotropic (lyotropic and thermotropic) LC behavior of the mixtures of Diene-GZI, acid and water, we have found that Diene-GZI self-organizes into Cubbi phases in the presence of suitable amounts of an acid, bis(trifluoromethanesulfonyl)imide (HTf2N, Fig. 1b), and water. For example, the mixture of Diene-GZI/HTf2N/water (1.0/0.5/14 by mol) (MX) exhibits a Cubbi phase at room temperature (Fig. 1c, left). The formation of the Cubbi phase has been characterized by powder XRD measurement and POM observation. The XRD pattern of MX shows two intense diffraction peaks in the small angle region and the reciprocal d-spacing ratio of the two peaks is √6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √8, which can be indexed as (211) and (220) reflections of an Ia
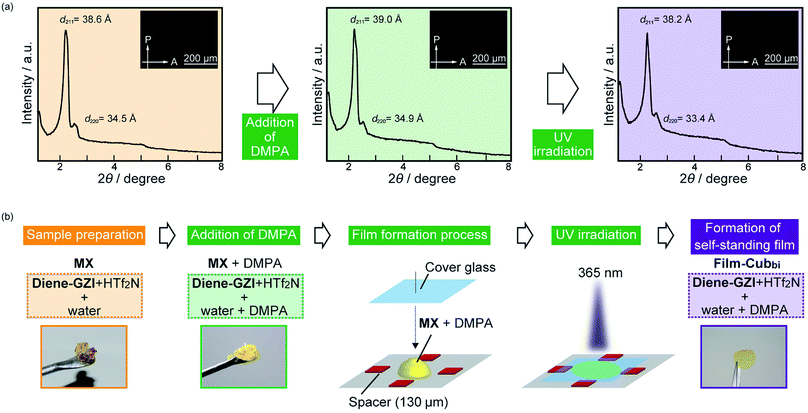
√8, which can be indexed as (211) and (220) reflections of an Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d-type (gyroid-type) Cubbi structure having a lattice parameter a of 94.4 Å (Fig. 2a). No birefringence is observed for MX under POM observation (Fig. 2a, inset), which is also indicative of the formation of the Cubbi nanostructure. Based on the insights into the molecular assemblies of the previously designed amphiphilic LC zwitterions,12,40–43 it is most plausible to assume that the sulfonic acid groups of Diene-GZI align regularly on the gyroid minimal surface.
d-type (gyroid-type) Cubbi structure having a lattice parameter a of 94.4 Å (Fig. 2a). No birefringence is observed for MX under POM observation (Fig. 2a, inset), which is also indicative of the formation of the Cubbi nanostructure. Based on the insights into the molecular assemblies of the previously designed amphiphilic LC zwitterions,12,40–43 it is most plausible to assume that the sulfonic acid groups of Diene-GZI align regularly on the gyroid minimal surface.
We performed in situ polymerization of MX in the Cubbi state. The detailed procedure is classified into 5 steps and is shown in Fig. 2b: step (I) preparation of MX; step (II) addition of 1 wt% of 2,2-dimethoxy-2-phenylacetophenone (DMPA) as a radical photo-polymerization initiator; step (III) film formation of the fluidic LC sample on a Teflon substrate; step (IV) UV irradiation; step (V) removal of the film from the substrate. These 5 steps produced a self-standing polymer film (Film-Cubbi). It is noteworthy that the polymerization has progressed while preserving the gyroid-nanostructure. This was confirmed by X-ray measurement and POM observation (Fig. 2a). By performing gravimetric analysis before and after the polymerization process, it was found that Film-Cubbi contains less than 10 wt% water, while the water content in MX was 18.7 wt%. The difference in the water content before and after polymerization is attributed to water evaporation during the polymerization process.
To obtain further insight into the nanostructure in Film-Cubbi, synchrotron XRD measurements were performed. By transforming the obtained diffraction patterns into scattered electron waves, we reconstructed the electron density map for Film-Cubbi (Fig. S11 in the ESI†). It was confirmed that the high electron density region forms a 3D-continuous domain along the gyroid minimal surface, which is occupied by the zwitterionic part, HTf2N and water. On the other hand, the electron lower domains formed by the alkyl chains are observed in the center of 3D-branched nanochannels. The microscopic structure in Film-Cubbi was investigated by scanning electron microscopy (SEM). Floor and cross-sectional views are shown in Fig. S8 in the ESI.† It can be seen that Film-Cubbi is a homogeneous polymer film with no micro-scale pores, suggesting that the polymerization proceeds homogeneously in the film.
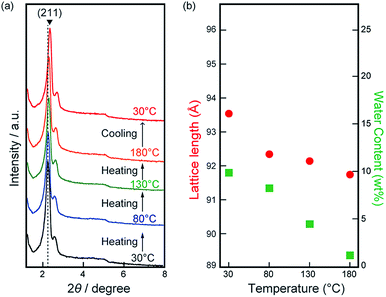
Our aim in the present study is to develop a 3D continuous proton conducting pathway in the film by installing water molecules densely on the gyroid minimal surface. For the realization of this aim, we have examined the structure preserving ability of Film-Cubbi under various conditions. To examine the temperature dependence of the gyroid-nanostructure of Film-Cubbi, we have carried out XRD measurements with varying temperatures from 30 to 180 °C on heating and 180 to 30 °C on cooling (Fig. 3a). It can be seen that little change of the XRD patterns occurs upon heating and cooling, indicating that Film-Cubbi maintains the gyroid-nanostructure in the temperature range whereas the unpolymerized MX monomer mixture starts to lose the Cubbi structure at around 35 °C (see Fig. S6 in the ESI†). Focusing on the peak positions, we have found a slight shift of the positions with the increase/decrease of the temperature. For example, the (211) peak gradually shifts to the wide-angle region upon heating from 2θ = 2.31° at 30 °C to 2θ = 2.36° at 180 °C. From these values, the cubic lattice parameter “a” in Film-Cubbi was calculated to be 93.5 Å at 30 °C and 91.7 Å at 180 °C (Fig. 3a), indicating a shrinkage of the lattice upon heating. This behavior is opposite to what would be expected due to thermal expansion. We assume that the shrinkage of the gyroid structure in Film-Cubbi results from water drying out of the sample.
To confirm this assumption, gravimetric analysis was performed on Film-Cubbi before and after the heating process. A weight decrease in Film-Cubbi was clearly observed as a function of the increasing temperature. Assuming that the weight decrease results from water drying out of the sample, we calculated the change of water content in Film-Cubbi, and these values are plotted in Fig. 3b. It can be seen that the water content decreases from 9.9 wt% in the initial state to 1.2 wt% at 180 °C. We conclude that water evaporates from Film-Cubbi on heating, which leads to weight loss accompanied by the slight shrinkage of the gyroid-nanostructure. On cooling, further shrinkage of the lattice from 91.7 to 89.6 Å was found when the sample was cooled from 180 to 30 °C, due to thermal contraction. The most notable point is that the gyroid-nanostructure in Film-Cubbi is maintained in the water-drying process, suggesting that the mean positions of the zwitterionic amphiphiles are strongly fixed by cross-linking polymerization while they still have molecular fluctuations. We assume that the creation of the well-stabilized gyroid-nanostructure is attributed to the progress of many more cross-linking reactions that are attainable for gemini-type molecular structures but unattainable for single-head + single-tail ones.48
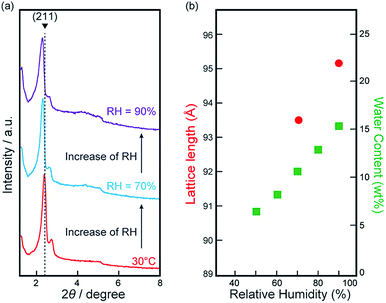
The next question we addressed is whether it is possible to incorporate a sufficient amount of water molecules into Film-Cubbi to form a 3D continuous hydrogen-bonding network of water molecules along a gyroid minimal surface. To help answer this question, we have prepared Film-Cubbi and placed it under RH controlled conditions (RH = 50 to 90%) for 12 h in order to reach an equilibrium state where water absorption and evaporation proceed at the same speed. The water contents (X wt%) in the treated films were evaluated from the weight change and henceforth they are denoted as Film-Cubbi(X). It was found that the water content X increases from 6.7 wt% at RH = 50% to 15.6 wt% at RH = 90% (Fig. 4b). To confirm whether Film-Cubbi maintains the gyroid structure or not during the water absorption and evaporation processes, XRD measurements have been performed for Film-Cubbi(X). Little difference in the shape of the XRD patterns was found for Film-Cubbi(X) while a slight shift of the peak positions was observed (Fig. 4a). These results indicate that it is possible to introduce/remove water molecules into/from Film-Cubbi(X) by controlling the RH without distorting the gyroid-nanostructure. In addition, there was no splitting of the (211) reflection peak upon water absorption, suggesting that the incorporated water molecules are homogeneously dispersed around the hydrophilic gyroid minimal surface in Film-Cubbi.
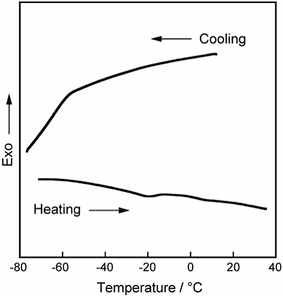
To obtain further insight into the position and state of the incorporated water molecules in the gyroid-nanostructures of Film-Cubbi(X), DSC measurement was performed for Film-Cubbi(15.6). No exothermic and endothermic peak is observed for Film-Cubbi(15.6) at around 0 °C on cooling, indicating that almost all the water molecules in the film exist not as free water but as bound water (Fig. 5). The number of water molecules per sulfonate group in Film-Cubbi(15.6) is calculated to be about 5.6. Taking into account the general knowledge that one sulfonic acid group has the ability to strongly interact with up to 8–14 water molecules,60–62 it is plausible that the absorbed water molecules are placed exclusively on the gyroid minimal surface where the sulfonate/sulfonic-acid groups of Diene-GZI are aligned densely.
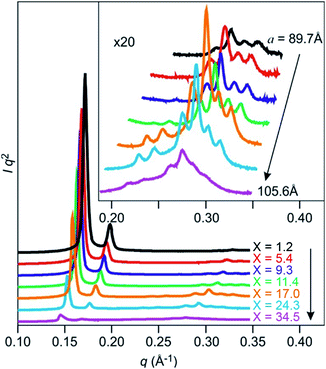
In order to obtain critical information on the positions of the incorporated water molecules, we performed synchrotron XRD measurements on samples of Film-Cubbi containing various amounts of water. The driest sample was obtained by drying at 110 °C and the wettest sample was prepared by immersing into water. Samples with a whole range of water contents were prepared by tuning these processes. The XRD patterns obtained for these samples clearly show that Film-Cubbi(X) can maintain the Cubbi periodic structure with the same Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d space group while increasing the water amount leads to expansion of the lattice (Fig. 6). In particular, the lattice parameter increases by 18% from the driest to the wettest conditions, which corresponds to an increase in the unit cell volume of 63%. Such an increase in the unit cell volume is attributed to the increase in the water content in Film-Cubbi(X). The density of the driest sample is measured to be 1.27 g cm−3 by means of flotation analysis (Fig. S12 in the ESI†). Using the measured lattice parameters and water content of the as-prepared, dried Film-Cubbi(X) samples, and assuming that the water density in the film is 1.00 g cm−3, we have derived a relationship between the lattice parameter and the amount of absorbed water by weight (Table S9 in the ESI†). It was thus estimated that the water content X in the prepared samples ranged from 1.2 to 34.5 wt%. The derived relationship suggests that the wettest sample has a density of 1.16 g cm−3, which is consistent with the experimentally obtained value (1.17 g cm−3).
d space group while increasing the water amount leads to expansion of the lattice (Fig. 6). In particular, the lattice parameter increases by 18% from the driest to the wettest conditions, which corresponds to an increase in the unit cell volume of 63%. Such an increase in the unit cell volume is attributed to the increase in the water content in Film-Cubbi(X). The density of the driest sample is measured to be 1.27 g cm−3 by means of flotation analysis (Fig. S12 in the ESI†). Using the measured lattice parameters and water content of the as-prepared, dried Film-Cubbi(X) samples, and assuming that the water density in the film is 1.00 g cm−3, we have derived a relationship between the lattice parameter and the amount of absorbed water by weight (Table S9 in the ESI†). It was thus estimated that the water content X in the prepared samples ranged from 1.2 to 34.5 wt%. The derived relationship suggests that the wettest sample has a density of 1.16 g cm−3, which is consistent with the experimentally obtained value (1.17 g cm−3).
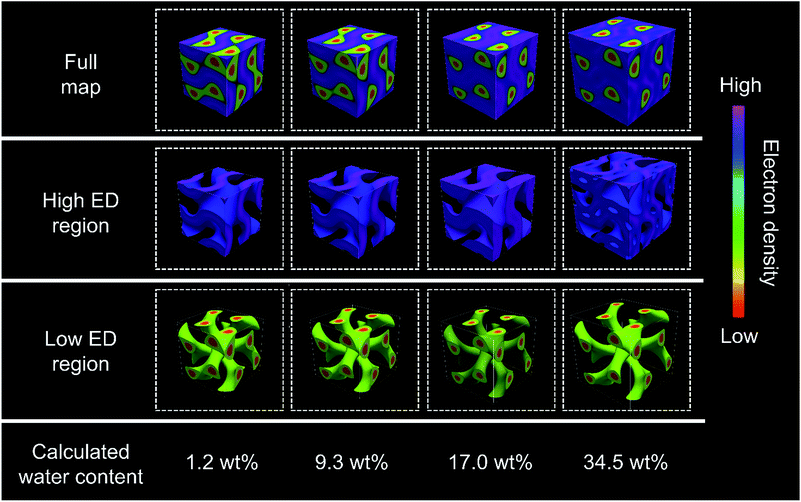
Another noteworthy feature in the XRD patterns is that the relative intensities of the first two diffraction peaks, (211) and (220), decrease dramatically with increasing water content in the Film-Cubbi(X) samples. It is expected that the increase in water content would expand the volume of high electron density regions (ionic + water) and reduce the relative volume of the lower electron density regions (alkyl chain); therefore the overall contrast in the structure is reduced leading to weaker diffraction peaks. This assumption is clearly confirmed from reconstructed electron density maps (Fig. 7 and S13 in the ESI†). The changes of the electron density are visualized with colour changes from red, yellow, green, aqua, and blue to purple as the electron density increases. Focusing on the gyroid minimal surface, it can be seen that, while it is coloured only purple when the water content is low (X = 1.2, 5.4, and 9.3), scattered blue domains appear when the water content is 17.0 wt%, and the blue domains gain 3D continuity when the water contents are 24.3 and 34.5 wt%. In other words, a blue coloured gyroid minimal surface appears upon water absorption between the purple domains with the highest electron density. This can be explained by assuming that the blue gyroid surface between the purple regions is formed by localization of some water molecules, and the purple domains are composed mostly of the ionic segments with high electron density. We attribute this local segregation between water and the ionic domains to two effects. One is the strong hydrophobicity of the Tf2N anion. The other is the strong hydrophilicity of the sulfonate anion of the zwitterionic parts. The number of water molecules per zwitterionic headgroup on the monomer in the films with 17.0, 24.3, and 34.5 wt% water can be calculated to be 6.2, 9.8, and 16.0. Recently, there have been several studies on the relationship between the ion structure of various ionic liquids and their water sorption ability.63 Considering this general insight, the maximum number of water molecules that form strong interactions with a pyridinium-cation is expected to be lower than 5. This led us to assume that there is an excess number of water molecules beyond the hydration limitation of the pyridinium cation when the water content X is ≥21 wt%. Consequently, the excess water molecules, being bound to the sulfonate groups and/or being shed by the Tf2N anions, form an extremely thin water sheet domain along the gyroid minimal surface. It is worth noting that the blue domain along the gyroid minimal surface can be named a “3D continuous aqua sub-nanosheet”.
It is well-known that proton conduction is induced by the combination of two mechanisms, the vehicle and the Grotthuss mechanisms.64,65 The former mechanism is proton migration based on the molecular diffusion of protonated water clusters. The latter mechanism is instantaneous transmittance of protons through water molecule networks as a result of breaking/forming of hydrogen-bonding, which provides exceptionally high-speed ion conduction. This mechanism can be recognized as a kind of Newton's cradle (NC) phenomenon where water molecules act as “balls”. Taking into account the geometrical character of a gyroid structure which is composed of patching of saddle-shaped plates, we expect that the present polymer film should act as a novel platform for water molecules to exhibit a 3D NC phenomenon and then show high proton conductivity in the order of 10−1 S cm−1, which is comparable to the limit value of the Grotthuss mechanism. Generally, it is essential for inducing effective NC phenomena to align “balls” one- or two-dimensionally without any space between them. With this in mind, it is considered that successive proton conduction via the Grotthuss mechanism along the gyroid minimal surface can be induced when water molecules are installed all over the surface (Fig. S14 in the ESI†). Roughly estimating the number of water molecules that is required to fulfill a gyroid minimal surface in a cubic lattice with a lattice length of 95 Å (see the ESI†), it is calculated to be about 4000. By converting the number of water molecules per unit cell into the weight ratio, it is expected that over 12 wt% of the water content is needed at least in the case of Film-Cubbi(X) to cover the gyroid minimal surface.
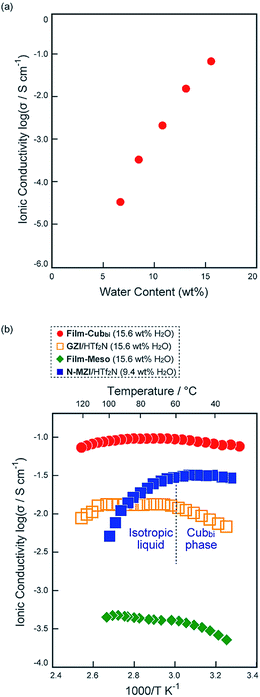
In order to examine the relationship between the ball filling rate (namely water content) and proton conductivity, impedance measurements were performed for Film-Cubbi(X) with various water contents. The ionic conductivity (σ) values are plotted against the water content X in Fig. 8a. It can be seen that the σ values depend largely on the water content. For example, the σ of Film-Cubbi(X) is 3.3 × 10−5 S cm−1 when X = 6.7 wt%, which is far lower than 10−1 S cm−1. The conduction mechanism in Film-Cubbi(6.7) is thus probably dominated by the vehicle mechanism because the gyroid minimal surface is not fully covered by water molecules enough to induce the Grotthuss mechanism. On the other hand, the σ of Film-Cubbi(13.1) is 1.5 × 10−2 S cm−1 and that of Film-Cubbi(15.6) is close to 10−1 S cm−1, which is an achievable value only when protons can be transported based on the Grotthuss mechanism over a long distance. These results led us to conclude that it is possible to induce a NC phenomenon along a gyroid minimal surface by adding an adequate amount of balls to fully cover the surface. We assume that, owing to the 2D nature of the gyroid structure, protons can show 2D NC-type fast transport in local ranges, which develops into 3D transport in larger ranges as a result of the 3D patching manner of the 2D plates.
To date, a variety of materials design methods have been employed for improving proton-conductive polymer electrolytes for fuel cell applications.66–68 The present materials design should be a new advantageous method for providing a new electrolyte design. Below, we compare the present materials and conventional polymer electrolytes, such as Nafion, from the viewpoint of ion conductive materials. The temperature dependence of σ of Film-Cubbi(15.6) was investigated under measurement conditions of a heating rate of 2 °C min−1 and RH = 40%. The σ linearly increases as temperature increases from ambient temperature to around 70 °C (Fig. 8b) and it reaches 9.9 × 10−2 S cm−1 at 70 °C, which is comparable to that of highly hydrated Nafion.69 The activation energy in this temperature range is calculated to be 2.2 kJ mol−1. This low activation energy strongly supports our conclusion that the proton conduction in Film-Cubbi(15.6) mostly depends on the Grotthuss mechanism. The activation energy of highly hydrated Nafion has been reported to be 10–15 kJ mol−1.68 Recently, there have been some reports that hydrogen-bonding networks of water molecules confined under specific conditions, such as in carbon nanotubes and in between mineral layers, exhibit exceptionally high ionic conductivity and low activation energy due to the formation of specifically coordinated structures of water molecules.70,71 Considering these examples, there is a possibility that a specific proton conduction mechanism is induced in the present materials in which water molecules are confined exclusively on a gyroid minimal surface.
In order to further discuss the effects of the polymerization on the ion conduction behavior, the σ of Film-Cubbi(15.6) was compared with that of an analogous non-polymerizable LC system. For such a non-polymerizable LC control sample, a mixture of a mono-type amphiphilic zwitterion with a normal alkyl chain (N-MZI)72 and HTf2N with a small amount of water was selected. The σ of the N-MZI/HTf2N mixture (1.0/1.0 by mol) with 9.4 wt% water (reported in our previous paper12) is also plotted in Fig. 8b. Comparing the σ of Film-Cubbi(15.6) and that of the N-MZI/HTf2N mixture, we found some significant differences. For example, Film-Cubbi(15.6) shows a σ of 8.4 × 10−2 S cm−1 at 35 °C, which is about 3 times higher than that of the N-MZI/HTf2N mixture. The large difference in σ mostly results from the difference in water content. The water content in Film-Cubbi(15.6) is about twice that in the N-MZI/HTf2N mixture. Here it should be noted that, while the N-MZI/HTf2N mixture forms gyroid-nanostructures when the water content is <10 wt%, it is impossible to increase the water content above 10 wt% for the N-MZI/HTf2N mixture while maintaining its molecular assembled gyroid-nanostructures because the increase of the water content results in phase changes and significant lowering of the isotropization temperature. Another noteworthy point is that the σ of the N-MZI/HTf2N mixture abruptly decreases at around 60 °C, which can be explained in two ways. One is the collapse of hydrogen-bonding networks of the water molecules, followed by phase transition to the isotropic phase. The other is decrease of the water content through evaporation. Compared to the ion conduction behaviour of the N-MZI/HTf2N mixture, Film-Cubbi(15.6) shows a gradual decrease of σ at temperatures higher than 70 °C. First, it is concluded that the polymerization of the amphiphilic zwitterions results in overcoming the disadvantage of the LC materials that they lose structural benefits in the temperature region higher than their isotropization temperature. Not only does it enhance the structural-benefits, it also contributes to the enhancement of the water-binding ability as a result of the preservation of a unique situation where the sulfonate/sulfonic-acid groups are densely aligned on a continuous surface. As another control sample, we prepared a polymeric sample by polymerizing the Diene-GZI/HTf2N mixture in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 with 1 wt% of DMPA at 130 °C in a disordered state under UV irradiation for 1 h. The obtained polymeric sample was mixed with water to obtain a water content of 15.6 wt% (Film-Meso(15.6)). The absence of a well-ordered nanostructure in Film-Meso(15.6) was confirmed by XRD measurement (see Fig. S15 in the ESI†). Comparing the σ of Film-Cubbi(15.6) and Film-Meso(15.6), it is notable that Film-Cubbi(15.6) shows higher σ than Film-Meso(15.6). For example, the σ of Film-Cubbi(15.6) is 9.7 × 10−2 S cm−1, which is 240 times larger than that of Film-Meso(15.6). These results suggest that the presence of the gyroid nanostructure is a critical factor that enhances the contribution of the Grotthuss mechanism in the polymerized amphiphilic zwitterionic systems.
0.5 with 1 wt% of DMPA at 130 °C in a disordered state under UV irradiation for 1 h. The obtained polymeric sample was mixed with water to obtain a water content of 15.6 wt% (Film-Meso(15.6)). The absence of a well-ordered nanostructure in Film-Meso(15.6) was confirmed by XRD measurement (see Fig. S15 in the ESI†). Comparing the σ of Film-Cubbi(15.6) and Film-Meso(15.6), it is notable that Film-Cubbi(15.6) shows higher σ than Film-Meso(15.6). For example, the σ of Film-Cubbi(15.6) is 9.7 × 10−2 S cm−1, which is 240 times larger than that of Film-Meso(15.6). These results suggest that the presence of the gyroid nanostructure is a critical factor that enhances the contribution of the Grotthuss mechanism in the polymerized amphiphilic zwitterionic systems.
To further confirm the importance of the polymerization for high proton conduction, we prepared a gemini-type amphiphilic zwitterion with normal alkyl chains (GZI, see the ESI†). By mixing GZI and HTf2N in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 molar ratio and adding 15.6 wt% of water, a control sample forming a fluidic LC state was prepared. The σ of the GZI/HTf2N mixture is also plotted in Fig. 8b. It can be seen that the σ of Film-Cubbi(15.6) is about an order of magnitude higher than that of the GZI/HTf2N mixture. These results lead us to assume that the polymerization of the gemini-amphiphilic zwitterion has an effect on limiting the position of water molecules in the system at the hydrophilic gyroid minimal surface. Consequently, it enhances the macroscopic continuity of hydrogen-bonding networks of water molecules, which enables a more efficient Grotthuss proton conduction mechanism over a long distance.
0.5 molar ratio and adding 15.6 wt% of water, a control sample forming a fluidic LC state was prepared. The σ of the GZI/HTf2N mixture is also plotted in Fig. 8b. It can be seen that the σ of Film-Cubbi(15.6) is about an order of magnitude higher than that of the GZI/HTf2N mixture. These results lead us to assume that the polymerization of the gemini-amphiphilic zwitterion has an effect on limiting the position of water molecules in the system at the hydrophilic gyroid minimal surface. Consequently, it enhances the macroscopic continuity of hydrogen-bonding networks of water molecules, which enables a more efficient Grotthuss proton conduction mechanism over a long distance.
In addition to these physicochemical properties, the present materials have great potential for exhibiting new advantageous aspects as polymer electrolytes for fuel cells. For example, since the proton conduction pathways in Film-Cubbi(X) have 3D continuity, this film is independent of the formation of “dead-ends” in proton conduction pathways. Another aspect is that the nanosegregated structure in Film-Cubbi(X) should contribute to the formation of an effective interface between catalysts and electrolytes. It is also expected that, by substituting the Tf2N anion with non-fluoridated anions after polymerization, Film-Cubbi(X) can be converted to fluorine-free polymer electrolytes, which may endow Film-Cubbi(X) with more bio-compatibility.
Conclusions
We have succeeded in creating a self-standing polymer film having a hydrophilic gyroid minimal surface by cross-linking polymerization of an amphiphilic zwitterionic gemini monomer in a bicontinuous cubic liquid-crystalline assembly. This polymer film has the ability to absorb water molecules while maintaining the gyroid nanostructure until the gyroid minimal surface is fully covered by water molecules. Under these water-fulfilled conditions, the film shows high ionic conductivity in the order of 10−1 S cm−1 at room temperature, suggesting that the Grotthuss mechanism, a type of Newton's cradle phenomenon, occurs successively along the 3D continuous gyroid minimal surface over a long distance.Experimental section
Materials
All the reagents were purchased from Sigma Aldrich and Tokyo Kasei corporation. The synthetic scheme for Diene-GZI is shown in the ESI.† The obtained compounds are characterized by 1H NMR, 13C NMR, and elemental analysis (see the ESI†).Polymerization
1 wt% of DMPA is added as a photo-polymerization initiator. MX was sandwiched by a Teflon sheet and a cover glass with 130 μm thickness spacers. UV irradiation was performed for DMPA-added MX for 2 h at 30 °C using a xenon lamp as a light source.Thermogravimetric analysis
The samples of Film-Cubbi were heated at 30, 80, 130, and 180 °C for 15 minutes, respectively. After that, their weights were measured immediately.Impedance measurement
Alternating current impedance measurements were performed using a Schlumberger Solartron 1260 impedance analyzer (frequency range = 10 Hz to 10 MHz, applied voltage = 0.1 V). Comb-shaped gold electrodes with a glass substrate were used as a cell for the measurements of ionic conductivity. To determine the cell constant of the cell, a 0.01 mol L−1 KCl aqueous solution was employed as a calibration standard.Conflicts of interest
There are no conflicts to declare.Acknowledgements
T. I. is grateful for financial support from the Precursory Research for Embryonic Science and Technology (PRESTO) from the Japan Science and Technology Agency (JST) (no. JPMJPR1413). This work was also partially supported by the Foundation for the Promotion of Ion Engineering. T. K. is grateful for financial support from the JSPS Research Fellowships for Young Scientists (no. JP 18J21088). The authors thank Takahiro Kuzumaki in RIGAKU Corporation for the XRD measurements under various relative humidities. We are grateful to Dr N. Terill and O. Shebanova at I22, Diamond Light Source for help with SAXS experiments. The authors acknowledge funding for this work from the EPSRC (EP-K034308, EP-P002250). Y. L. thanks the CSC for the stipend and the UoS for waiving the tuition fee.Notes and references
- K. D. Kreuer, J. Membr. Sci., 2001, 185, 29 CrossRef CAS.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535 CrossRef CAS PubMed.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587 CrossRef CAS PubMed.
- B. Lafitte and P. Jannasch, Adv. Funct. Mater., 2007, 17, 2823 CrossRef CAS.
- B. Bae, T. Yoda, K. Miyatake, H. Uchida and M. Watanabe, Angew. Chem., Int. Ed., 2010, 49, 317 CrossRef CAS PubMed.
- P. Nimmanpipug, K. Kodchakorn, V. S. Lee, J. Yana, C. Jarumaneeroj, S. Phongtamrug and S. Chirachanchai, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 1625 CrossRef CAS.
- M. J. Park and N. P. Balsara, Macromolecules, 2010, 43, 292 CrossRef CAS.
- M. Michau and M. Barboiu, J. Mater. Chem., 2009, 19, 6124 RSC.
- C.-F. Chow, V. A. L. Roy, Z. Ye, M. H. W. Lam, C. S. Lee and K. C. Lau, J. Mater. Chem., 2010, 20, 6245 RSC.
- Y. Chen, M. Thorn, S. Christensen, C. Versek, A. Poe, R. C. Hayward, M. T. Tuominen and S. Thayumanavan, Nat. Chem., 2010, 2, 503 CrossRef CAS PubMed.
- S. Ueda, J. Kagimoto, T. Ichikawa, T. Kato and H. Ohno, Adv. Mater., 2011, 23, 3071 CrossRef CAS PubMed.
- T. Ichikawa, T. Kato and H. Ohno, J. Am. Chem. Soc., 2012, 134, 11354 CrossRef CAS PubMed.
- E. Tunkara, C. Albayrak, E. O. Polat, C. Kocabas and Ö. Dag, ACS Nano, 2014, 8, 11007 CrossRef CAS PubMed.
- Y. Nagao, Langmuir, 2017, 33, 12547 CrossRef CAS PubMed.
- J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski and G. K. H. Shimizu, Nat. Chem., 2009, 1, 705 CrossRef CAS PubMed.
- L. Cao, H. Wu, P. Yang, X. He, J. Li, Y. Li, M. Xu, M. Qiu and Z. Jiang, Adv. Funct. Mater., 2018, 28, 1804944 CrossRef.
- P. Garstecki and R. Hołyst, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 64, 021501 CrossRef CAS PubMed.
- S. Diele, Curr. Opin. Colloid Interface Sci., 2002, 7, 333 CrossRef CAS.
- M. Impéror-Clerc, Curr. Opin. Colloid Interface Sci., 2005, 9, 370 CrossRef.
- D. W. Bruce, Acc. Chem. Res., 2000, 33, 831 CrossRef CAS PubMed.
- C. Tschierske, Angew. Chem., Int. Ed., 2013, 52, 8828 CrossRef CAS PubMed.
- C. Tschierske and G. Ungar, ChemPhysChem, 2016, 17, 9 CrossRef CAS PubMed.
- S. Kutsumizu, Isr. J. Chem., 2012, 52, 844 CrossRef CAS.
- X. Zeng, G. Ungar and M. Impéror-Clerc, Nat. Mater., 2005, 4, 562 CrossRef CAS PubMed.
- T. Kato, M. Yoshio, T. Ichikawa, B. Soberats, H. Ohno and M. Funahashi, Nat. Rev. Mater., 2017, 2, 17001 CrossRef.
- D. L. Gin, W. Gu, B. A. Pindzola and W.-J. Zhou, Acc. Chem. Res., 2001, 34, 973 CrossRef CAS PubMed.
- B.-K. Cho, RSC Adv., 2014, 4, 395 RSC.
- B.-K. Cho, A. Jain, S. M. Gruner and U. Wiesner, Science, 2004, 305, 1598 CrossRef CAS PubMed.
- T. Ichikawa, M. Yoshio, A. Hamasaki, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2007, 129, 10662 CrossRef CAS PubMed.
- D. L. Gin, X. Lu, P. R. Nemade, C. S. Pecinovsky, Y. Xu and M. Zhou, Adv. Funct. Mater., 2006, 16, 865 CrossRef CAS.
- A. J. Boydston, C. S. Pecinovsky, S. T. Chao and C. W. Bielawski, J. Am. Chem. Soc., 2007, 129, 14550 CrossRef CAS PubMed.
- R. L. Kerr, S. A. Miller, R. K. Shoemaker, B. J. Elliott and D. L. Gin, J. Am. Chem. Soc., 2009, 131, 15972 CrossRef CAS PubMed.
- H. Zhang, L. Li, M. Möller, X. Zhu, J. J. Hernandez Rueda, M. Rosenthal and D. A. Ivanov, Adv. Mater., 2013, 25, 3543 CrossRef CAS PubMed.
- J. C. Shah, Y. Sadhale and D. M. Chilukuri, Adv. Drug Delivery Rev., 2001, 47, 229 CrossRef CAS PubMed.
- T. Kato, J. Uchida, T. Ichikawa and T. Sakamoto, Angew. Chem., Int. Ed., 2018, 57, 4355 CrossRef CAS PubMed.
- J. W. Goodby, I. M. Saez, S. J. Cowling, V. Görtz, M. Draper, A. W. Hall, S. Sia, G. Cosquer, S.-E. Lee and E. P. Raynes, Angew. Chem., Int. Ed., 2008, 47, 2754 CrossRef CAS PubMed.
- K. V. Axenov and S. Laschat, Materials, 2011, 4, 206 CrossRef CAS PubMed.
- K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643 CrossRef CAS PubMed.
- T. Ichikawa, K. Fujimura, M. Yoshio, T. Kato and H. Ohno, Chem. Commun., 2013, 49, 11746 RSC.
- T. Kobayashi, T. Ichikawa, T. Kato and H. Ohno, Adv. Mater., 2017, 29, 1604429 CrossRef PubMed.
- T. Ichikawa, A. Okafuji, T. Kato and H. Ohno, ChemistryOpen, 2016, 5, 439 CrossRef CAS PubMed.
- T. Matsumoto, A. Ono, T. Ichikawa, T. Kato and H. Ohno, Chem. Commun., 2016, 52, 12167 RSC.
- A. Ono, H. Ohno, T. Kato and T. Ichikawa, Solid State Ionics, 2018, 317, 39 CrossRef CAS.
- D. J. Broer, C. M. W. Bastiaansen, M. G. Debije and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2012, 51, 7102 CrossRef CAS PubMed.
- U. Beginn, G. Zipp and M. Möller, Adv. Mater., 2000, 12, 510 CrossRef CAS.
- H.-K. Lee, H. Lee, Y. H. Ko, Y. J. Chang, N.-K. Oh, W.-C. Zin and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2669 CrossRef CAS PubMed.
- D. Yang, D. F. O'Brien and S. R. Marder, J. Am. Chem. Soc., 2002, 124, 13388 CrossRef CAS PubMed.
- B. A. Pindzola, J. Jin and D. L. Gin, J. Am. Chem. Soc., 2003, 125, 2940 CrossRef CAS PubMed.
- L. Y. Jin, J. Bae, J.-H. Ryu and M. Lee, Angew. Chem., Int. Ed., 2006, 45, 650 CrossRef CAS PubMed.
- M. Yoshio, T. Kagata, K. Hoshino, T. Mukai, H. Ohno and T. Kato, J. Am. Chem. Soc., 2006, 128, 5570 CrossRef CAS PubMed.
- D. Batra, D. N. T. Hay and M. A. Firestone, Chem. Mater., 2007, 19, 4423 CrossRef CAS.
- T. Ichikawa, M. Yoshio, A. Hamasaki, J. Kagimoto, H. Ohno and T. Kato, J. Am. Chem. Soc., 2011, 133, 2163 CrossRef CAS PubMed.
- H. Takeuchi, T. Ichikawa, M. Yoshio, T. Kato and H. Ohno, Chem. Commun., 2016, 52, 13861 RSC.
- M. Henmi, K. Nakatsuji, T. Ichikawa, H. Tomioka, T. Sakamoto, M. Yoshio and T. Kato, Adv. Mater., 2012, 24, 2238 CrossRef CAS PubMed.
- F. M. Menger and C. A. Littau, J. Am. Chem. Soc., 1993, 115, 10083 CrossRef CAS.
- M. Zhou, P. R. Nemade, X. Lu, X. Zeng, E. S. Hatakeyama, R. D. Noble and D. L. Gin, J. Am. Chem. Soc., 2007, 129, 9574 CrossRef CAS PubMed.
- E. S. Hatakeyama, B. R. Wiesenauer, C. J. Gabriel, R. D. Noble and D. L. Gin, Chem. Mater., 2010, 22, 4525 CrossRef CAS.
- G. P. Sorenson, K. L. Coppage and M. K. Mahanthappa, J. Am. Chem. Soc., 2011, 133, 14928 CrossRef CAS PubMed.
- G. P. Sorenson and M. K. Mahanthappa, Soft Mater., 2016, 12, 2408 RSC.
- B. Guo, S. W. Tay, Z. Liu and L. Hong, Polymers, 2012, 4, 1499 CrossRef.
- I. Nicotera, L. Coppola, C. O. Rossi, M. Youssry and G. A. Ranieri, J. Phys. Chem. B, 2009, 113, 13935 CrossRef CAS PubMed.
- A. Siu, J. Schmeisser and S. Holdcroft, J. Phys. Chem. B, 2006, 110, 6072 CrossRef CAS PubMed.
- H. Ohno, K. Fujita and Y. Kohno, Phys. Chem. Chem. Phys., 2015, 17, 14454 RSC.
- T. Ueki and M. Watanabe, Macromolecules, 2008, 41, 3739 CrossRef CAS.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- A. J. Kim, M. S. Kaucher, K. P. Davis, M. Peterca, M. R. Imam, N. A. Christian, D. H. Levine, F. S. Bates, V. Percec and D. A. Hammer, Adv. Funct. Mater., 2009, 19, 2930 CrossRef CAS.
- E. B. Trigg, T. W. Gaines, M. Maréchal, D. E. Moed, P. Rannou, K. B. Wagener, M. J. Stevens and K. I. Winey, Nat. Mater., 2018, 17, 725 CrossRef CAS PubMed.
- A. Kusoglu and A. Z. Weber, Chem. Rev., 2017, 117, 987 CrossRef CAS PubMed.
- Z. Cao, Y. Peng, T. Yan, S. Li, A. Li and G. A. Voth, J. Am. Chem. Soc., 2010, 132, 11395 CrossRef CAS PubMed.
- D. Muonz-Santiburcio, C. Wittekindt and D. Marx, Nat. Commun., 2013, 4, 2349 CrossRef PubMed.
- The molecular structure of N-MZI is shown in the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc00131j |
| This journal is © The Royal Society of Chemistry 2019 |