Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid chemiexcitation of phenoxy-dioxetane luminophores yields ultrasensitive chemiluminescence assays†
Nir
Hananya‡
a,
Jolene P.
Reid‡
b,
Ori
Green
a,
Matthew S.
Sigman
*b and
Doron
Shabat
 *a
*a
aSchool of Chemistry, Faculty of Exact Sciences, Tel Aviv University, Tel Aviv 69978, Israel. E-mail: chdoron@post.tau.ac.il
bDepartment of Chemistry, University of Utah, 315 South 1400 East, Salt Lake City, Utah 84112, USA. E-mail: sigman@chem.utah.edu
First published on 19th November 2018
Abstract
The utility of dioxetane-based chemiluminescent probes in biosensing and bioimaging is being increasingly recognized. While phenoxy-dioxetane luminophores with fast chemiexcitation kinetics are highly desired, current luminophores suffer from slow chemiexcitation. Herein we describe a rational, computationally-supported design of phenoxy-dioxetanes with fast chemiexcitation kinetics. These new luminophores were designed to contain a substituent that promotes rapid chemiexcitation, emitting light up to 100-fold faster than currently known dioxetanes. We demonstrate the superiority of the new phenoxy-dioxetanes by preparing three chemiluminescent probes for NAD(P)H, which differ from each other in the rate of the chemiexcitation. Comparison of these probes reveals a correlation between the chemiexcitation rate and the probe sensitivity. We anticipate that these new phenoxy-dioxetanes could serve as an ideal platform for designing chemiluminescence probes with enhanced sensitivity for numerous bioassays.
Introduction
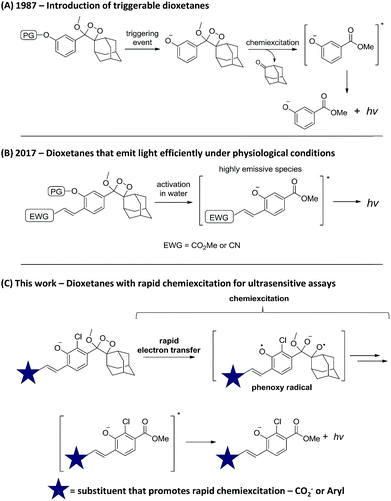
Chemiluminescence (CL) is increasingly recognized as a powerful tool for sensing and imaging.1,2 Its main advantage over fluorescence lies in the fact that irradiation by an external light source is not required, thus the background signal is extremely low and the obtained sensitivity is considerably enhanced.3,4 Two major breakthroughs in the chemistry of chemiluminescent compounds have established this strategy as a general and efficient tool for bioimaging. The first, which took place >30 years ago, was the introduction of triggerable dioxetanes by Schaap.5 With these compounds, light emission is a consequence of phenolate formation following phenol deprotection. Thus, by selecting the appropriate phenol-protecting group, one can directly determine the event that triggers light emission (Scheme 1A). This modularity of the dioxetanes enabled the preparation of numerous dioxetane-based chemiluminescent probes;6–8 the most recognized of which is the alkaline phosphatase substrate CDP-Star®.9,10 The second breakthrough was reported last year by our group, when a subtle change in the phenoxy-dioxetanes structure enabled the development of remarkably efficient emitters under physiological conditions.11 Specifically, it was demonstrated that by introducing an electron-withdrawing acrylic group at the ortho position of the phenol donor, a 3000-fold increase in CL quantum yield (ΦCL) could be obtained (Scheme 1B). This new class of phenoxy-dioxetane-based luminophores has been applied by us and others to construct CL probes for detection and imaging of various enzymes and analytes.12–18 Herein, we present next generation phenoxy-dioxetanes, which through a rational, computationally-supported design were equipped with substituents that promote an increased rate of chemiexcitation (Scheme 1C).Rapid chemiexcitation is highly desirable, as it is anticipated to improve the sensitivity of chemiluminescent analytical bioassays. As photons are being released within a short period of time, signal to background ratio (S/B) is higher and sensitivity is better.19 Thus, the new luminophores reported here were specifically designed to undergo fast light-emitting chemiexcitation following phenolate formation. Furthermore, we demonstrate how this improvement of light-emission kinetics yields CL assays with superior sensitivity.
Results
Rational design and synthesis of phenoxy-dioxetane luminophores with rapid chemiexcitation
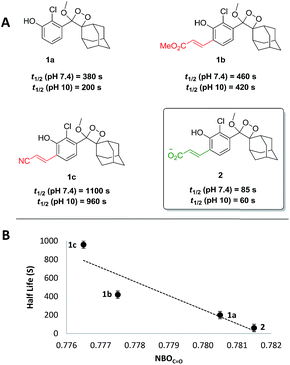
As a starting point, the chemiexcitation kinetics of recently reported luminophores 1b and 1c,11 which are sufficiently bright under physiological conditions, were evaluated.§ Noticeably, the observed kinetics are relatively slow, in comparison to the simple unsubstituted luminophore 1a (Fig. 1A). We therefore sought to rationally modify the electronic structure of the luminophore in order to achieve faster kinetics, based on mechanistic analysis of chemiexcitation. Although the details of the chemiexcitation process remain controversial,20–23 there is a consensus that its first step is an electron transfer from the phenolate donor to the dioxetane moiety, generating a phenoxy-radical species (see Scheme 1C; see ref. 23 for a comprehensive review). Therefore, the rate of the chemiexcitation is controlled at least by two effects. First, increased electron density of the phenolate should promote faster chemiexcitation, while the converse, presence of an electron-withdrawing substituent, retards this process. This is consistent with the slower chemiexcitation of the substituted phenoxy-dioxetanes 1b and 1c, compared to the unsubstituted 1a. Second, as the radical-stabilizing nature of the phenoxy moiety is increased, the chemiexcitation rate is accelerated.As such, our initial efforts to enhance chemiexcitation rate were focused on increasing the phenolate electron density. Therefore, phenol-dioxetane 2 was prepared with an acrylic acid ortho substituent. Under physiological conditions the acrylic acid exists in the form of acrylate anion, which is the least electron-withdrawing substituent among all other acrylate derivatives. The CL kinetic profile of 2 under physiological conditions was measured, and, as expected, was found to be 5.5-fold faster than 1b and 13-fold faster than 1c (Fig. 1A, t1/2 = 85 s at pH 7.4). Since the active species in the chemiexcitation process is the phenolate anion, chemiexcitation kinetics were further measured at pH 10 (Fig. 1A). In this manner, the chemiexcitation rate of the phenolate species could be evaluated, eliminating the effect of phenol pKa differences. Changing the pH from 7.4 to 10 did not significantly impact the general trend among the half-lives of luminophores, thus confirming that the differences in the chemiexcitation rate are intrinsic and do not stem from different pKa values.
To further understand the effect of the substituent's electron-withdrawing character on the rate of the chemiexcitation, a study of the phenolate chemiexcitation rate as a function of electronic effects of luminophores was investigated. Hammett values have typically been employed to afford a quantitative measure of the electron withdrawing/donating ability of aromatic substituents.24,25 Previous efforts have shown that NBO charges and IR carbonyl stretching frequencies can be used as an alternative to empirically derived Hammett values, σp.26 Considering the substituents under evaluation, we employed the NBOC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, the charge on the benzoic acid moiety (see ESI for details†).¶ As depicted in Fig. 1B, NBOC
O, the charge on the benzoic acid moiety (see ESI for details†).¶ As depicted in Fig. 1B, NBOC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O values correlate well with chemiexcitation kinetics; as the substituent is more electron withdrawing, the chemiexcitation rate of the luminophore becomes slower.
O values correlate well with chemiexcitation kinetics; as the substituent is more electron withdrawing, the chemiexcitation rate of the luminophore becomes slower.
Encouraged by these results, we sought to design luminophores with even faster chemiexcitation kinetics. Replacing the acryl-substituents on the phenoxy-dioxetanes with styryl-substituents (Fig. 2A) was hypothesized to be a handle to improve the performance for the following two reasons. First, the phenyl ring can serve as a modular platform for modifying the electron withdrawing/donating nature of the conjugated substituent. Second, extended conjugation of the styryl substituent is anticipated to increase the radical stabilizing nature of the phenoxy moiety. As noted above, a more stabilized phenoxy radical should lead to faster chemiexcitation (see Scheme 1C). To test this hypothesis, luminophores 3a–c were synthesized, based on a Wittig reaction between aldehyde 4 and substituted benzyltriphenyl-phosphonium bromides 5a–c (Fig. 2B, see ESI for synthetic details†). A carboxylic acid was incorporated in each luminophore, to increase aqueous solubility. The CL kinetic profiles of luminophores 3a–c were measured and compared to those of luminophores 1a–c and 2. Remarkably, chemiexcitation of the styryl derivatives occurred extremely rapidly. For example, 3b (as a median representative of the styryl derivatives) has a half-life shorter by more than 200-fold than that of 1c, and by more than 15-fold than that of 2 (Fig. 2C). It should be noted that this outstanding performance of luminophores 3a–c appears not only at pH 10 but also under physiologically relevant conditions (pH 7.4; see Table 1 below. For full kinetic data see ESI†).
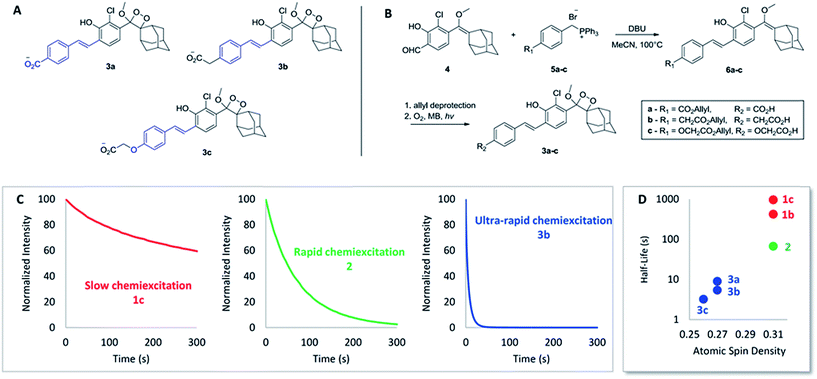 | ||
| Fig. 2 (A) Structure of styryl-substituted luminophores 3a–c. (B) General synthetic pathway of 3a–c. (C) CL kinetic profiles of 1c, 2 and 3b. 20 μL of luminophore solution in DMSO [10 μM] were injected to 180 μL of aqueous buffer and CL was measured immediately. See ESI for full data.† (D) Relationship between radical-stabilizing character of the luminophores and their chemiexcitation kinetics. | ||
| λmax (nm) | t 1/2,7.4 (s) | t 1/2,10 (s) | Φ CL (%) | NBOC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
O–H BDEa (kcal mol−1) | Spin Densitya | |
|---|---|---|---|---|---|---|---|
| a Simplified compounds were used for calculations. See ESI for details. | |||||||
| 1a | 470 | 380 | 200 | 0.003 | 0.780 | 88.1 | 0.34 |
| 1b | 550 | 460 | 420 | 2.3 | 0.777 | 86.7 | 0.31 |
| 1c | 525 | 1100 | 960 | 13.8 | 0.776 | 86.9 | 0.31 |
| 2 | 510 | 85 | 60 | 9.8 | 0.781 | 85.0 | 0.31 |
| 3a | 535 | 8.4 | 7.0 | 4.2 | 0.780 | 84.9 | 0.27 |
| 3b | 500 | 5.1 | 4.2 | 1.4 | 0.779 | 83.8 | 0.27 |
| 3c | 490 | 3.2 | 2.7 | 0.6 | 0.780 | 82.6 | 0.26 |
To further explore the relationship between the phenoxy-radical stability and the chemiexcitation kinetics, radical stabilizing properties of the acryl- and the styryl-substituted dioxetanes were examined using DFT calculations. We used a slightly simplified structural surrogate in our calculations, focusing on the conserved phenol conjugated π-system wherein the methoxy-dioxetane-adamantane portion was replaced with a hydrogen (see ESI†). The O–H bond dissociation energies (BDEs) of these compounds were calculated at the SMD-water-UM06-2X/def2-TZVP level of theory (see ESI for details†). It was determined that the introduction of a phenyl group has a dramatic radical stabilizing effect, as the styryl-substituted phenoxy-radicals (BDE = 82.6–84.9 kcal mol−1) were found to be more stable than the acryl-substituted series (BDE = 85.0–86.9 kcal mol−1). To further clarify and contextualize these energetic results, natural bond orbitals were used to evaluate the atomic spin distribution in the molecule. As expected, the atomic spin density at the radical center for the acrylate series (0.31) is significantly higher than those derived from the styryl-substituted compounds (0.26–0.27), consistent with the BDE results. Altogether the calculations support our assumption that the styryl substituents better stabilize the phenoxy radical, compared to the acryl substituents. An evident relationship is revealed between the chemiexcitation kinetics and the radical stability, supporting our hypothesis that the styryl substituents promote rapid chemiexcitation by stabilizing the radical to a greater extent, as opposed to the acryl derivatives (Fig. 2D).
Table 1 summarizes the CL properties and the computational parameters of acryl- and styryl-substituted luminophores. Overall, the combination of experiment and calculation suggest that radical stability can explain the differences between the kinetics of acryl- and styryl-substituted luminophores. However, it is also clear that radical stability is not the sole structural effect that determines chemiexcitation kinetics. For example, BDEs and atomic spin density calculations clearly show that the unsubstituted derivative 1a stabilizes the radical the least (BDE = 88.1 kcal mol−1, spin density = 0.34); however, its chemiexcitation rate is faster than those of 1b and 1c with greater radical stabilizing properties (BDE = 86.7–86.9 kcal mol−1, spin density = 0.31). As proposed above, polar effects (electron donation/withdrawal) also have an impact on the kinetics, and this can explain the trend well when radical stabilizing groups are not present (see Fig. 1). Both effects are difficult to delineate, since BDEs and NBOC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O values are sensitive to similar structural components in many cases. In sum, the performed calculations clearly support our rational design of dioxetanes with rapid chemiexcitation.
O values are sensitive to similar structural components in many cases. In sum, the performed calculations clearly support our rational design of dioxetanes with rapid chemiexcitation.
Our strategy successfully resulted in phenoxy-dioxetanes with improved chemiexcitation kinetics. However, for these luminophores to be effective in the construction of chemiluminescent probes, it was essential to verify that they maintain sufficiently high ΦCL. ΦCL of the luminophores was examined by measuring their total light emission. All new luminophores exhibit ΦCL at least 200-fold higher than that of Schaap's dioxetane 1a (Table 1); luminophore 2 has ΦCL of approximately 10%, and luminophores 3a–c are somewhat less bright with their ΦCL ranges from 0.6% to 4.2%. However, as will be discussed further below, the absolute ΦCL is not a crucial parameter when using the dioxetanes as chemiluminescent probes in test-tube bioassays (as long as the luminophore stands at a minimum threshold of brightness). This is because the key value in these assays is the signal to background ratio and not the absolute amount of light emitted (see Discussion).
Ultrasensitive chemiluminescent probes for NAD(P)H
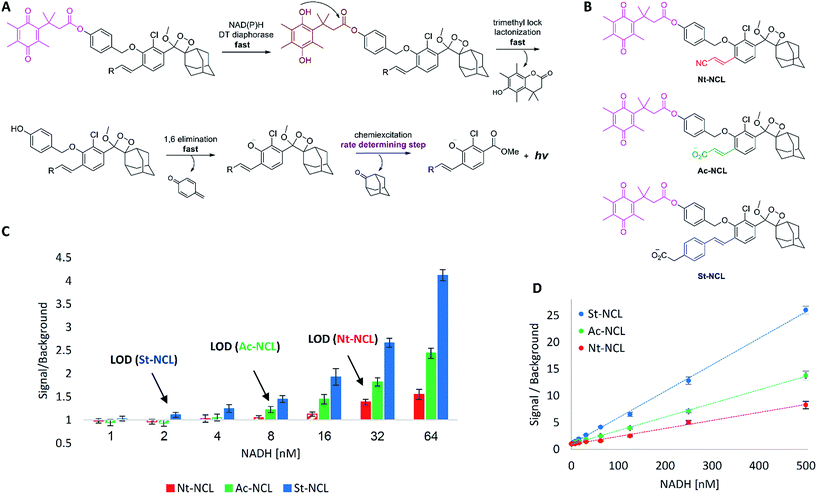
Next, we sought to demonstrate the advantage of the fast chemiexcitation observed for the new acrylate-anion-substituted dioxetane and the styryl-substituted dioxetane by the generation of CL probes for NADH and NADPH. These dinucleotides are important electron carriers found in all living cells. They participate in multiple redox reactions such as NAD(P)/NAD(P)H-dependent energy metabolic pathways,27,28 or maintenance of intracellular redox status.29 Therefore, the ability to measure NAD(P)H levels quantitatively provides an useful tool for investigating metabolism and for monitoring oxidative stress response. Several methods have been developed for NAD(P)H detection.30,31 One promising approach relies on an NAD(P)H-dependent quinone reduction, which leads to the release of a reporter moiety in the form of free aniline or phenol (Fig. 3A).32 DT diaphorase utilizes NAD(P)H present in the analyzed sample to reduce a quinone substrate. The resulting hydroquinone has a unique “trimethyl-lock” structure, which induces fast lactonization.33 Subsequent 1,6-elimination yields free phenol-dioxetane that decompose through chemiexcitation to emit light. At high enzyme concentration, the first three steps should take place within seconds, making the chemiexcitation rate determining.Three chemiluminescent probes were synthesized (see ESI for synthetic procedures†): acrylonitrile-substituted Nt-NCL, acrylate-substituted Ac-NCL, and styryl-substituted St-NCL (Fig. 3B). The probes were incubated along with DT diaphorase (NQO1) and tested for their ability to detect different NADH concentrations (Fig. 3C and D). Nt-NCL, with slow chemiexcitation kinetics following activation by NADH, demonstrated modest sensitivity (LOD = 32 nM). However, Ac-NCL, which underwent rapid chemiexcitation following activation, was 4-fold more sensitive with an LOD of 8 nM. Remarkably, St-NCL, which exhibited ultra-rapid chemiexcitation, was found as the most sensitive NAD(P)H probe. It was 16-fold more sensitive than Nt-NCL and 4-fold more sensitive than Ac-NCL (LOD = 2 nM). These results highlight the superior performance of the new rapidly light-emitting dioxetanes.
Discussion
Chemiluminescence is becoming more and more popular as an imaging tool in bioassays. Compared to fluorescence, it has one major advantage and one major drawback. On the one hand, the background signal in chemiluminescence is significantly lower; since an external light source is not required. On the other hand, chemiluminescent probes generate considerably lower number of photons than fluorescent probes; since fluorescent molecules can be repeatedly excited by the irradiation of external light to emit numerous photons. Consequently, the superiority of chemiluminescence is expressed predominantly in applications in which the absolute amount of light is not decisive.It is therefore not surprising that chemiluminescence has primarily been used for bioanalytical assays performed in a test-tube setting. For such assays, in which the probe concentration is generally not limited, the absolute amount of light emitted by each molecule does not have substantial implication on the signal/background. As long as the signal is intense enough to be readily quantified by the detector, the effect of ΦCL of the chemiluminescent probe on the signal/background is negligible. The reason for this lies in the fact that for dioxetane-based chemiluminescent assays, the background originates almost exclusively from the slow spontaneous hydrolysis of the triggering moiety and not from any external interference. Therefore, luminophore with higher ΦCL also generates a higher background.
In light of the above, our main aim in this work was to develop luminophores with faster chemiexcitation rate (as we realized that rapid chemiexcitation will improve the signal/background for such assays). We anticipated that improving the chemiexcitation kinetics will yield probes with higher sensitivity, even if the rapid chemiexcitation comes to a certain extent at the expense of high ΦCL. Indeed, we were able to develop new phenoxy-dioxetane luminophores with up to 100-fold more rapid chemiexcitation kinetics. Despite the fact that the styryl-substituted dioxetanes are in general less bright than the acryl-substituted derivatives, the styryl-based probe St-NCL yields significantly more sensitive assay for NADH than Nt-NCL and Ac-NCL.
The incorporation of the acrylate anion or styryl substituents increases the reactivity of the phenolate species towards electron transfer-initiated chemiexcitation. However, the stability of the phenol-dioxetanes was not impaired. We found that even the unprotected phenols (i.e. luminophores 2, 3a–c) are stable for days at room temperature, or for months at −20 °C, with only negligible decomposition. NAD(P)H probes that are based on these dioxetanes found to be stable at aqueous solution (pH 7.4) for several weeks at 4 °C. In addition, these compounds were found to be stable under normal room illumination conditions.
The aqueous solubility of our phenoxy-adamantyl-dioxetane probes is significantly increased, by the presence of a substituent with ionizable carboxylic acid. In the current work we describe a test-tube assay for NADH, in which high probe concentration, of 50 μM, is required to increase the assay sensitivity. In such assay, relatively high DMSO percentage (5%) can be used. For using dioxetane probes in living cells or animals, typically much lower probe concentration is applied (1–5 μM). Under such conditions, biologically compatible concentration of DMSO (0.1%), is sufficient for carboxylate-substituted dioxetane probes, to exhibit satisfactory aqueous solubility.
We believe that the importance of this work extends even beyond the report on the specific phenoxy-dioxetanes with rapid chemiexcitation. The significance of chemiexcitation kinetics as a key parameter was addressed here for the first time. Furthermore, by placing a rational method to control chemiexcitation rate, this work paves the way for the design of other luminophores with higher degree of control over their chemiexcitation properties.
Conclusions
In summary, we were able to design and synthesize new phenoxy-dioxetanes with up to 100-fold more rapid chemiexcitation kinetics. Our rational design was further supported by computational analysis of the key structural elements. Chemiluminescent probes based on such phenoxy-dioxetanes proved up to 16-fold more sensitive than probes with slower chemiexcitation. Furthermore, the insights placed here regarding the relationship between dioxetane electronic structure and its chemiexcitation kinetics can assist in the design of other luminophores with higher degree of control over their characteristics. We anticipate that our new phenoxy-dioxetanes luminophores will serve as an ideal platform for designing more sensitive chemiluminescent probes for various bioanalytical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. S. thanks the Israel Science Foundation (ISF), the Binational Science Foundation (BSF) and the German Israeli Foundation (GIF) for financial support. M. S. S. is grateful to the NIH (1 R01 GM121383) for partial financial support. Computational resources were provided by the Center for High Performance Computing at the University of Utah and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the NSF (ACI-1548562).Notes and references
- A. Roda, P. Pasini, M. Mirasoli, E. Michelini and M. Guardigli, Trends Biotechnol., 2004, 22, 295–303 CrossRef CAS PubMed.
- H. Wynberg, E. W. Meijer and J. C. Hummelen, in Bioluminescence and Chemiluminescence, ed. M. A. Deluca and W. D. McElroy, Academic Press, 1981, pp. 687–689 Search PubMed.
- N. Hananya and D. Shabat, Angew. Chem., Int. Ed., 2017, 56, 16454–16463 CrossRef CAS PubMed.
- N. Hananya, A. Eldar Boock, C. R. Bauer, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2016, 138, 13438–13446 CrossRef CAS PubMed.
- A. P. Schaap, T. Chen and R. S. Handley, Tetrahedron Lett., 1987, 28, 1155–1158 CrossRef CAS.
- S. Sabelle, P. Y. Renard, K. Pecorella, S. De Suzzoni-Dézard, C. Créminon, J. Grassi and C. Mioskowski, J. Am. Chem. Soc., 2002, 124, 4874–4880 CrossRef CAS PubMed.
- J. A. Richard, L. Jean, A. Romieu, M. Massonneau, P. Noack-Fraissignes and P. Y. Renard, Org. Lett., 2007, 9, 4853–4855 CrossRef CAS PubMed.
- J. Cao, R. Lopez, J. M. Thacker, J. Y. Moon, C. Jiang, S. N. S. Morris, J. H. Bauer, P. Tao, R. P. Mason and A. R. Lippert, Chem. Sci., 2015, 6, 1979–1985 RSC.
- B. Irena, E. Brooks and J. C. Voyta, J. Biolumin. Chemilumin., 1989, 4, 99–111 CrossRef PubMed.
- I. Bronstein, B. Edwards, A. Sparks, WO Patent 9426726, 1994.
- O. Green, T. Eilon, N. Hananya, S. Gutkin, C. R. Bauer and D. Shabat, ACS Cent. Sci., 2017, 3, 349–358 CrossRef CAS PubMed.
- M. E. Roth-Konforti, C. R. Bauer and D. Shabat, Angew. Chem., Int. Ed., 2017, 56, 15633–15638 CrossRef CAS PubMed.
- O. Green, S. Gnaim, R. Blau, A. Eldar-Boock, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2017, 139, 13243–13248 CrossRef CAS PubMed.
- T. Eilon-Shaffer, M. Roth-Konforti, A. Eldar-Boock, R. Satchi-Fainaro and D. Shabat, Org. Biomol. Chem., 2018, 16, 1708–1712 RSC.
- N. Hananya, O. Green, R. Blau, R. Satchi-Fainaro and D. Shabat, Angew. Chem., Int. Ed., 2017, 56, 11793–11796 CrossRef CAS PubMed.
- K. J. Bruemmer, O. Green, A. S. Timothy, D. Shabat and J. C. Chang, Angew. Chem., Int. Ed., 2018, 57, 7508–7512 CrossRef CAS PubMed.
- J. Cao, W. An, A. G. Reeves and A. R. Lippert, Chem. Sci., 2018, 9, 2552–2558 RSC.
- S. Gnaim, O. Green and D. Shabat, Chem. Commun., 2018, 54, 2073–2085 RSC.
- J. Lampinen, M. Raitio, A. Perälä, V. Kytöniemi and R.-R. Harinen, Appl. Note AP-MIB-VARIO12-0108 – Thermo Sci., 2008 Search PubMed.
- W. Adam, I. Bronstein, A. V. Trofimov and R. F. Vasil'ev, J. Am. Chem. Soc., 1999, 121, 958–961 CrossRef CAS.
- W. Adam and A. V. Trofimov, J. Org. Chem., 2000, 65, 6474–6478 CrossRef CAS PubMed.
- L. Yue and Y. J. Liu, J. Chem. Theory Comput., 2013, 9, 2300–2312 CrossRef CAS PubMed.
- M. Vacher, I. Fdez Galván, B. W. Ding, S. Schramm, R. Berraud-Pache, P. Naumov, N. Ferré, Y. J. Liu, I. Navizet, D. Roca-Sanjuán, W. J. Baader and R. Lindh, Chem. Rev., 2018, 118, 6927–6974 CrossRef CAS PubMed.
- L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- C. B. Santiago, A. Milo and M. S. Sigman, J. Am. Chem. Soc., 2016, 138, 13424–13430 CrossRef CAS PubMed.
- K. Eto, Y. Tsubamoto, Y. Terauchi, T. Sugiyama, T. Kishimoto, N. Takahashi, N. Yamauchi, N. Kubota, S. Murayama, T. Aizawa, Y. Akanuma, S. Aizawa, H. Kasai, Y. Yazaki and T. Kadowaki, Science, 1999, 283, 981–985 CrossRef CAS PubMed.
- M. Noda, S. Yamashita, N. Takahashi, K. Eto, L. M. Shen, K. Izumi, S. Daniel, Y. Tsubamoto, T. Nemoto, M. Iino, H. Kasai, G. W. G. Sharp and T. Kadowaki, J. Biol. Chem., 2002, 277, 41817–41826 CrossRef CAS PubMed.
- A. Gaikwad, D. J. Long, J. L. Stringer and A. K. Jaiswal, J. Biol. Chem., 2001, 276, 22559–22564 CrossRef CAS PubMed.
- Y. Zhou, Z. Xu and J. Yoon, Chem. Soc. Rev., 2011, 40, 2222–2235 RSC.
- R. Freeman, R. Gill, I. Shweky, M. Kotler, U. Banin and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 309–313 CrossRef CAS PubMed.
- W. Zhou, D. Leippe, S. Duellman, M. Sobol, J. Vidugiriene, M. O'Brien, J. W. Shultz, J. J. Kimball, C. DiBernardo, L. Moothart, L. Bernad, J. Cali, D. H. Klaubert and P. Meisenheimer, ChemBioChem, 2014, 15, 670–675 CrossRef CAS PubMed.
- O. A. Okoh and P. Klahn, ChemBioChem, 2018, 19, 1668–1694 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Chemiluminescence measurements protocols and data, computational methods and details, synthetic procedures and spectra of key compounds. See DOI: 10.1039/c8sc04280b |
| ‡ These authors contributed equally to this work. |
| § As expected, the decomposition profile of all luminophores described in this paper followed first-order kinetics; thus, the rate constant, as well as t1/2, were derived from the linear graph of ln(RLU) vs. time. See ESI.† |
| ¶ We also considered HOMO energies as a descriptor. However, no correlation could be established. |
| This journal is © The Royal Society of Chemistry 2019 |