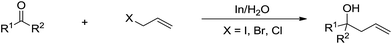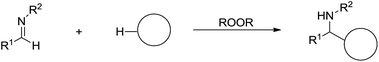Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
En route to metal-mediated and metal-catalysed reactions in water
Feng
Zhou
 and
Chao-Jun
Li
and
Chao-Jun
Li
 *
*
Department of Chemistry, FRQNT Center for Green Chemistry and Catalysis, McGill University, Montreal, Quebec H3A 0B8, Canada. E-mail: cj.li@mcgill.ca
First published on 5th November 2018
Abstract
This perspective report presents the key approaches for the development of various organometallic reactions in aqueous media. In view of future sustainability, the efficient use of natural resources, such as renewable biomass-based feedstocks, constitutes an important aspect for sustainable chemical industry. The exploration and discovery of efficient organometallic reactions or equivalents in water enrich the toolbox of organic chemists for the direct conversion of biomass-derived feedstocks into high-valued chemicals and the direct modification of biomolecules in their native aqueous environment, which contributes to future sustainability.
Introduction
Carbon–carbon bond formation plays the central role in synthetic organic chemistry. For polar routes, such transformations necessarily involve carbon nucleophiles and electrophiles.1 One class of the most commonly used nucleophiles is organometallic compounds. Since the discovery of alkylzinc compounds by Frankland from the reaction of iodoalkanes with metallic zinc,2 organometallic reagents serving as nucleophiles for organic syntheses have been rapidly evolved both in scope and application.3–7 The early discoveries of Reformatsky,8 Barbier,9 Grignard,10 and Gilman11 are among the important milestones in the development of classical organometallic reactions. Subsequently, there have been great progresses in the use of alkali12 and other metals since the 1930s. Recently, organometallic reactions catalysed by transition metals have become increasingly important in the synthesis of organic molecules,13 underscored by three Nobel Prizes: for palladium-catalysed cross-coupling in 2010,14–16 olefin metathesis in 2005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17–19 and asymmetric catalysis in 2001
17–19 and asymmetric catalysis in 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–22 within the last decade.
20–22 within the last decade.
In spite of the enormous progress being made, there are obvious shortcomings for classical organometallic reactions in terms of chemical sustainability, such as the extensive use of organic solvents, stoichiometric metals, moisture and functional group intolerances, which are partially ascribed to the limitation of the historical development of classical reactions from fossil based feedstocks23 bearing no functional groups and being insoluble in water. Accordingly, classical organic reactions were intuitively developed in fossil originated organic solvents. With the increasing concerns of the depletion of non-renewable fossil resources and the environmental deterioration,24 developing products from renewable resources and improving resources/energy utilization efficiency are the key measures towards future chemical sustainability.25
Biomass, as a sustainable and renewable feedstock provided by nature could be a practical alternative towards future chemicals.26–28 In contrast to the fossil-based feedstocks, biomass-based ones are generally over-functionalized and often soluble or soluble after depolymerisation in water. As a result, further processing is required to adapt them to the reaction conditions that were initially developed for fossil-based classical organometallic reactions in organic solvents. One of the most common strategies for such purposes is the exhaustive protecting group manipulations, which in turn limiting the possibility of using water as solvent and leading to extra steps and waste etc. Conversely, exploration of organometallic reactions directly in water could be a possible solution in terms of various functional groups tolerance and direct chemical modification of biomolecules. Hence, exploring such fundamentally novel chemistry would potentially simplify chemical synthesis significantly, improve synthetic efficiency, provide valuable tools for chemical biology, and contribute to the future chemical sustainability29 with respect to atom-economy,30 the E-factor31 and step-economy.32
General design concept
Most classical organometallic compounds are highly polarized as carbanions, which are also strong bases. Consequently, they are unstable towards active protons (moisture or functional groups) and must be synthesized/used under strictly anhydrous/aprotic conditions. Conceptually, the key to developing successful organometallic reactions in water (aqueous media) is to attenuate or prevent the protonation of carbon–metal bond once the organometallic species is generated (eqn (1)). | (1) |
There are several conceivable approaches to achieve this objective. The most straightforward approach is to tune the relative electronegativity33 between carbon and metal atoms to form the more covalent C–M bond. A second approach is to design radical pathways, as the strong O–H bond (enthalpy 436 kJ mol−1) is very difficult to break homolytically. The third approach is to mimic nature's lipid bilayer membrane34 by physically segregating and temporarily stabilizing the organometallic species from water via micelle formation using surfactants35 or on water strategy.36–38 The fourth approach is to bypass the stoichiometric organometallic reagents by transition-metal-catalysed organic transformations in water, such as via C–H bond activation and hydrazone umpolung.39,40 This perspective article will illustrate these aspects using the classical nucleophilic additions as examples.
Various approaches
Moving towards more covalent C–M bond
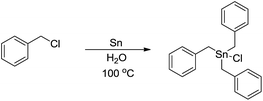
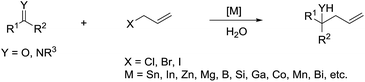
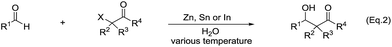
The main reason for the necessary anhydrous conditions in classical organometallic reactions was due to the highly polarized and reactive C–M bonds towards water,3 especially the organometallic compounds of s-block elements. As C–M bonds become more covalent, such as the organometallic compounds of group 14 and group 15, they have less carbanion character and thus are less prone to acidic proton and relatively more stable in aqueous media. Different from s-block and late p-block organometallic compounds, the ones from group 13 elements take up a special position within the main group due to their moderate reactivity towards water.41 Furthermore, certain classes of organometallics remain viable in the presence of water. For example, the preparation of arylmercuric chlorides in aqueous media has been known since 1905.42 In the 1960's, tribenzylstannyl halide was prepared in large scale in water (Scheme 1).43–45 The Wurtz-type reductive coupling of allyl halides proceeded in aqueous alcohol.46 These reports opened up new perspectives regarding the metal-mediated organic reactions and it has been increasingly realized that these kinds of reactions can be conducted in water under special circumstances. Indeed, there has been great progress in this research area over the past decades, such as allylation,47–49 allenylation,50,51 propargylation,52,53 benzylation,54 phenylation55,56etc.The most explored reaction is the allylation of carbonyls and other electrophiles with allyl halides mediated by various metals (Scheme 2),57 among which the use of indium to mediate the Barbier–Grignard-type reactions in water reported by Li and Chan received particular attention (Scheme 3).48 This was attributed to the fact that on one hand, indium possesses the lowest first ionization potential among the metallic elements near it in the periodic table; and on the other hand, it does not form oxides readily in air and is not sensitive to boiling water or alkali. Consequently, indium was shown to be the most effective for such transformations in water, proceeding smoothly at room temperature without any promoter. This methodology has found wide applications in aqueous synthetic chemistry,58–61 particularly important in carbohydrate chemistry.
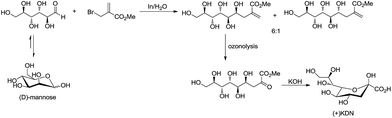
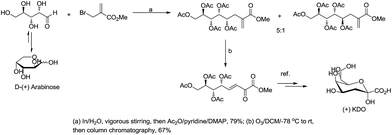
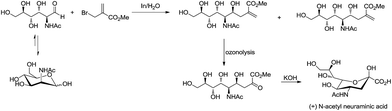
For example, Chan and Li62 reported a concise and stereoselective synthesis of (+)-3-deoxy-D-glycero-D-galacto-nonulosonic acid (KDN) from D-mannose (Scheme 4) and a formal synthesis63 of KDO (Scheme 5), whereas Whitesides reported the synthesis of N-acetyl-neuraminic acid (Scheme 6)64 and other sialic acid derivatives by the method.65,66 This chemistry has also been extended to six-carbon sialic acid derivatives by Chappell and Halcomb67 and the protocol has been further improved by Warwel and Fessner.68
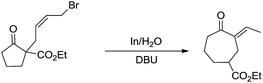
Besides being used successfully in carbohydrates chemistry, the metal-mediated allylation has also been applied to the synthesis of other scaffolds such as 1,3-butadienes,69 vinyloxiranes, 2-methylenetetrahydrofurans,70 trimethylenemethane equivalent,71 cyclopentane derivatives.72 A carbocyclic ring enlargement methodology was also developed (Scheme 7),73,74 by using the indium-mediated Barbier-type reaction in water, in which 5-, 6-, 7-, 8-, and 12-membered rings are expanded by two carbon atoms into 7-, 8-, 9-, 10-, and 14-membered ring derivatives respectively. The use of water as a solvent was found to be critical for the success of the reaction and similar ring enlargement in organic solvents was not successful. Such a ring expansion strategy can also be applied to heterocyclic medium ring and75 one carbon-ring expansion.76
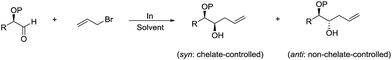
The diastereoselectivity of such reaction was studied in detail by Parquette and co-workers, and found that the free hydroxyl derivatives react with excellent diastereofacial control to give significantly heightened levels of syn-1,2-diols and anti-diols (Scheme 8).77 Relative reactivities were determined in the α-series and the hydroxyl aldehyde proved to be the most reactive substrate. This reactivity ordering suggests that the selectivity stems from chelated intermediates. The rate acceleration observed in water can be heightened by initial acidification. Then they reported a variety of diastereoselective allylations in aqueous conditions and their synthetic applications such as a practical alternative to the Knoevenagel reaction of aliphatic aldehydes, the formation of α-methylene-γ-lactones fused to medium and large rings and the intercalation of multiple carbon atoms between the carbonyls of α-diketones.78
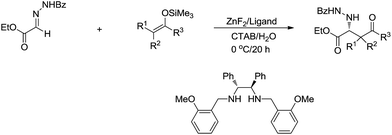
In 2002, Delgado and co-workers reported a Barbier-type diastereoselective allylation of α-amino aldehydes with an enantiopure 2-sulfinylallyl building block in aqueous media mediated by zinc (Scheme 9).79 High levels of diastereoinduction can be achieved from α-amino aldehydes configurationally related to natural α-amino acids.
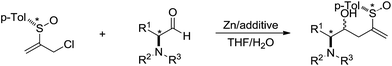 | ||
| Scheme 9 Zinc-mediated Barbier-type diastereoselective allylation of α-amino aldehydes with 2-sulfinylallyl chloride in aqueous media. | ||
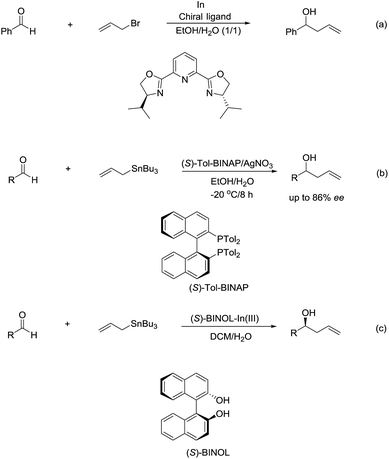
The enantioselectivity of such reactions in water was also possible. In 1999, an enantioselective allylation reaction of aldehydes in an aqueous media using chiral pyridine bis(oxazoline) ligand was reported by Loh and co-workers (Scheme 10a).80 Subsequently, a AgNO3/(S)-Tol-BINAP-catalysed enantioselective allylation of aldehydes using allyltributylstannane was achieved by the same group (Scheme 10b).81 Later, they reported an enantioselective allylation of aldehyde catalysed by a moisture-tolerant chiral (S)-BINOL–In(III) complex. The allylation of a variety of aromatic, α,β-unsaturated and aliphatic aldehydes resulted in both moderate to good yields and high enantioselectivities (Scheme 10c).82
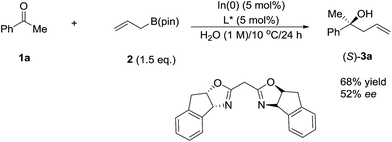
In 2003, Kobayashi and co-workers reported a catalytic asymmetric allylation of aldehydes using allyltributyltin in aqueous media via the combination of cadmium bromide and chiral diamine ligands. Interestingly, these ligands were found to accelerate the reactions significantly (Scheme 11).83 Later, they reported an In-catalysed allylation of ketone with allyl boronates in water (Scheme 12).84 Preliminary asymmetric study using chiral bis-oxazoline ligand showed that a moderate enantioselectivity can be obtained.
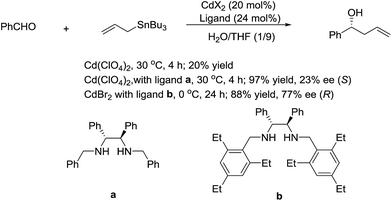 | ||
| Scheme 11 Cd-catalysed asymmetric allylation of aldehyde using allyltributylstannane in an aqueous media. | ||
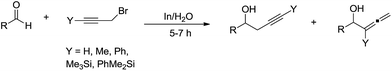
In addition to allyl halides, structurally related propargyl halides are also effective for such type of reactions. In 1995, Issac and Chan reported their studies of the propargylation of aldehydes in aqueous medium mediated by indium (Scheme 13).52 They found that simple prop-2-yn-1-yl bromide reacted with both aliphatic and aromatic aldehydes in water to afford mainly the homopropargyl alcohols. In contrast, when propargyl bromide was γ-substituted, the coupling products were predominantly, or exclusively, the allenylic alcohols. Such couplings also proceed with α-chloropropargyl phenyl sulfide.85
The indium-mediated coupling of propargyl bromide with a variety of imines and imine oxides afforded homopropargylamine derivatives in aqueous media under mild conditions (Scheme 14).86
Propargylation of glyoxylic oxime ether in the presence of a catalytic amount of palladium(0) complex and indium(I) iodide in aqueous media was also studied.87
The indium-mediated highly diastereoselective allenylation in aqueous medium was also highly successfully applied to the total synthesis of (+)-goniofufurone (Scheme 15).50,51
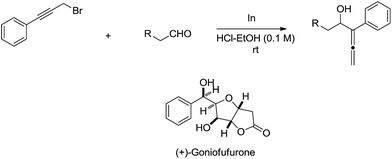 | ||
| Scheme 15 Indium-mediated allenylation in aqueous media and its application to the synthesis of (+)-goniofufurone. | ||
Benzyl halides and allyl(propargyl) halides are structurally similar but have distinctively different chemical reactivities in the aqueous Barbier–Grignard-type reactions. Although tribenzyl and dibenzyltin derivatives have been prepared in aqueous conditions since the 1960s, they do not add onto carbonyls, most likely because it is not possible to form a six-membered cyclic transition state with the carbonyl group in a ‘two-component’ fashion. Still, a zinc-mediated benzylation of carbonyl compounds in aqueous media was reported by Wang and co-workers in 2005 (Scheme 16).88
 | ||
| Scheme 16 Zn-mediated benzylation of carbonyl compounds in aqueous media in the presence of CdCl2 and InCl3. | ||
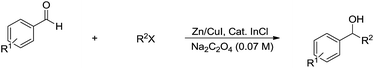
Compared with allyl and propargyl metal species, p-block aryl-metal (and metalloids) (aryl-tin, aryl-boron, aryl-bismuth, aryl-lead, and aryl-silicon) and vinyl-metal species are highly covalent and very unreactive by themselves. However, by utilizing a transition-metal catalyst, such aryl (and vinyl) derivatives become highly reactive, yet stable towards water and protonic functional groups. This strategy has been widely applied for their various cross-coupling reactions with organic halides.89 By using a similar strategy, rhodium90–93 and palladium94,95 catalysed arylation of carbonyls and (asymmetric) conjugate additions96–101 with aryl-metal reagents in water and under an air atmosphere are successful (Scheme 17a).55 A strong electronic effect on Rh-catalysed carbonyl additions and conjugated additions with various arylmetallic reagents was observed (Scheme 17b).56
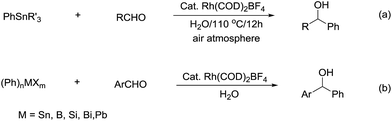 | ||
| Scheme 17 (a) Rh-catalysed carbonyl phenylation in water; (b) the electronic effect on Rh-catalysed carbonyl additions with arylmetallic reagents. | ||
Moving towards more radical character
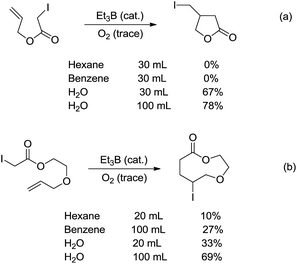
Radicals are neutral species with one unpaired valence electron, which, in contrast to organic anions and cations, make them chemically “stable” towards water. Indeed, the aqueous radical chemistry predominates in biological processes.102,103 As radicals are nonpolar, their additions to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds were first realized in water. In 1998, Oshima and co-workers reported that the Et3B-induced atom transfer radical cyclization of allyl iodoacetate proceeded much more smoothly in water at ambient temperature than in benzene or hexane (Scheme 18a).104 Treatment of the allylic iodoacetate in water with Et3B (metalloid) at room temperature for 3 h provided β-iodomethyl-γ-butyrolactone in 67% yield. In contrast, in benzene the desired product was not obtained at all and oligomeric by-products were formed.
C bonds were first realized in water. In 1998, Oshima and co-workers reported that the Et3B-induced atom transfer radical cyclization of allyl iodoacetate proceeded much more smoothly in water at ambient temperature than in benzene or hexane (Scheme 18a).104 Treatment of the allylic iodoacetate in water with Et3B (metalloid) at room temperature for 3 h provided β-iodomethyl-γ-butyrolactone in 67% yield. In contrast, in benzene the desired product was not obtained at all and oligomeric by-products were formed.
The remarkable solvent effect of water was also observed in the case of medium and large ring construction. For example, Oshima and co-workers reported an intramolecular cyclization mediated by Et3B in water provided the 9-membered lactone in 69% yield (Scheme 18b). The same reaction carried out in benzene afforded much inferior yield.105 Although the exact role of water was not clear at that stage, a hydrogen bonding between water and the carbonyl oxygen could be formed to facilitate the abstraction of iodine, generating the (alkoxycarbonyl)methyl radical. Hydrophobic interaction may also accelerate the cyclization.
With regard to metal-mediated conjugate additions involving alkyl groups, Luche and coworkers reported that alkyl halides in the presence of zinc-copper couple reacted smoothly with conjugated carbonyl compounds and nitriles to give 1,4-addition products in good yields under sonication conditions in aqueous conditions (Scheme 19a).106–108 A moderate diastereoselectivity was obtained in those reactions where a mixture of diastereomers could be generated.109,110 The reactivity of the halides followed the order of tertiary > secondary ![[right double angle bracket]](https://www.rsc.org/images/entities/char_27eb.gif) primary, and iodide > bromide (chlorides did not react). The preferred solvent system was aqueous ethanol. The reaction was proposed to undergo a free radical mechanism occurring on the metal surface under sonochemical conditions (Scheme 19b). Efforts to trap the intermediate intramolecularly only gave a very low yield of the cyclization product.111 Similar additions also occurred on vinylphosphine oxides. When optically active vinylphosphine oxide was used, P-chiral alkylphosphine oxide was obtained with retention of configuration (Scheme 20).112
primary, and iodide > bromide (chlorides did not react). The preferred solvent system was aqueous ethanol. The reaction was proposed to undergo a free radical mechanism occurring on the metal surface under sonochemical conditions (Scheme 19b). Efforts to trap the intermediate intramolecularly only gave a very low yield of the cyclization product.111 Similar additions also occurred on vinylphosphine oxides. When optically active vinylphosphine oxide was used, P-chiral alkylphosphine oxide was obtained with retention of configuration (Scheme 20).112
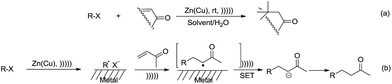 | ||
| Scheme 19 Zinc–copper couple-mediated conjugate additions of alkyl halides to carbonyl compounds in aqueous media and the proposed radical mechanism. | ||
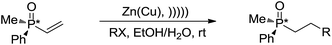 | ||
| Scheme 20 Zinc–copper couple-mediated conjugate addition of alkyl halides to vinylphosphine oxides in aqueous media. | ||
Giese and co-workers studied the diastereoselectivity associated with a related addition in water113 and found that the anti-isomer was the main product if the attacking radical is bulky (Scheme 21).
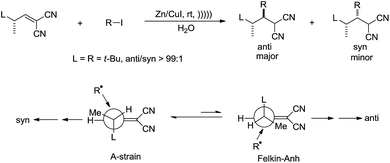 | ||
| Scheme 21 Zinc–copper couple-mediated conjugate addition of alkyl halides and electron-deficient alkenes in water. | ||
The authors rationalized the high diastereoselectivity by proposing that the more stable ‘A-strain’ conformer of the alkene reacts much slower with bulky alkyl radical than the less stable ‘Felkin–Anh’ conformer.
The diastereoselective ultrasonically induced 1,4-addition of alkyl iodides in the presence of zinc–copper couple to chiral α,β-unsaturated systems in aqueous media was studied by Suarez and co-workers (Scheme 22). They observed that good diastereoselectivities were obtained with the Z-isomer while the reactions with the E-isomer were non-stereoselective.114,115
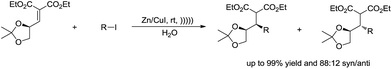 | ||
| Scheme 22 Zinc–copper couple-mediated diastereoselective addition of alkyl iodides and α,β-unsaturated compounds in water. | ||
For the more challenging nucleophilic addition of radical-based alkyl-metal species to polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X bonds, the key is to prevent the more facile reversed reaction to break the C–C bonds of the radical addition intermediate. One approach is via the stabilization of the radical. Thus, in 2002, Li and co-workers reported a zinc-mediated conjugate addition reaction of alkyl halides to α-phthalimidoacylate derivatives and nucleophilic addition to imines for the efficient synthesis of α-amino acid derivatives and amines in the presence of NH4Cl in water (Scheme 23). Notably, no reaction was observed in absence of water.116
X bonds, the key is to prevent the more facile reversed reaction to break the C–C bonds of the radical addition intermediate. One approach is via the stabilization of the radical. Thus, in 2002, Li and co-workers reported a zinc-mediated conjugate addition reaction of alkyl halides to α-phthalimidoacylate derivatives and nucleophilic addition to imines for the efficient synthesis of α-amino acid derivatives and amines in the presence of NH4Cl in water (Scheme 23). Notably, no reaction was observed in absence of water.116
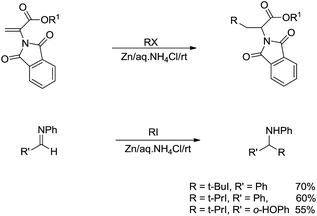 | ||
| Scheme 23 Zinc-mediated conjugate addition of alkyl halides to α-phthalimidoacylate and imine derivatives in aqueous media. | ||
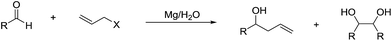
It is noteworthy that Li and co-workers also reported a magnesium-mediated Barbier-type allylation of aldehyde using water as a sole solvent, which proceeds most likely via a radical process on the metal surface (Scheme 24).117
In 2002, Naito and co-workers reported an intermolecular alkyl radical addition to imine derivatives and electron-deficient C–C double bond in aqueous media by using indium as a single-electron-transfer radical initiator (Scheme 25).118 The one-pot reaction provided a convenient method for preparing α-amino acids.
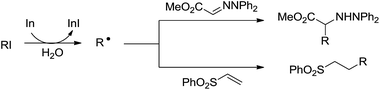 | ||
| Scheme 25 In-mediated alkyl radical addition to imine and phenyl vinyl sulfone derivatives in aqueous media. | ||
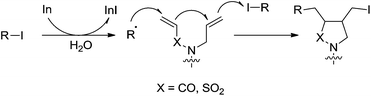
In 2003, they reported an indium-mediated cascade reaction, in which the addition–cyclization–trapping sequences efficiently generated the cyclized products in aqueous media (Scheme 26).119 The substrates bearing vinylsulfonamide and hydrazone proceeded smoothly in aqueous media to provide the functionalized cyclic products.
For the even more challenging Barbier–Grignard-type carbonyl alkylation using unactivated alkyl halides in water, in absence of radical stabilization, the “reductive Lewis acid” concept was used by Li and co-workers successfully, in which conceptually the addition is accompanied by reduction and “free radical intermediate” is not “free” during the reaction progress (Scheme 27).120
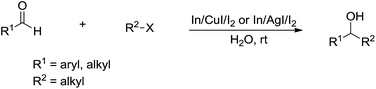
In 2008, Loh and co-workers further developed this Barbier–Grignard-type alkylation reaction of aldehydes including aliphatic version using unactivated alkyl halides in water catalysed by In/CuI or In/AgI catalysis (Scheme 28).121 The reactions proceeded more efficiently in water than in organic solvent.
Another type of reactions is the metal-mediated Reformatsky-type reaction. The reaction of a metal with an α-halogen carbonyl compound would generate an organometallic intermediate that can equilibrate between the carbanion form and the enolate form. Like the case of allyl and propargyl, the enolate form would allow the reaction to proceed through a six-membered cyclic transition state and thus could be energetically favorable. Indeed, the reaction of an α-halogen carbonyl compound with an aldehyde in the presence of zinc, tin, or indium in water provided a direct cross-aldol reaction product (Scheme 29).122,123 While a direct Reformatsky-type reaction occurred in low yields in the case of aromatic aldehydes were used in water mediated by zinc.124 Later Lee and co-workers reported that the reactions of aldehydes or ketones with ethyl bromoacetate in the presence of indium promoted by ultrasound afforded β-hydroxyesters in good to excellent yields under mild conditions.125 Bieber and co-workers found that even catalytic amounts of benzoyl peroxide or peracids can greatly enhance the reactions of bromoacetates and aromatic aldehydes, giving satisfactory yields. A radical chain mechanism, initiated by electron abstraction from the organometallic Reformatsky reagent, was proposed (Scheme 30).126
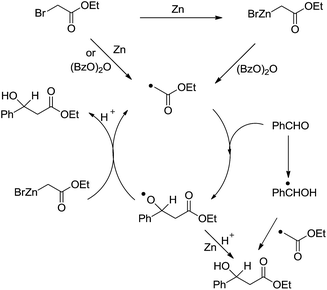 | ||
| Scheme 30 Zinc-mediated Reformatsky-type reaction of bromoacetates and aromatic aldehydes and the proposed radical chain mechanism. | ||
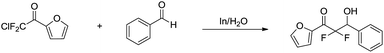
The indium-mediated aqueous Reformatsky reaction was successfully applied to the synthesis of α,α-difluoro-β-hydroxy ketones by Welch and co-workers in 2001 (Scheme 31).127
Via temporarily stabilization (surfactant) or on water strategy
One strategy that nature frequently adopts to perform reactions in aqueous media is encapsulation via lipid bilayer membranes.34 Chemists, in turn, have developed surfactants that can be self-assembled in water to form micelles,128 which provide the lipophilic interior to serve, in essence, as the solvent for catalysis. Organic solutes interact with micelles according to their polarity: non-polar solutes are encapsulated in the interior of the micelle, while polar solutes locate themselves at the surface of the micelle and moderately polar molecules would be positioned closer to the polar surface. This compartmentalization of solutes lays the foundation and is responsible for many organic reactions in aqueous media, particularly assisting with organometallic processes, as the lipophilic interior of the surfactants provides the hydrophobic area to temporarily stabilize polar carbon–metal bond and facilitate the chemical transformation, such as in oxidation, reduction and C–C coupling reactions.129Recently, the application of surfactants for aqueous catalysis has been demonstrated in the development of catalytic organometallic reactions in water. For example, in 2004, Kobayashi and co-workers reported a Zn-mediated enantio and diastereoselective, stereospecific Mannich-type reaction in water, in which the surfactant CTAB (cetyltrimethylammonium bromide) significantly improved the yield (Scheme 32).130
In 2012, Lipshutz and co-workers reported a copper-catalysed conjugate addition of alkyl halides to enones mediated by zinc in aqueous media using TPGS-750-M as surfactant (Scheme 33).131 Various cross-coupling reactions catalysed by transition metals in aqueous media using surfactants were also reported by his group.132,133
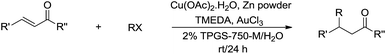 | ||
| Scheme 33 Cu-catalysed and zinc-mediated conjugate additions of alkyl halides to enones in aqueous media using TPGS-750-M as surfactant. | ||
In 2014, Li and co-workers reported the Rh-catalysed and Zn-mediated Barbier–Grignard-type arylation of aldehydes using unactivated aryl iodides in water, in which the organometallic species could be temporarily stabilized by encapsulation into the lipophilic interior of the surfactant BrijC10 (Scheme 34).134
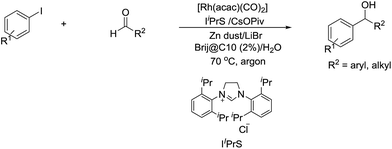 | ||
| Scheme 34 Rh-catalysed and Zn-mediated Barbier–Grignard-type arylation of aldehydes in water using BrijC10 as surfactant. | ||
In 2018, Lipshutz and co-workers developed an environmentally responsible, mild method for the synthesis of functionalized 1,3-butadienes via Pd-catalyzed cross-coupling of substituted allenic esters in water in the presence of the surfactant TPGS-750-M (Scheme 35).135 Various sp–sp2, sp2–sp2, and sp2–sp3 coupling reactions were realized and these transformations tolerated broad functional groups.
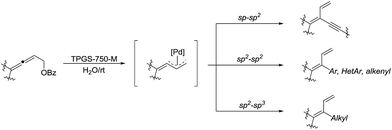 | ||
| Scheme 35 Pd-catalysed cross-coupling of substituted allenic esters for the synthesis of functionalized 1,3-butadienes in water in the presence of the surfactant TPGS-750-M. | ||
As for the reactivity of polar organometallic compounds on water, recently Capriati and co-workers reported the nucleophilic addition of Grignard reagents and lithium reagents to γ-chloroketone on water at room temperature and under air for efficient formation of THF derivatives, in which water may play an important role as demonstrated by the solvent isotope effect and the control experiments that indicate the strong intermolecular hydrogen bonds jointly with trans-phase H-bonding with the substrate, thus (a) shielding the organometallic reagent from competitive protonolysis processes and (b) activating the carbonyl derivative towards nucleophilic addition (Scheme 36a).136 Later, they further developed the nucleophilic additions of organolithium and organomagnesium reagents to imines and nitriles using bulk water as a privileged reaction medium, working under air, at room temperature, with vigorous stirring (Scheme 36b).137 The significant solvent D/H isotope effect observed for the on-water nucleophilic additions of organolithium compounds to imines suggests the on-water catalysis arises from proton transfer across the organic–water interface. The strong intermolecular hydrogen bonds between water molecules may play a key role in disfavouring protonolysis, which occurs extensively in other protic media such as methanol.138,139
Bypass stoichiometric reagents
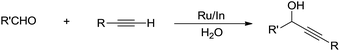
In the past two decades, there have been great advances in aqueous C–H bond activation catalysed by transition metals. Since C(sp)–H bonds are the most acidic, hence the easiest to activate in water. In 2002, Li and co-workers reported that a bimetallic Ru–In catalytic system could catalyse alkynylation of aldehydes in water (Scheme 37).145
Then, by using Cu/Ru or Cu/pyridine-oxazoline catalyst, they succeeded the addition and asymmetric addition of arylacetylenes to imines to form propargylamines in excellent yields and enantioselectivity in water (Scheme 38).146,147 Furthermore a series of transition-metal-catalysed C(sp)–H bond activation reactions in water were reported by his group.148,149
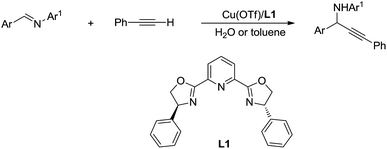 | ||
| Scheme 38 Cu/pyridine-oxazoline catalysed asymmetric addition of phenylacetylene to imine in water via C(sp)–H activation. | ||
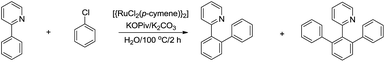
For the less acidic C(sp2)–H bonds, one strategy to facilitate their reaction in water is via chelation. In 2010, Dixneuf and co-workers reported a Ru-catalysed and pyridine-directed C(sp2)–H bond activation in water for efficient ortho-phenylation (Scheme 39).150 The selectivity of mono-phenylation and bis-phenylations was found to be better in water than in organic solvent.
In 2012, Ackermann and co-workers developed the Ru-catalysed tandem cyclization of aniline derivative and alkyne for efficient indole synthesis via C(sp2)–H bond activation in water (Scheme 40).151
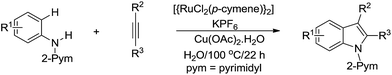 | ||
| Scheme 40 Ru-catalysed tandem cyclization of aniline derivative and alkyne for indole synthesis in water via C(sp2)–H bond activation. | ||
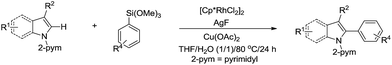
In 2014, Loh and co-workers presented the Rh-catalysed and pyrimidine-directed 2-phenylation of indole derivatives via C(sp2)–H bond activation in water using trimethoxyphenylsilane as phenylation reagent (Scheme 41).152 In 2015, Li and co-workers reported the Rh-catalysed homo-coupling of aryl carboxylic acid in water via two-fold C(sp2)–H bond activation using MnO2 as terminal oxidant (Scheme 42).153
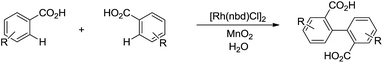 | ||
| Scheme 42 Rh-catalysed homo-coupling of aryl carboxylic acid in water via two-fold C(sp2)–H bond activation. | ||
Such a chelation strategy can also be applied towards the least acidic C(sp3)–H bonds. For example, in 2014, Chen and co-workers reported a Pd-catalysed N-quinolylcarboxamide directed β-arylation of alanine at room temperature via C(sp3)–H bond activation, in which water is used as a co-solvent (Scheme 43).154 This method provided a convenient approach for the synthesis of both natural and unnatural aromatic α-amino acids.
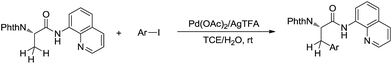 | ||
| Scheme 43 Pd-catalysed N-quinolylcarboxamide directed β-arylation of alanine at room temperature via C(sp3)–H bond activation using water as a co-solvent. | ||
In 2015, Rao and coworkers reported the Pd-catalysed β-C(sp3)–H bond oxidation of amides using 8-aminoquinoline as directing group in water (Scheme 44).155 Interestingly, the isotope labelling experiment indicates that the oxygen originates from water.
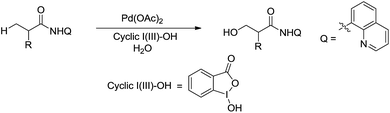 | ||
| Scheme 44 Pd-catalysed β-C(sp3)–H bond oxidation of amides using 8-aminoquinoline as directing group in water. | ||
An alternative strategy for the C(sp3)–H bonds activation is via radical process. In 2008, Li and co-workers reported a direct addition of cycloalkanes to imines mediated by peroxide and the reaction tolerates a wide range of functionalities as well as aqueous conditions (Scheme 45).156
In 2017, Li and co-workers reported the ruthenium catalysed umpolung strategy for the nucleophilic addition to carbonyl164 (Scheme 48a) and aryl imine165 (Scheme 48b) compounds through hydrazone intermediates using aldehydes as carbanion equivalents. The unique chemoselectivity exhibited by carbonyl-derived carbanion equivalents is demonstrated by their tolerance to protic reaction media and good functional group compatibility.
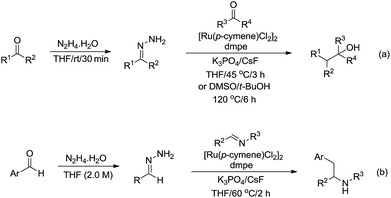 | ||
| Scheme 48 Ru-catalysed nucleophilic addition of carbonyl and aryl imine compounds via hydrazone intermediate using aldehydes as carbanion equivalents. | ||
Subsequently, they described a ruthenium catalysed conjugate additions via hydrazone approach, in which water was tolerated in the transformation (Scheme 49).166
Recently, Li and coworkers reported a nickel-catalysed C(sp2)–C(sp3) cross-coupling reaction from two sustainable biomass-based feedstocks: phenol derivatives with umpolung aldehydes through moisture/air-stable hydrazones intermediate generated in situ (Scheme 50).167 Water tolerance, functional group compatibility and late-stage elaboration of complex biological molecules exemplified its practicability and unique chemoselectivity over stoichiometric organometallic reagents. Further development of such reactions in water is foreseen and being actively pursued in our lab.
Conclusions
The past few decades have witnessed the rapid development of water-based organic synthesis, in which the organometallic reactions in water has also made considerable progress. This article aims to summarize the key approaches for realizing the various organometallic reactions focusing on nucleophilic additions in aqueous media, which provides the readers with perspectives for further developments in this field. In view of future sustainability, the use of renewable biomass-based feedstocks constitutes an important part for sustainable development of chemical industry. The development of efficient organometallic reactions in water provides important tools for the direct conversion of biomass-derived feedstocks into high-valued chemicals and direct modification of biomolecules under native aqueous environment, and constitutes overall synthetic efficiency.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We are grateful to the Canada Research Chair Foundation (to C.-J. L.), the Canadian Foundation for Innovation, FRQNT Centre in Green Chemistry and Catalysis, and the Natural Science and Engineering Research Council of Canada for support of our researches.Notes and references
- R. V. Hoffman, in Organic Chemistry: An Intermediate Text, John Wiley & Sons, Inc., 2nd edn, 2004, ch. 8 Search PubMed.
- E. von Frankland, Liebigs Ann., 1849, 71, 171–213 CrossRef.
- E. W. Abel, F. G. A. Stone and G. Wilkinson, Comprehensive Organometallic Chemistry II: A Review of the Literature 1982–1994, Pergamon, Oxford; New York, 1995 Search PubMed.
- D. M. P. Mingos and R. H. Crabtree, Comprehensive Organometallic Chemistry III, Elsevier, Amsterdam, Boston, 2007 Search PubMed.
- P. Knochel and I. Wiley, Handbook of Functionalized Organometallics: Applications in Synthesis, Wiley-VCH, Weinheim, 2005 Search PubMed.
- D. H. B. Ripin, in Practical Synthetic Organic Chemistry, ed. S. Caron, 2011, ch. 12 Search PubMed.
- R. Chinchilla, C. Nájera and M. Yus, Chem. Rev., 2004, 104, 2667–2722 CrossRef CAS PubMed.
- S. Reformatsky, Ber. Dtsch. Chem. Ges., 1887, 20, 1210–1211 CrossRef.
- P. Barbier, C. R. Acad. Sci., 1899, 128, 110 CAS.
- V. Grignard, C. R. Acad. Sci., 1900, 130, 1322 CAS.
- H. Gilman, R. G. Jones and L. A. Woods, J. Org. Chem., 1952, 17, 1630–1634 CrossRef CAS.
- M. J. Harvey, in Alkali Metals: Organometallic Chemistry, Encyclopedia of Inorganic and Bioinorganic Chemistry, 2014 Search PubMed.
- B. C. G. Söderberg, Coord. Chem. Rev., 2008, 252, 57–133 CrossRef.
- R. F. Heck, Acc. Chem. Res., 1979, 12, 146–151 CrossRef CAS.
- E.-I. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738–6764 CrossRef CAS PubMed.
- A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef CAS PubMed.
- R. H. Grubbs, Angew. Chem., Int. Ed., 2006, 45, 3760–3765 CrossRef CAS PubMed.
- R. R. Schrock, Angew. Chem., Int. Ed., 2006, 45, 3748–3759 CrossRef CAS PubMed.
- Y. Chauvin, Angew. Chem., Int. Ed., 2006, 45, 3740–3747 CrossRef PubMed.
- W. S. Knowles, Adv. Synth. Catal., 2003, 345, 3–13 CrossRef CAS.
- N. Ryoji, Angew. Chem., Int. Ed., 2002, 41, 2008–2022 CrossRef.
- K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2024–2032 CrossRef CAS PubMed.
- W. Keim, Fossil Feedstocks–What Comes After?, Springer-Verlag, Berlin Heidelberg, 2014 Search PubMed.
- M. Z. Jacobson, Air Pollution and Global Warming: History, Science, and Solutions, Cambridge University Press, 2nd edn, 2012 Search PubMed.
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148–173 RSC.
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- F. Cherubini, Energy Convers. Manage., 2010, 51, 1412–1421 CrossRef CAS.
- J. H. Clark, J. Chem. Technol. Biotechnol., 2007, 82, 603–609 CrossRef CAS.
- C.-J. Li, Green Chem., 2016, 18, 1836–1838 RSC.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- R. A. Sheldon, Chem. Tech., 1994, 24, 38–47 CAS.
- P. A. Wender, V. A. Verma, T. J. Paxton and T. H. Pillow, Acc. Chem. Res., 2008, 41, 40–49 CrossRef CAS PubMed.
- A. L. Allred, J. Inorg. Nucl. Chem., 1961, 17, 215–221 CrossRef CAS.
- G. van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112 CrossRef CAS PubMed.
- X. Cui, S. Mao, M. Liu, H. Yuan and Y. Du, Langmuir, 2008, 24, 10771–10775 CrossRef CAS PubMed.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed.
- R. N. Butler and A. G. Coyne, Org. Biomol. Chem., 2016, 14, 9945–9960 RSC.
- Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492–5502 CrossRef CAS PubMed.
- C.-J. Li and T. H. Chan, Organic Reactions in Aqueous Media, John Wiely & Sons, New York, 1997 Search PubMed.
- C.-J. Li, Chem. Rev., 2005, 105, 3095–3166 CrossRef CAS PubMed.
- F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry: A Comprehensive Text, Wiley, New York, 1980 Search PubMed.
- W. Peters, Ber. Dtsch. Chem. Ges., 1905, 38, 2567 CrossRef CAS.
- K. Sisido, Y. Takeda and Z. Kinugawa, J. Am. Chem. Soc., 1961, 83, 538–541 CrossRef.
- K. Sisido, S. Kozima and T. Hanada, J. Organomet. Chem., 1967, 9, 99–107 CrossRef CAS.
- K. Sisido and S. Kozima, J. Organomet. Chem., 1968, 11, 503–513 CrossRef CAS.
- J. Nosek, Collect. Czech. Chem. Commun., 1964, 29, 597–602 CrossRef CAS.
- J. Nokami, J. Otera, T. Sudo and R. Okawara, Organometallics, 1983, 2, 191–193 CrossRef CAS.
- C.-J. Li and T. H. Chan, Tetrahedron Lett., 1991, 32, 7017–7020 CrossRef CAS.
- T.-P. Loh, Q.-Y. Hu, Y.-K. Chok and K.-T. Tan, Tetrahedron Lett., 2001, 42, 9277–9280 CrossRef CAS.
- X.-H. Yi, Y. Meng and C.-J. Li, Chem. Commun., 1998, 449–450 RSC.
- X.-H. Yi, Y. Meng, X.-G. Hua and C.-J. Li, J. Org. Chem., 1998, 63, 7472–7480 CrossRef CAS PubMed.
- M. B. Isaac and T.-H. Chan, J. Chem. Soc., Chem. Commun., 1995, 1003–1004 RSC.
- T. M. Mitzel, C. Palomo and K. Jendza, J. Org. Chem., 2001, 67, 136–145 CrossRef.
- L. W. Bieber, E. C. Storch, I. Malvestiti and M. F. da Silva, Tetrahedron Lett., 1998, 39, 9393–9396 CrossRef CAS.
- C.-J. Li and Y. Meng, J. Am. Chem. Soc., 2000, 122, 9538–9539 CrossRef CAS.
- T. Huang, Y. Meng, S. Venkatraman, D. Wang and C.-J. Li, J. Am. Chem. Soc., 2001, 123, 7451–7452 CrossRef CAS PubMed.
- C.-J. Li, Tetrahedron, 1996, 52, 5643–5668 CrossRef CAS.
- L. Rodolfo, C. Oscar, B. Joan, O. Modesto and L. F. Javier, Eur. J. Org. Chem., 2001, 3719–3729 Search PubMed.
- D. Backhaus, Tetrahedron Lett., 2000, 41, 2087–2090 CrossRef CAS.
- J. S. Yadav and C. Srinivas, Tetrahedron Lett., 2002, 43, 3837–3839 CrossRef CAS.
- A. Jeevanandam and Y.-C. Ling, Tetrahedron Lett., 2001, 42, 4361–4362 CrossRef CAS.
- T. H. Chan and C.-J. Li, J. Chem. Soc., Chem. Commun., 1992, 747–748 RSC.
- A. Dondoni, P. Merino and J. Orduna, Tetrahedron Lett., 1991, 32, 3247–3250 CrossRef CAS.
- D. M. Gordon and G. M. Whitesides, J. Org. Chem., 1993, 58, 7937–7938 CrossRef CAS.
- E. Kim, D. M. Gordon, W. Schmid and G. M. Whitesides, J. Org. Chem., 1993, 58, 5500–5507 CrossRef CAS.
- J. Gao, R. Haerter, D. M. Gordon and G. M. Whitesides, J. Org. Chem., 1994, 59, 3714–3715 CrossRef CAS.
- M. D. Chappell and R. L. Halcomb, Org. Lett., 2000, 2, 2003–2005 CrossRef CAS PubMed.
- M. Warwel and W.-D. Fessner, Synlett, 2000, 865–867 CAS.
- T. H. Chan and C.-J. Li, Organometallics, 1990, 9, 2649–2650 CrossRef CAS.
- C.-J. Li and T. H. Chan, Organometallics, 1991, 10, 2548–2549 CrossRef CAS.
- B. M. Trost and S. A. King, J. Am. Chem. Soc., 1990, 112, 408–422 CrossRef CAS.
- Y.-Q. Lu and C.-J. Li, Tetrahedron Lett., 1996, 37, 471–474 CrossRef CAS.
- C.-J. Li, D.-L. Chen, Y.-Q. Lu, J. X. Haberman and J. T. Mague, J. Am. Chem. Soc., 1996, 118, 4216–4217 CrossRef CAS.
- C.-J. Li, D.-L. Chen, Y.-Q. Lu, J. X. Haberman and J. T. Mague, Tetrahedron, 1998, 54, 2347–2364 CrossRef CAS.
- C.-J. Li and D.-L. Chen, Synlett, 1999, 735–736 CrossRef CAS.
- J. X. Haberman and C.-J. Li, Tetrahedron Lett., 1997, 38, 4735–4736 CrossRef CAS.
- L. A. Paquette and T. M. Mitzel, J. Am. Chem. Soc., 1996, 118, 1931–1937 CrossRef CAS.
- L. A. Paquette, Synthesis, 2003, 765–774 CrossRef CAS.
- F. Márquez, R. Montoro, A. Llebaria, E. Lago, E. Molins and A. Delgado, J. Org. Chem., 2002, 67, 308–311 CrossRef.
- T.-P. Loh and J.-R. Zhou, Tetrahedron Lett., 1999, 40, 9115–9118 CrossRef CAS.
- T.-P. Loh and J.-R. Zhou, Tetrahedron Lett., 2000, 41, 5261–5264 CrossRef CAS.
- Y.-C. Teo, E.-L. Goh and T.-P. Loh, Tetrahedron Lett., 2005, 46, 6209–6211 CrossRef CAS.
- S. Kobayashi, N. Aoyama and K. Manabe, Chirality, 2003, 15, 124–126 CrossRef CAS PubMed.
- U. Schneider, M. Ueno and S. Kobayashi, J. Am. Chem. Soc., 2008, 130, 13824–13825 CrossRef CAS PubMed.
- T. M. Mitzel, C. Palomo and K. Jendza, J. Org. Chem., 2002, 67, 136–145 CrossRef CAS PubMed.
- D. Prajapati, D. D. Laskar, B. J. Gogoi and G. Devi, Tetrahedron Lett., 2003, 44, 6755–6757 CrossRef CAS.
- H. Miyabe, Y. Yamaoka, T. Naito and Y. Takemoto, J. Org. Chem., 2004, 69, 1415–1418 CrossRef CAS PubMed.
- C. Zhou, J. Jiang, Y. Zhou, Z. Xie, Q. Miao and Z. Wang, Lett. Org. Chem., 2005, 2, 61–64 CrossRef CAS.
- B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2008, 41, 59 CAS.
- M. Ueda and N. Miyaura, J. Org. Chem., 2000, 65, 4450–4452 CrossRef CAS PubMed.
- C.-J. Li, Acc. Chem. Res., 2002, 35, 533–538 CrossRef CAS PubMed.
- M. Sakai, M. Ueda and N. Miyaura, Angew. Chem., Int. Ed., 1998, 37, 3279–3281 CrossRef CAS PubMed.
- K. Fagnou and M. Lautens, Chem. Rev., 2003, 103, 169–196 CrossRef CAS PubMed.
- T. Yamamoto, T. Ohta and Y. Ito, Org. Lett., 2005, 7, 4153–4155 CrossRef CAS PubMed.
- S. Lin and X. Lu, J. Org. Chem., 2007, 72, 9757–9760 CrossRef CAS PubMed.
- T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829–2844 CrossRef CAS PubMed.
- P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95–119 CrossRef CAS.
- X. Lu and S. Lin, J. Org. Chem., 2005, 70, 9651–9653 CrossRef CAS PubMed.
- S. Lin and X. Lu, Org. Lett., 2010, 12, 2536–2539 CrossRef CAS PubMed.
- K. Kikushima, J. C. Holder, M. Gatti and B. M. Stoltz, J. Am. Chem. Soc., 2011, 133, 6902 CrossRef CAS PubMed.
- C.-G. Feng, M.-H. Xu and G.-Q. Lin, Synlett, 2011, 1345–1356 CAS.
- C. L. Hawkins and M. J. Davies, Biochim. Biophys. Acta, Bioenerg., 2001, 1504, 196–219 CrossRef CAS.
- M. Gutowski and S. Kowalczyk, Acta Biochim. Pol., 2013, 60, 1–16 CAS.
- H. Yorimitsu, T. Nakamura, H. Shinokubo and K. Oshima, J. Org. Chem., 1998, 63, 8604–8605 CrossRef CAS.
- H. Yorimitsu, T. Nakamura, H. Shinokubo, K. Oshima, K. Omoto and H. Fujimoto, J. Am. Chem. Soc., 2000, 122, 11041–11047 CrossRef CAS.
- C. Petrier, C. Dupuy and J. L. Luche, Tetrahedron Lett., 1986, 27, 3149–3152 CrossRef CAS.
- J. L. Luche and C. Allavena, Tetrahedron Lett., 1988, 29, 5369–5372 CrossRef CAS.
- C. Dupuy, C. Petrier, L. A. Sarandeses and J. L. Luche, Synth. Commun., 1991, 21, 643–651 CrossRef CAS.
- B. Giese, W. Damm, M. Roth and M. Zehnder, Synlett, 1992, 441–443 CrossRef CAS.
- P. Erdmann, J. Schäfer, R. Springer, H.-G. Zeitz and B. Giese, Helv. Chim. Acta, 1992, 75, 638–644 CrossRef CAS.
- J. L. Luche, C. Allavena, C. Petrier and C. Dupuy, Tetrahedron Lett., 1988, 29, 5373–5374 CrossRef CAS.
- K. Michał Pietrusiewicz and M. Zabłocka, Tetrahedron Lett., 1988, 29, 937–940 CrossRef.
- M. Roth, W. Damm and B. Giese, Tetrahedron Lett., 1996, 37, 351–354 CrossRef CAS.
- R. M. Suárez, J. P. Sestelo and L. A. Sarandeses, Synlett, 2002, 1435–1438 Search PubMed.
- R. M. Suárez, J. Pérez Sestelo and L. A. Sarandeses, Chem.–Eur. J., 2003, 9, 4179–4187 CrossRef PubMed.
- T. Huang, C. C. K. Keh and C.-J. Li, Chem. Commun., 2002, 2440–2441 RSC.
- C.-J. Li and W.-C. Zhang, J. Am. Chem. Soc., 1998, 120, 9102–9103 CrossRef CAS.
- H. Miyabe, M. Ueda, A. Nishimura and T. Naito, Org. Lett., 2002, 4, 131–134 CrossRef CAS PubMed.
- M. Ueda, H. Miyabe, A. Nishimura, O. Miyata, Y. Takemoto and T. Naito, Org. Lett., 2003, 5, 3835–3838 CrossRef CAS PubMed.
- C. C. K. Keh, C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 4062–4063 CrossRef CAS PubMed.
- Z.-L. Shen, Y.-L. Yeo and T.-P. Loh, J. Org. Chem., 2008, 73, 3922–3924 CrossRef CAS PubMed.
- T. H. Chan, C.-J. Li and Z. Y. Wei, J. Chem. Soc., Chem. Commun., 1990, 505–507 RSC.
- J.-Y. Zhou, Y. Jia, Q.-Y. Shao and S.-H. Wu, Synth. Commun., 1996, 26, 769–775 CrossRef CAS.
- T. H. Chan, C.-J. Li, M. C. Lee and Z. Y. Wei, Can. J. Chem., 1994, 72, 1181–1192 CrossRef CAS.
- P. H. Lee, K. Bang, K. Lee, S.-y. Sung and S. Chang, Synth. Commun., 2001, 31, 3781–3789 CrossRef CAS.
- L. W. Bieber, I. Malvestiti and E. C. Storch, J. Org. Chem., 1997, 62, 9061–9064 CrossRef CAS.
- W. J. Chung, S. Higashiya and J. T. Welch, J. Fluorine Chem., 2001, 112, 343–347 CrossRef CAS.
- J. B. F. N. Engberts, in Methods and Reagents for Green Chemistry, ed. P. Tundo, A. Perosa and F. Zecchini, John Wiley & Sons, Inc., NJ, 2007, ch. 7, pp. 159–170 Search PubMed.
- D. Torsten, P. Eckhard and O. Günther, Angew. Chem., Int. Ed., 2005, 44, 7174–7199 CrossRef PubMed.
- T. Hamada, K. Manabe and S. Kobayashi, J. Am. Chem. Soc., 2004, 126, 7768–7769 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Huang, W. W. Y. Leong, G. Zhong and N. A. Isley, J. Am. Chem. Soc., 2012, 134, 19985–19988 CrossRef CAS PubMed.
- B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2012, 45, 3 CAS.
- B. H. Lipshutz, S. Ghorai and M. Cortes-Clerget, Chem.–Eur. J., 2018, 24, 6672–6695 CrossRef CAS PubMed.
- F. Zhou and C.-J. Li, Nat. Commun., 2014, 5, 4254 CrossRef CAS PubMed.
- D. J. Lippincott, R. T. H. Linstadt, M. R. Maser, F. Gallou and B. H. Lipshutz, Org. Lett., 2018, 20, 4719–4722 CrossRef CAS PubMed.
- L. Cicco, S. Sblendorio, R. Mansueto, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Sci., 2016, 7, 1192–1199 RSC.
- G. Dilauro, M. Dell'Aera, P. Vitale, V. Capriati and F. M. Perna, Angew. Chem., Int. Ed., 2017, 56, 10200–10203 CrossRef CAS PubMed.
- J. García-Álvarez, E. Hevia and V. Capriati, Eur. J. Org. Chem., 2015, 6779–6799 CrossRef.
- J. García-Álvarez, E. Hevia and V. Capriati, Chem.–Eur. J., 2018, 24, 14854–14863 CrossRef PubMed.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- J.-Q. Yu, L. Ackermann and Z. Shi, in C–H Activation, Topics in Current Chemistry, Springer, Berlin, 2010, ch. 292 Search PubMed.
- P. H. Dixneuf and H. Doucet, in C–H Bond Activation and Catalytic Functionalization I Topics in Organometallic Chemistry, Springer, Cham, 2015 Search PubMed.
- P. H. Dixneuf and H. Doucet, in C–H Bond Activation and Catalytic Functionalization II Topics in Organometallic Chemistry, Springer, Cham, 2016 Search PubMed.
- C. I. Herrerías, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed.
- C. Wei and C.-J. Li, Green Chem., 2002, 4, 39–41 RSC.
- C.-J. Li and C. Wei, Chem. Commun., 2002, 268–269 RSC.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2002, 124, 5638–5639 CrossRef CAS PubMed.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed.
- (a) X. Yao and C.-J. Li, Org. Lett., 2005, 7, 4395–4398 CrossRef CAS PubMed; (b) C.-J. Li, Acc. Chem. Res., 2010, 43, 581–590 CrossRef CAS PubMed.
- P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Angew. Chem., Int. Ed., 2010, 49, 6629–6632 CrossRef CAS PubMed.
- L. Ackermann and A. V. Lygin, Org. Lett., 2012, 14, 764–767 CrossRef CAS PubMed.
- M.-Z. Lu, P. Lu, Y.-H. Xu and T.-P. Loh, Org. Lett., 2014, 16, 2614–2617 CrossRef CAS PubMed.
- H. Gong, H. Zeng, F. Zhou and C.-J. Li, Angew. Chem., Int. Ed., 2015, 54, 5718–5721 CrossRef CAS PubMed.
- B. Wang, W. A. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952–3957 RSC.
- J. Hu, T. Lan, Y. Sun, H. Chen, J. Yao and Y. Rao, Chem. Commun., 2015, 51, 14929–14932 RSC.
- G. Deng and C.-J. Li, Tetrahedron Lett., 2008, 49, 5601–5604 CrossRef CAS.
- D. Seebach and E. J. Corey, J. Org. Chem., 1975, 40, 231–237 CrossRef CAS.
- B.-T. GrÖBel and D. Seebach, Synthesis, 1977, 357–402 CrossRef.
- S. Gustavo, Curr. Org. Chem., 2000, 4, 283–304 Search PubMed.
- J. Sukumaran and U. Hanefeld, Chem. Soc. Rev., 2005, 34, 530–542 RSC.
- M. Pohl, G. A. Sprenger and M. Müller, Curr. Opin. Biotechnol., 2004, 15, 335–342 CrossRef CAS PubMed.
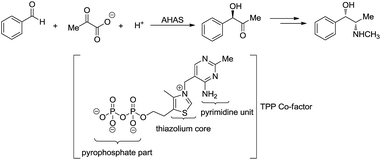
- R. S. Menon, A. T. Biju and V. Nair, Beilstein J. Org. Chem., 2016, 12, 444–461 CrossRef CAS PubMed.
- D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534–541 CrossRef CAS PubMed.
- H. Wang, X.-J. Dai and C.-J. Li, Nat. Chem., 2016, 9, 374–378 CrossRef PubMed.
- N. Chen, X.-J. Dai, H. Wang and C.-J. Li, Angew. Chem., Int. Ed., 2017, 56, 6260–6263 CrossRef CAS PubMed.
- X.-J. Dai, H. Wang and C.-J. Li, Angew. Chem., Int. Ed., 2017, 56, 6302–6306 CrossRef CAS PubMed.
- L. Lv, D. Zhu, J. Tang, Z. Qiu, C.-C. Li, J. Gao and C.-J. Li, ACS Catal., 2018, 8, 4622–4627 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |