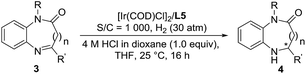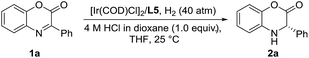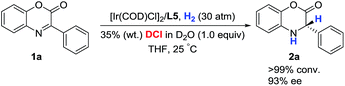Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives with a Brønsted acid cocatalyst†
Zhengyu
Han
a,
Gang
Liu
a,
Rui
Wang
a,
Xiu-Qin
Dong
 *a and
Xumu
Zhang
*a and
Xumu
Zhang
 *ab
*ab
aKey Laboratory of Biomedical Polymers, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei 430072, P. R. China. E-mail: xiuqindong@whu.edu.cn
bDepartment of Chemistry, Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen, Guangdong 518055, P. R. China. E-mail: zhangxm@sustc.edu.cn
First published on 19th March 2019
Abstract
The Ir-catalyzed highly efficient asymmetric hydrogenation of benzoxazinones and derivatives was successfully developed with N-methylated ZhaoPhos L5 as the ligand, which may display a new activation mode with a single anion-binding interaction among the substrate, cocatalyst Brønsted acid and ligand. This synthetic approach afforded a series of chiral dihydrobenzoxazinones and derivatives with excellent results (>99% conversion, 88–96% yields, 91–>99% ee, up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TON). A key to success is the utilization of a strong Brønsted acid as the cocatalyst, such as hydrochloric acid, to form a possible single anion-binding interaction with the substrate and catalyst, which greatly contributed to the improvement of reactivity and enantioselectivity. Importantly, a creative and efficient synthetic route was developed to construct the important intermediate for the potential IgE/IgG receptor modulator through our catalytic methodology system.
500 TON). A key to success is the utilization of a strong Brønsted acid as the cocatalyst, such as hydrochloric acid, to form a possible single anion-binding interaction with the substrate and catalyst, which greatly contributed to the improvement of reactivity and enantioselectivity. Importantly, a creative and efficient synthetic route was developed to construct the important intermediate for the potential IgE/IgG receptor modulator through our catalytic methodology system.
Introduction
Chiral dihydrobenzoxazinones and derivatives are important and unique building blocks in the biologically active molecule discovery process (Fig. 1).1 Chiral dihydrobenzoxazinone derivatives A are potential IgE/IgG receptor modulators for the treatment of autoimmune diseases.2 Chiral 1,2,3,4-tetrahydroquinoxaline compounds B–C and 2,3-dihydro-3,8-diphenylbenzo[1,4]oxazine D are disclosed as active and promising cholesteryl ester transfer protein inhibitors.3–6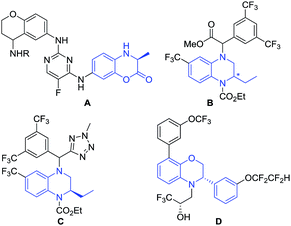 | ||
| Fig. 1 Selected examples of bioactive compounds containing the framework of chiral dihydrobenzoxazinones and derivatives. | ||
Taking into account the growing importance of these compounds, great attention had been paid to the development of efficient enantioselective synthetic methodologies. Among various synthetic approaches for the construction of these chiral dihydrobenzoxazinones and derivatives,7 the direct asymmetric hydrogenation of prochiral benzoxazinones and derivatives was paid great attention with the advantages of high atom economy, a relatively simple procedure and easy work-up. Zhou and co-workers realized Ru-catalyzed biomimetic asymmetric hydrogenation of benzoxazinones and derivatives in the presence of chiral phosphoric acid through the NAD(P)H mode.7c In 2015, Beller and co-workers described asymmetric hydrogenation of benzoxazinones via a relay iron/chiral Brønsted acid catalysis with good to excellent enantioselectivities.7e However, the reactivity of most catalytic systems is not very high with less than 2000 TON (turnover numbers). It is well known that the substrate activation strategy with noncovalent interactions has been widely used in the field of asymmetric organic catalysis, which played an important role in greatly improving the reactivity and stereoselectivity.8,9 The thiourea motif as the hydrogen bonding donor can recognize suitable guest molecules, which usually works on the direct activation of neutral substrates bearing hydrogen bonding acceptor groups.10 Compared with the hydrogen bonding interaction, the anion-binding ion-pairing strategy did not draw much attention until recent development in organocatalysis.9b–d,11 In 2013, our group successfully developed a series of bifunctional bisphosphine–thiourea ligands, which extended the powerful hydrogen-bonding and anion-binding activation strategy in organocatalysis to transition-metal-catalyzed asymmetric hydrogenation, and a variety of functionalized substrates had been hydrogenated well.12 Herein, we successfully realized Ir-catalyzed highly enantioselective hydrogenation of prochiral benzoxazinones and derivatives using N-methylated ZhaoPhos L5 as the ligand, which may exhibit a single anion-binding activation mode among the substrate, cocatalyst Brønsted acid hydrochloric acid and ligand. A variety of hydrogenation products, chiral dihydrobenzoxazinones and derivatives, can be obtained with excellent results (>99% conversion, 88–96% yields, 91–>99% ee), and our catalytic system displayed extremely high activity with up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TON (Scheme 1). In addition, a highly efficient synthetic route was successfully developed to prepare the important intermediate for the potential IgE/IgG receptor modulator with our asymmetric hydrogenation methodology as the key reaction step.
500 TON (Scheme 1). In addition, a highly efficient synthetic route was successfully developed to prepare the important intermediate for the potential IgE/IgG receptor modulator with our asymmetric hydrogenation methodology as the key reaction step.
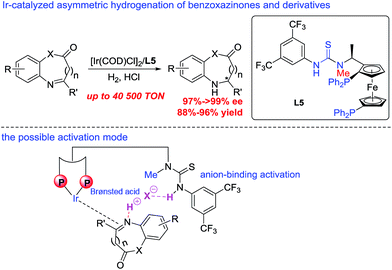 | ||
| Scheme 1 Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives, and the possible activation mode. | ||
Results and discussion
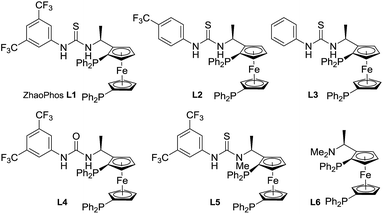
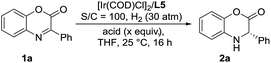
Inspired by the powerful performance of the ligand ZhaoPhos L1 in Rh-catalyzed asymmetric hydrogenation of iminium salts, isoquinoline hydrochloride and N-unprotected indoles,12b,d,e,g we initially investigated the hydrogenation of model substrate 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a catalyzed by Rh(NBD)2BF4/ZhaoPhos L1 in toluene with 1.0 equiv. HCl (4 M in dioxane) as the additive, affording the hydrogenation product 2a with 63% conversion and 82% ee (Table 1, entry 1). Other metal precursors were then screened, and [Ir(COD)Cl]2 was proved to be the best with 90% conversion and 94% ee (Table 1, entry 3). Solvents played an important role in asymmetric reactions, and always affected the reactivity and enantioselectivity. This asymmetric hydrogenation was then conducted in different solvents. Moderate to high conversions and excellent enantioselectivities were observed in CH2Cl2, tetrahydrofuran (THF), 1,4-dioxane, CHCl3, ethyl acetate (EA) and CH3CN (55–>99% conversions, 93–98% ee, Table 1, entries 4–9). And the hydrogenation product 2a can be obtained with the best result in THF (>99% conversion, 98% ee, Table 1, entry 5).| Entry | Metal precursor | Solvent | Conv.b (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.05 mmol 1a in 1.0 mL solvent, S/C = 100, 45 atm H2, 1.0 equiv. HCl (4 M in dioxane), 25 °C, 24 h. b Determined by 1H NMR analysis. c Determined by HPLC analysis using a chiral stationary phase. | ||||
| 1 | Rh(NBD)2BF4 | Toluene | 63 | 82 |
| 2 | [Rh(COD)Cl]2 | Toluene | 71 | 64 |
| 3 | [Ir(COD)Cl]2 | Toluene | 90 | 94 |
| 4 | [Ir(COD)Cl]2 | CH2Cl2 | 97 | 93 |
| 5 | [Ir(COD)Cl]2 | THF | >99 | 98 |
| 6 | [Ir(COD)Cl]2 | 1,4-Dioxane | 60 | 95 |
| 7 | [Ir(COD)Cl]2 | CHCl3 | 95 | 95 |
| 8 | [Ir(COD)Cl]2 | Ethyl acetate | 95 | 93 |
| 9 | [Ir(COD)Cl]2 | CH3CN | 55 | 94 |
A series of bisphosphine–(thio)urea ligands (Fig. 2) were then applied to this Ir-catalyzed asymmetric hydrogenation of 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a in THF. Full conversions and excellent enantioselectivities can be obtained in the presence of ZhaoPhos L1 and N-methylated ZhaoPhos L5, and N-methylated ZhaoPhos L5 provided higher enantioselectivity (>99% conversion, 98–99% ee, Table 2, entries 1 and 5). The ligand L2 containing one trifluoromethyl group and ligand L3 without any trifluoromethyl group on the phenyl ring provided poor conversions and excellent enantioselectivities (33–45% conversions, 97% ee, Table 2, entries 2 and 3). The ligand L4 displayed very poor reactivity and enantioselectivity, which changed the thiourea motif to the urea motif (11% conversion, 13% ee, Table 2, entry 4). In addition, no reaction was observed in the presence of ligand L6 without the thiourea motif (Table 2, entry 6). This indicated that the thiourea motif may make a great contribution to activate our substrate through anion-binding interactions. When the catalyst loading is decreased from 1.0 mol% to 0.2 mol%, full conversion and excellent enantioselectivity can be obtained with ligand L5 (>99% conversion, 99% ee, Table 2, entry 8). Interestingly, it is better than ZhaoPhos L1 (65% conversion, 98% ee, Table 2, entry 7 vs. entry 8). It is possible that a single anion-binding interaction in a precise position is sufficient in this asymmetric reaction, which is different from previous reports.11,12b,d,e,g
| Entry | Ligand | Conv.b (%) | eec (%) |
|---|---|---|---|
| a Reaction conditions: 0.05 mmol 1a in 1.0 mL THF, S/C = 100, 45 atm H2, 1.0 equiv. HCl (4 M in dioxane), 25 °C, 24 h. b Determined by 1H NMR analysis. c Determined by HPLC with a chiral stationary phase. d 30 atm H2, S/C = 500, 16 h. | |||
| 1 | ZhaoPhos L1 | >99 | 98 |
| 2 | L2 | 45 | 97 |
| 3 | L3 | 33 | 97 |
| 4 | L4 | 11 | 13 |
| 5 | L5 | >99 | 99 |
| 6 | L6 | NR | NA |
| 7d | ZhaoPhos L1 | 65 | 98 |
| 8d | L5 | >99 | 99 |
A series of representative Brønsted acids were then deeply inspected in this Ir/ligand L5-catalyzed asymmetric hydrogenation of 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a, and we found that there is a significant correlation between the reactivity, enantioselectivity and acid strength of the Brønsted acid. When hydrochloric acid, HCl (4 M in dioxane), was switched to a strong acid, CF3SO3H, this hydrogenation proceeded smoothly to provide the same result with full conversion and 99% ee (Table 3, entries 1 and 7). CF3COOH and H3PO4 gave high conversions and good enantioselectivities (95–>99% conversions, 81–84% ee, Table 3, entries 2 and 3). As expected, the weaker acids HCOOH and AcOH afforded poor reactivities and enantioselectivities (21–30% conversions, 57–58% ee, Table 3, entries 4–5). In addition, the amount of Brønsted acid HCl was further investigated in this asymmetric hydrogenation. We found that the amount of HCl had little effect on the reactivity and enantioselectivity, and the reaction results remained excellent, when the amount of HCl was gradually reduced from 2.0 equiv. to 0.01 equiv. (>99% conversion, 95–99% ee, Table 3, entries 6–10). However, no reaction was observed in the absence of the cocatalyst HCl (Table 3, entry 11), which showed that HCl was involved in this transformation with great importance. These results also displayed that the acid strength of the Brønsted acid cocatalyst is very important to achieve excellent results in this asymmetric hydrogenation, which may affect the formation of anion-binding activation among the substrate, Brønsted acid and ligand. To our delight, this asymmetric hydrogenation still proceeded smoothly with the same result even when catalyzed by only the 0.1 mol% [Ir(COD)Cl]2/ligand L5 catalyst with 1.0 equiv. HCl (4 M in dioxane) (Table 3, entry 12).
| Entry | Acid | pKa (in H2O)b | x (equiv.) | Conv.c (%) | eed (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.05 mmol substrate 1a in 1.0 mL THF, 30 atm H2, 25 °C; S/C = 100, 16 h. b The pKa data are available from Evans's pKa table. c Conversion was determined by 1H NMR analysis. d ee was determined by HPLC with a chiral stationary phase. e S/C = 1000. NR is no reaction, NA is not available. | |||||
| 1 | CF3SO3H | −14 | 1.0 | >99 | 99 |
| 2 | CF3COOH | −0.25 | 1.0 | >99 | 84 |
| 3 | H3PO4 | 2.12 | 1.0 | 95 | 81 |
| 4 | HCOOH | 3.77 | 1.0 | 30 | 58 |
| 5 | CH3COOH | 4.76 | 1.0 | 21 | 57 |
| 6 | HCl | −8 | 2.0 | >99 | 99 |
| 7 | HCl | −8 | 1.0 | >99 | 99 |
| 8 | HCl | −8 | 0.5 | >99 | 99 |
| 9 | HCl | −8 | 0.1 | >99 | 99 |
| 10 | HCl | −8 | 0.01 | >99 | 95 |
| 11 | HCl | −8 | 0 | NR | NA |
| 12e | HCl | −8 | 1.0 | >99 | 99 |
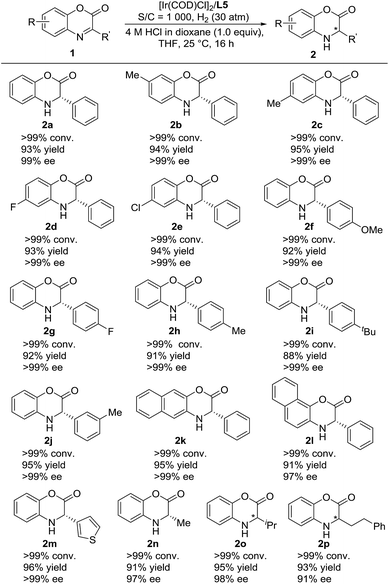
We then continued to investigate the substrate generality of this Ir-catalyzed asymmetric hydrogenation of prochiral benzoxazinones using N-methylated ZhaoPhos L5 as the ligand. These reaction results are summarized in Table 4. A series of prochiral benzoxazinones were hydrogenated smoothly in this catalytic system to prepare various chiral dihydrobenzoxazinones with excellent results (>99% conversion, 88–96% yields, 91–>99% ee). The benzoxazinone substrates with different substituents on the benzo ring (1b–1e) were hydrogenated well with >99% conversion, 93–95% yields and >99% ee. In addition, regardless of the position or electronic properties of the substituents on the phenyl ring of the benzoxazinone substrates (1f–1j), the corresponding hydrogenation products (2f–2j) were obtained with 88–95% yields and >99% ee. The naphthyl substituted substrates with bulky steric hindrance (1k–1l) were obtained smoothly with excellent results (91–95% yields, 97–>99% ee). It is worth noting that the heteroaromatic substrate 3-(thiophen-3-yl)-2H-benzo[b][1,4]oxazin-2-one (1m) was well tolerated to produce the desired product (2m) with 96% yield and >99% ee. Moreover, the alkyl substrates (1n–1p) were hydrogenated smoothly with excellent results (>99% conversion, 91–95% yields, and 91–98% ee).
a Unless otherwise noted, all reactions were carried out with a [Ir(COD)Cl]2/ligand L5/substrate 1 (0.05 mmol) ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in 1.0 mL THF with 1.0 equiv. HCl (4 M in dioxane) at room temperature under 30 atm H2 for 16 h. Conversion was determined by 1H NMR analysis, ee was determined by HPLC with a chiral stationary phase, and the yield was isolated yield. 1000 in 1.0 mL THF with 1.0 equiv. HCl (4 M in dioxane) at room temperature under 30 atm H2 for 16 h. Conversion was determined by 1H NMR analysis, ee was determined by HPLC with a chiral stationary phase, and the yield was isolated yield.
|
|---|
|
Encouraged by the success of the Ir/N-methylated ZhaoPhos L5-catalyzed asymmetric hydrogenation of prochiral benzoxazinones, the asymmetric hydrogenation of several quinoxalinones was subsequently explored. As shown in Table 5, the asymmetric hydrogenation of quinoxalinones (3a–3c) proceeded efficiently, affording the desired products (4a–4c) with >99% conversion, 90–91% yields and 98–>99% ee (Table 5, entries 1–3). To our delight, the hydrogenation of benzo-seven-membered cyclic imine 4-phenyl-1H-benzo[b][1,4]diazepin-2(3H)-one (3d) proceeded smoothly to obtain the hydrogenation product (4d) with excellent results (>99% conversion, 91% yield, >99% ee, Table 5, entry 4). In addition, our catalytic system displayed extremely high activity in this asymmetric hydrogenation, and when the catalyst loading was reduced from 0.1 mol% to 0.02 mol% (S/C = 5000), the asymmetric hydrogenation of benzo-seven-membered cyclic imine (3d) can be finished with >99% conversion, 93% yield and >99% ee (Table 5, entry 5).
| Entry | R | R′ | n | Sub. | Prod. | Conv.b (%) | Yieldc (%) | eed (%) |
|---|---|---|---|---|---|---|---|---|
a Unless otherwise noted, all reactions were carried out with a [Ir(COD)Cl]2/ligand L5/substrate 3 (0.05 mmol) ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in 1.0 mL THF at room temperature under 30 atm H2 for 16 h.
b Determined by 1H NMR analysis.
c Isolated yield.
d Determined by HPLC analysis using a chiral stationary phase.
e S/C = 5000. 1000 in 1.0 mL THF at room temperature under 30 atm H2 for 16 h.
b Determined by 1H NMR analysis.
c Isolated yield.
d Determined by HPLC analysis using a chiral stationary phase.
e S/C = 5000.
|
||||||||
| 1 | H | Ph | 0 | 3a | 4a | >99 | 90 | >99 |
| 2 | H | Et | 0 | 3b | 4b | >99 | 91 | 99 |
| 3 | Me | Ph | 0 | 3c | 4c | >99 | 91 | 98 |
| 4 | H | Ph | 1 | 3d | 4d | >99 | 91 | >99 |
| 5e | H | Ph | 1 | 3d | 4d | >99 | 93 | >99 |
In order to further investigate the high activity of this Ir/N-methylated ZhaoPhos L5 catalytic system, the model substrate 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a was applied in this asymmetric hydrogenation with very low catalyst loading under 40 atm H2. These results are summarized in Table 6. The hydrogenation product 2a can be obtained with full conversion, 91% yield and 99% ee when the catalyst loading is decreased from 0.1 mol% (S/C = 1000) to 0.01 mol% (S/C = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (Table 6, entries 1 and 2). Our catalytic system still exhibited high activity and excellent enantioselectivity with further diminution of the catalyst loading to 0.0025 mol% (S/C = 40
000) (Table 6, entries 1 and 2). Our catalytic system still exhibited high activity and excellent enantioselectivity with further diminution of the catalyst loading to 0.0025 mol% (S/C = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), affording the product 2a with excellent results (>99% conversion, 89% yield and 99% ee, Table 6, entry 3). In addition, good conversion and excellent enantioselectivity can be obtained even in the presence of 0.002 mol% (S/C = 50
000), affording the product 2a with excellent results (>99% conversion, 89% yield and 99% ee, Table 6, entry 3). In addition, good conversion and excellent enantioselectivity can be obtained even in the presence of 0.002 mol% (S/C = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) catalyst (81% conversion, TON = 40
000) catalyst (81% conversion, TON = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, 72% yield, 99% ee, Table 6, entry 4).
500, 72% yield, 99% ee, Table 6, entry 4).
| Entry | S/C | Time (h) | Conv.b (%) | Yieldc (%) | eed (%) |
|---|---|---|---|---|---|
| a Reaction conditions: substrate 1a (5.0 mmol) in 20.0 mL THF, 40 atm H2, 1.0 equiv. HCl (4 M in dioxane), 25 °C. b Conversion was determined by 1H NMR analysis. c Isolated yield. d ee was determined by HPLC with a chiral stationary phase. | |||||
| 1 | 1000 | 16 | >99 | 93 | 99 |
| 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
48 | >99 | 91 | 99 |
| 3 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72 | >99 | 89 | 99 |
| 4 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
72 | 81 | 72 | 99 |
The deuterium-labeling experiment was conducted to verify the hydrogen atom source of the hydrogenation product dihydrobenzoxazinone. As shown in Scheme 2, the asymmetric hydrogenation of 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a proceeded in the presence of DCl in D2O, and the product 2a without deuterium was obtained. This observation displayed that the hydrogen atom of the hydrogenation product was from H2 and not HCl to a great extent.
A series of asymmetric reductions of model substrate 3-phenyl-2H-benzo[b][1,4]oxazin-2-one 1a were performed using ligand L5 with varying ee values. As shown in Fig. 3, there is no nonlinear effect in this transformation, which indicated that there should be no catalyst self-aggregation or ligand–substrate agglomeration in this catalytic system.13
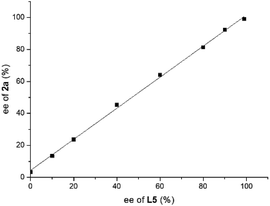 | ||
| Fig. 3 Investigation of the nonlinear effect of the hydrogenation of substrate 1a using ligand L5 with different ee values. | ||
IgE/IgG is one of the most important immunoglobulins, which are associated with the release of vasoactive amines stored in basophils and tissue mast cell granules to cause allergic effects. Our catalytic hydrogenation methodology showed great synthetic application. As shown in Scheme 3, 7-((2-chloro-5-fluoropyrimidin-4-yl)amino)-3-methyl-2H-benzo[b][1,4]oxazin-2-one 1q was efficiently obtained within four steps using the easily commercially available 2-amino-5-nitrophenol as the starting material.14 The Ir-catalyzed asymmetric hydrogenation of compound 1q was efficiently accomplished to obtain the chiral compound 2q with 92% yield and 91% ee, which is the key intermediate to construct the potential IgE/IgG receptor modulator for the treatment of autoimmune diseases.2
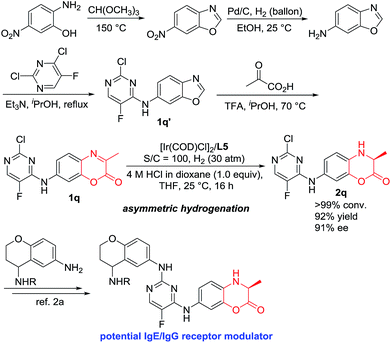 | ||
| Scheme 3 Synthetic application for the construction of the key intermediate of biologically active molecules. | ||
Conclusions
In summary, the Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives was successfully developed with N-methylated ZhaoPhos L5 as the ligand through a new anion-binding activation strategy. A series of chiral dihydrobenzoxazinones and derivatives were obtained with excellent results (88–96% yields, 91–>99% ee, up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TON). The utilization of a strong Brønsted acid, such as hydrochloric acid even at a small catalytic amount, as the cocatalyst is very important to form a single anion-binding interaction with the substrate and ligand L5, which strongly improved the reactivity. Moreover, a highly efficient synthetic route was developed to construct the key intermediate to synthesize a potential IgE/IgG receptor modulator through our catalytic methodology system.
500 TON). The utilization of a strong Brønsted acid, such as hydrochloric acid even at a small catalytic amount, as the cocatalyst is very important to form a single anion-binding interaction with the substrate and ligand L5, which strongly improved the reactivity. Moreover, a highly efficient synthetic route was developed to construct the key intermediate to synthesize a potential IgE/IgG receptor modulator through our catalytic methodology system.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Grant No. 21432007 and 21502145), Wuhan Morning Light Plan of Youth Science and Technology (Grant No. 2017050304010307), Shenzhen Nobel Prize Scientists Laboratory Project (Grant No. C17783101) and the Fundamental Research Funds for and the Central Universities (Grant No. 2042018kf0202). The Program of Introducing Talents of Discipline to Universities of China (111 Project) is also appreciated.Notes and references
- (a) F. Grant, S. Bartulis, L. Brogley, M. S. Dappan, R. Kasar, A. Khan, M. Neitzel, M. A. Pleiss, E. D. Thorsett and J. Tucker, PCT Int. Appl. WO 2003093245 A1, 2003; (b) Z. Jones, R. Groneberg, M. Drew and C. T. Eary, U.S. Pat., 20050282812, 2005; (c) D.-S. Su and M. G. Bock, U.S. Pat. Appl. Publ. US 20050020591 A1, 2005; (d) J. Ilaš, P. Š. Anderluh, M. S. Dolenc and D. Kikelj, Tetrahedron, 2005, 61, 7325–7348 CrossRef; (e) C. J. Abraham, D. H. Paull, M. T. Scerba, J. W. Grebinski and T. Lectka, J. Am. Chem. Soc., 2006, 128, 13370–13371 CrossRef CAS PubMed; (f) D. V. Smil, S. Manku, Y. A. Chantigny, S. Leit, A. Wahhab, T. P. Yan, M. Fournel, C. Maroun, Z. Li, A.-M. Lemieux, A. Nicolescu, J. Rahil, S. Lefebvre, A. Panetta, J. M. Besterman and R. Déziel, Bioorg. Med. Chem. Lett., 2009, 19, 688–692 CrossRef CAS PubMed; (g) M. Rueping, M. Stoeckel, E. Sugiono and T. Theissmann, Tetrahedron, 2010, 66, 6565–6568 CrossRef CAS.
- (a) R. Singh, A. Argade, D. G. Payan, J. Clough, H. Keim, S. Bhamidipati, C. Sylvain and H. Li, WO2004014382 A1, 2004; (b) R. Singh, A. Argade, D. G. Payan, J. Clough, H. Keim, S. Bhamidipati, C. Sylvain and H. Li, US 7517886B2, 2009.
- A. Kuhl, P. Kolkhof, L. Telan, J. G. Peters, K. Lustig, R. Kast, K. Muenter, J. P. Stasch and H. Tinel, WO 2005028451, 2005.
- S. Fleischer, S. Zhou, S. Werkmeister, K. Junge and M. Beller, Chem.–Eur. J., 2013, 19, 4997–5003 CrossRef CAS PubMed.
- C. T. Eary, Z. S. Jones, R. D. Groneberg, L. E. Burgess, D. A. Mareska, M. D. Drew, J. F. Blake, E. R. Laird, D. Balachari, M. O'Sullivan, A. Allen and V. Marsh, Bioorg. Med. Chem. Lett., 2007, 17, 2608–2613 CrossRef CAS PubMed.
- A. Wang, C. P. Prouty, P. D. Pelton, M. Yong, K. T. Demarest, W. V. Murray and G.-H. Kuo, Bioorg. Med. Chem. Lett., 2010, 20, 1432–1435 CrossRef CAS PubMed.
- (a) R. I. Storer, D. E. Carrera and D. W. C. Macmillan, J. Am. Chem. Soc., 2006, 128, 84–86 CrossRef CAS PubMed; (b) X. Chen, Y. Zheng, C. Shu, W. Yuan, B. Liu and X. Zhang, J. Org. Chem., 2011, 76, 9109–9115 CrossRef CAS PubMed; (c) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442–2448 CrossRef CAS PubMed; (d) J. L. Nunez-Rico and A. Vidal-Ferran, Org. Lett., 2013, 15, 2066–2069 CrossRef CAS PubMed; (e) L.-Q. Lu, Y. Li, K. Junge and M. Beller, J. Am. Chem. Soc., 2015, 137, 2763–2768 CrossRef CAS PubMed; (f) Y. Zhang, R. Zhao, R. L.-Y. Bao and L. Shi, Eur. J. Org. Chem., 2015, 3344–3351 CrossRef CAS; (g) X. Zhang, B. Xu and M.-H. Xu, Org. Chem. Front., 2016, 3, 944–948 RSC.
- Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, 1999 Search PubMed.
- For reviews, see: (a) R. R. Knowles and E. N. Jacobsen, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20678–20685 CrossRef CAS PubMed; (b) R. J. Phipps, G. L. Hamilton and F. D. Toste, Nat. Chem., 2012, 4, 603–614 CrossRef CAS PubMed; (c) K. Brak and E. N. Jacobsen, Angew. Chem., Int. Ed., 2013, 52, 534–561 CrossRef CAS PubMed; (d) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518–533 CrossRef CAS PubMed.
- (a) M. S. Sigman and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 4901–4902 CrossRef CAS; (b) A. G. Wenzel and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 12964–12965 CrossRef CAS PubMed; (c) T. Okino, Y. Hoashi and Y. Takemoto, Tetrahedron Lett., 2003, 44, 2817–2821 CrossRef CAS; (d) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 10558–10559 CrossRef CAS PubMed; (e) D. J. Maher and S. J. Connon, Tetrahedron Lett., 2004, 45, 1301–1305 CrossRef CAS; (f) H. Huang and E. N. Jacobsen, J. Am. Chem. Soc., 2006, 128, 7170–7171 CrossRef CAS PubMed; (g) M. P. Lalonde, Y. Chen and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 6366–6370 CrossRef CAS PubMed; (h) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520–1543 CrossRef CAS PubMed; (i) X. Yu and W. Wang, Chem.–Asian J., 2008, 3, 516–532 CrossRef CAS PubMed; (j) P. M. Pihko, Hydrogen Bonding in Organic Synthesis, Wiley, 2009 CrossRef.
- (a) Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187–1198 RSC; (b) C. Zhao, C. A. Sojdak, W. Myint and D. Seidel, J. Am. Chem. Soc., 2017, 139, 10224–10227 CrossRef CAS PubMed; (c) K. Eichstaedt, J. Jaramillo-Garcia, D. A. Leigh, V. Marcos, S. Pisano and T. A. Singleton, J. Am. Chem. Soc., 2017, 139, 9376–9381 CrossRef CAS PubMed; (d) A. E. Wendlandt, P. Vangal and E. N. Jacobsen, Nature, 2018, 556, 447–451 CrossRef CAS PubMed.
- Selected examples: (a) Q. Zhao, S. Li, K. Huang, R. Wang and X. Zhang, Org. Lett., 2013, 15, 4014–4017 CrossRef CAS PubMed; (b) Q. Zhao, J. Wen, R. Tan, K. Huang, P. Metola, R. Wang, E. V. Anslyn and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 8467–8470 CrossRef CAS PubMed; (c) Z. Han, P. Li, Z. Zhang, C. Chen, Q. Wang, X.-Q. Dong and X. Zhang, ACS Catal., 2016, 6, 6214–6218 CrossRef CAS; (d) J. Wen, R. Tan, S. Liu, Q. Zhao and X. Zhang, Chem. Sci., 2016, 7, 3047–3051 RSC; (e) P. Li, Y. Huang, X. Hu, X.-Q. Dong and X. Zhang, Org. Lett., 2017, 19, 3855–3858 CrossRef CAS PubMed; (f) X. Li, C. You, Y. Yang, Y. Yang, P. Li, G. Gu, L. W. Chung, H. Lv and X. Zhang, Chem. Sci., 2018, 9, 1919–1924 RSC; (g) J. Wen, X. Fan, R. Tan, H.-C. Chien, Q. Zhou, L. W. Chung and X. Zhang, Org. Lett., 2018, 20, 2143–2147 CrossRef CAS PubMed; (h) X. Yin, Y. Huang, Z. Chen, Y. Hu, L. Tao, Q. Zhao, X.-Q. Dong and X. Zhang, Org. Lett., 2018, 20, 4173–4177 CrossRef CAS PubMed.
- (a) D. Guillaneux, S.-H. Zhao, O. Samuel, D. Rainford and H. B. Kagan, J. Am. Chem. Soc., 1994, 116, 9430–9439 CrossRef CAS; (b) C. Girard and H. B. Kagan, Angew. Chem., Int. Ed., 1998, 37, 2922–2959 CrossRef.
- (a) S. Yan, L. Ye, M. Liu, J. Chen, J. Ding, W. Gao, X. Huang and H. Wu, RSC Adv., 2014, 4, 16705–16709 RSC; (b) D.-J. Zhang, W.-F. Sun, Z.-J. Zhong, R.-M. Gao, H. Yi, Y.-H. Li, Z.-G. Peng and Z.-R. Li, Molecules, 2014, 19, 925–939 CrossRef PubMed; (c) Y. C. Teo, S. N. Riduan and Y. Zhang, Green Chem., 2013, 15, 2365–2368 RSC; (d) Y. Kamada, N. Sakai, S. Sogabe, K. Ida, H. Oki, K. Sakamoto, W. Lane, G. Snell, M. Iida, Y. Imaeda, J. Sakamoto and J. Matsui, J. Med. Chem., 2017, 60, 4358–4368 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c8sc05797d |
| This journal is © The Royal Society of Chemistry 2019 |