Representations of chemical phenomena in secondary school chemistry textbooks
Johnson
Enero Upahi
 *ab and
Umesh
Ramnarain
*ab and
Umesh
Ramnarain
 a
a
aDepartment of Science and Technology Education, Faculty of Education, University of Johannesburg, Auckland Park Kingsway Campus, Johannesburg, South Africa. E-mail: johnsonenero@yahoo.com; upahie@uj.ac.za
bDepartment of Science Education, Faculty of Education, University of Ilorin, Ilorin, Nigeria
First published on 1st September 2018
Abstract
The difficulties encountered by students in learning chemistry range from human factors to the intrinsic nature of chemistry. To enhance students’ understanding of chemistry, there is a wide consensus within the community of chemistry educators on the importance of and need to integrate different levels of representations in chemistry teaching and learning resources. As learning resources, textbooks are ubiquitous and usually readily available to both students and teachers. Therefore, this study investigated how chemical phenomena are represented or depicted in secondary school chemistry textbooks. We adopted a rubric developed by Gkitzia et al. (Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12, 5–14) to analyze the textbooks for types of representations; relatedness of chemical representations to text; and the appropriateness of captions. The results indicated the dominance of symbolic representations, followed by sub-microscopic, then hybrid and multiple representations. In all three textbooks, there was no evidence of mixed representation. While many of the chemical representations were completely related to the texts, some were unlinked. The germaneness of suitable captions in textbooks is in the explicit, brief and concise explanation that captions give to an entire representation. While our results indicated that more than half of the representations had suitable captions, there was evidence of representations that were problematic and had no captions. The implication of these results for students’ cognitive load, and the need for textbook-users to explore alternative resources that depict phenomena in 2D or 3D representations are discussed.
Introduction
Textbooks play important roles as sources of information for both teachers and students to support science teaching and learning (Chiappetta and Koballa, 2002; Abd-El-Khalick et al., 2008). Around the world, chemistry teachers rely on textbooks as their primary teaching instruments (e.g., as curriculum, guidebooks) on what chemical concepts to teach and how students are expected to learn these concepts (Chiappetta and Fillman, 2007; Gkitzia et al., 2011; Upahi and Jimoh, 2015). Given that textbooks have remained a major source of information for chemistry instruction, the credibility of the information offered in the textbooks should be of major concern to researchers and science educators. Do chemical representations convey meanings that can inform effective learning? Mohammed and Kumari (2007) analyzed teachers’ experiences with the use of science textbooks and reported that teachers had difficulties recognizing mistakes in the textbooks. In a specific-domain discipline, researchers have also shown that inappropriate use of language in chemistry constituted barriers that manifested as misconceptions or alternative conceptions for learners in certain chemical concepts such as chemical bonding (Bergqvist et al., 2013), chemical equilibrium (Pedrosa and Dias, 2000) and electrochemistry (Sanger and Greenbowe, 1999). While the issue of language in science (particularly in chemistry) education has remained a dominant area of research, researchers have also continued to explore ways to reduce learning difficulties that arise from the way teachers and learning resources (e.g. textbooks) present chemical concepts.There is a popular notion among students that chemistry is an abstract and ‘a conceptually difficult subject in the school curriculum’ (Childs and Sheehan, 2009, p. 204). To address the abstract nature of chemistry, Alex H. Johnstone thought of chemistry in three forms namely, the macro and tangible: as what can be seen, touched and smelled; the submacro: as atoms, molecules, ions and structures; and the representational: as symbols, formulae, equations, molarity, mathematical manipulation and graphs. He argued that if chemistry is to be fully understood, there must be flexible movement or translation to the sub-microscopic level, where the behaviour of substances are interpreted in terms of the unseen and molecular, and recorded in some symbolic language and notations (Johnstone, 2000a). These ideas have since influenced chemistry education research (Johnstone, 2000a, 2000b; Talanquer, 2011; Taber, 2013). Research outcomes have shown that when chemistry is presented at these three levels of representations, learners tend to develop conceptual understandings of chemical phenomena (e.g., Treagust et al., 2003; Ainsworth, 2006).
Given that representations play a central role in facilitating learning, appropriate representations in chemistry textbooks could minimize students’ difficulties in understanding abstract chemical concepts. The extent to which textbooks clearly depict scientifically correct and relevant images, can help students develop correct mental models of chemical phenomena, and enable them to translate among the different levels of representation. Therefore, the aim of this study was to investigate how chemical phenomena are represented or depicted in secondary school chemistry textbooks. We adopted suitable criteria (discussed in a later section) to examine chemical representations in textbooks. Specifically, the study examined the: types of representations; relatedness of representations to texts; and the properties of captions in representations. The study was guided by the following research questions: (1) What types of representations are featured in the Nigerian chemistry textbooks? (2) To what extent do the chemical representations relate to the texts? and (3) How suitable are the properties of captions?
Theoretical background
The concepts and principles in chemistry range from concrete to abstract. Johnstone (1991, 2000a) noted that chemistry can be represented at three levels: the macroscopic, the sub-microscopic and symbolic. Johnstone (2000b) described these three levels as corners of a triangle, with each vertex of the triangle corresponding to a chemistry level, which are considered together as levels of chemical representations (Jaber and BouJaoude, 2012). According to Johnstone (1991), when students are engaged in chemistry at a “multi-level thinking” (Taber, 2013), they are most like to find the subject difficult and challenging.As noted earlier, chemistry education research has benefitted tremendously from Johnstone's model of the “chemistry triplet” as it has informed curriculum reforms and classroom practice (Taber, 2013, p. 156). However, researchers have subjected the ideas of the chemistry triplet presented by Johnstone to different “adaptations and reinterpretations” (Taber, 2013, p. 158). For instance, the original levels described by Johnstone have been interpreted as levels of instruction (Gabel, 1999), types of representations (Gilbert and Treagust, 2009), and three major ways of chemical representations (Talanquer, 2011). Gilbert and Treagust (2009) also modified the original chemistry levels of macroscopic, sub-microscopic and representational as phenomenological, model and symbolic respectively. However, Talanquer (2011) observed and cautioned that the different connotations ascribed to the levels of representations by researchers could be another source of confusion, especially if the connotations are meant to convey the same meaning for each of the chemistry triplet.
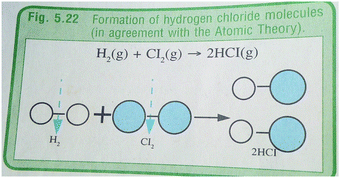
As useful as Johnstone's (1991) model of chemical representations may appear, scholars have criticized it, and noted that while the three levels of representation are thought to be located in the vertices of a triangle, the nature of a chemical representation located in a given corner of the triangle may exhibit characteristics that goes beyond the vertex to which it is assigned (Nyachwaya and Wood, 2014). Put in context, Taber (2013) draws on the example of a chemical equation representing a reaction to show that the equation transcends the ‘different entities’ involved in the reaction to include ‘relationships in a chemical process’. For an equation with symbolic representation such as: H2(g) + Cl2(g) → 2HCl(g), the macroscopic level is depicted in the equation by the substances that react to form a new product, while the sub-microscopic level shows the symbols of molecules in the reactants and product, and in the proportions (mole/volume ratios) in which the reaction occurs. The failure of this explanation to show that a representation transcends its originally assigned corner in Johnstone's triangle, increases the chances of it being misinterpreted by students, especially if a teacher or textbook does not clarify the context in which a chemical equation is used. This further confirms how students' difficulties in chemistry emerge from the “intrinsic nature of the subject” (Johnstone, 2000b, p. 9).
Researchers have proposed additional levels to those conceptualized by Johnstone. Dori and Hameiri (2003) proposed the process level that incorporates the translation of macro, micro and symbolic levels in the process of chemical reactions. Gkitzia et al. (2011) proposed and validated other levels of representations at which chemical phenomena on two or three levels of chemistry depict either multiple, hybrid or mixed representations (see full description in later section). Dangur et al. (2014) also proposed the quantum level that describe the electronic configuration of ions, molecules, atoms, the solid state and how they relate to quantum mechanics.
Given that students find certain chemistry concepts difficult to understand. The sources of students’ difficulties in learning chemistry are diverse. However, Johnstone (1991) noted that understanding chemistry in a meaningful way requires that students are informed about the three different levels of representations chemical phenomena exist in: the macroscopic, sub-microscopic and the symbolic levels (Chandrasegaran et al., 2007). Building on Johnstone's triplet chemistry, Gkitzia et al. (2011) model provides a theoretical background that informs the types of representations we expect to see in chemistry instruction and textbooks.
Review of related literature on chemical representations
Within chemistry education, there is a widespread of literature that has documented students’ difficulties in transforming or translating among the levels of representations (e.g.Nyachwaya et al., 2011; Özmen, 2011; Naah and Sanger, 2012; Ramnarain and Joseph, 2012). Conversely, Gabel (1999) noted that while teachers are able to translate among the different representational levels in their courses, they do not make a deliberate effort to integrate these levels in chemistry teaching. Consequently, this contribute to the learning difficulty experienced by high school students in chemistry (Ramnarain and Joseph, 2012). Researchers have suggested that when teachers scaffold learners to move among the representational levels, there may be improvement in their conceptual understanding and they may become more successful in chemistry (Johnstone, 1993; Gabel, 1999; Chandrasegaran et al., 2008; Ramnarain and Joseph, 2012). However, one must question whether it will not be too challenging for teachers who operate within the confines of a curriculum that is driven by high-stakes testing and summative assessments to engage learners at the different levels of representations of chemical phenomena. A solution to this concern could be to turn to textbooks, which usually contain a reasonable number of representations, many of which are pictures or photographs.For meaningful understanding of the abstract concepts in chemistry education, representations in textbooks play a very significant role in reducing students’ difficulties with such concepts (Özalp and Kahveci, 2011). Given the critical role played by textbooks in science learning, we examined how textbooks portray chemical representations and the overall meaning they convey. Studies have analyzed textbooks for representations in different countries and contexts, and at different levels of education (Chiappetta et al., 1991; Harrison, 2001; Nyachwaya and Gillaspie, 2016). For instance, Chiappetta et al. (1991) investigated pictures or diagrams in seven high school chemistry textbooks in the United States (US). Harrison (2001) examined models used in Australian high school chemistry textbooks and reported that iconic and symbolic models were frequently used. Nyachwaya and Gillaspie (2016) examined representations in college chemistry textbooks for the number of representations, physical integration with the text, figure indexing, extended captions, labelling, representation function, and conceptual integration. However, more studies are reported on textbook analyses for representations of chemical phenomena that have used the same rubric (Gkitzia et al., 2011; Nyachwaya and Wood, 2014; Kapici and Acikalin-Savasci, 2015; Demirdöğen, 2017; Shehab and BouJaoude, 2017). These are reviewed here.
Gkitzia et al. (2011) developed a rubric that was applied in the analysis of five Greek chemistry textbooks for grade 10. Specifically, the authors applied the rubric to evaluate chemistry representations in textbooks for grades 9 and 10 in Greek high schools and in two other Greek and one American university textbooks. However, the study only reported findings for grade 10 Greek chemistry textbook. The results showed a dispersion of the representational types in the textbook, with an equal proportion of macroscopic and sub-microscopic at 23.6% each as the highest, followed by multiple representations. It is significant to note that while many of the multiple representations had a combination of two out of the three levels of chemical representations, only one multiple representation was at three levels. With respect to relatedness to text, more than half of the images were completely related to the corresponding chemical phenomena. On the other hand, findings on the existence and properties of captions showed that about half of the visual representations were either problematic or had no caption.
Nyachwaya and Wood (2014) adopted the rubric developed by Gkitzia et al. (2011) to investigate the nature and types of representations used in physical chemistry textbooks in the US. The results indicated that 85% of the representations were symbolic representations. The sub-microscopic was slightly higher than the macroscopic and multiple representations, which collectively accounted for the remaining 15%. It is noteworthy that all the identified representations in the analyzed textbooks were completely related and linked to the corresponding text. In relation to captions, the researchers did not explicitly specify the proportion of representations with captions but mentioned that representations with captions had 100% of the captions described as concise, explicit and suitable.
Similarly, Kapici and Acikalin-Savasci (2015) examined visual representations about the particulate nature of matter across grades 6–8 science textbooks used in Turkey and reported that macroscopic representations were the most prevalent, followed by sub-microscopic, then symbolic and multiple. The fewest representations used were the hybrid and mixed. With respect to the degree of relatedness to text, the result indicates that 4 out of 10 images were completely related and linked. A significant finding was that more than half (63%) of the images had no captions.
In Lebanon, Shehab and BouJaoude (2017) applied the same rubric to examine grades 9, 11 and 12 high school chemistry textbooks and reported that the textbooks were focused on macroscopic, sub-microscopic, and symbolic levels with implicit or ambiguous labels. In terms of properties of captions, approximately two-third of the representations were problematic. This means that the interpretation of surface features of chemical phenomena is left to the readers and only one-third of the representations mentioned the surface features explicitly. In terms of relation to the text content, many of the representations were completely related to the texts. Also, most of the representations were appropriately captioned.
More recently, Demirdöğen (2017) also examined chemical representations presented in four Turkish high school chemistry textbooks. The results are not too different from the earlier studies. Findings revealed that images frequently used were at macroscopic, symbolic and hybrid levels. In relation to surface features, representations were explicit to the text content with appropriate captions. Representations were completely related to the texts, and multiple representations identified in the texts had sufficient links to the text content.
Despite the significant role played by textbooks as the most readily available resources for science teaching, Bunce and Gabel (2002) contend that chemistry education does not emphasize the sub-microscopic level of chemical concepts. In recent studies, this assertion is still illustrated in research findings on textbook analysis (Nyachwaya and Wood, 2014; Kapici and Acikalin-Savasci, 2015; Demirdöğen, 2017). Our assumption is that if chemistry is understood at the sub-microscopic level; on the basis that this level of chemical representation could help students develop mental models of vast chemical phenomena that are intangible, the prevalent representations in the analyzed textbooks should ordinarily favour sub-microscopic representations (Chittleborough and Treagust, 2007; Davidowitz and Chittleborough, 2009).
In addition, representations such as hybrid, multiple and mixed that combine elements from the three levels of chemistry described by Johnstone (1991) have been confirmed to stimulate students’ attention and enable them to translate or move among the representational levels (Ainsworth, 1999). Their use to depict chemical phenomena plays an essential role to complement the different representations to impart the same knowledge, constrain interpretation using the inherent properties of the representations and to ultimately achieve a deeper understanding of an abstract chemical concept. While Kapici and Acikalin-Savasci (2015) reported that multiple representations had lowest prevalence, Nyachwaya and Wood (2014) reported the non-existence of mixed or hybrid representations in the textbooks. Given these findings, our study intends to contribute to the body of knowledge on how chemistry textbooks used in Nigeria address and incorporate chemical representations at multiple levels.
Rationale for the study
Following the previous sections on theoretical background/framework and the discussions in the literature review, we suggest that students have difficulties in understanding chemistry at each representational level and in moving effortlessly among the different levels (Chittleborough and Treagust, 2008; Davidowitz and Chittleborough, 2009; Kern et al., 2010; Nyachwaya et al., 2011; Naah and Sanger, 2012; Ramnarain and Joseph, 2012). Wu and Shah (2004) noted that ‘chemistry is a representative, symbolic and a visual science’ that requires representations to help learners construct mental images of the behaviours of chemical entities (Mathewson, 2005). This is because visuals facilitate learners’ interpretation of chemistry at sub-microscopic level, if they understand that macroscopic substances are composed of sub-microscopic molecules that can be translated into symbolic representations (O’Dwyer and Childs, 2014). Uttal and O’Doherty (2008) described the construct of “visualization” as any kind of physical representation devised to make an abstract concept perceptible (Kozma and Russell, 2005). Similarly, Gkitzia et al. (2011) suggested that visualization in chemistry could help learners to create correct mental images of chemical phenomena in their minds to aid meaningful understanding. Since the formation of a mental image of a chemical concept is central for learning, the extent to which textbooks for chemistry learning depict or portray these phenomena requires investigation.In Nigeria, research with respect to the analysis of science textbooks, particularly in chemistry is not widespread. There are comparatively fewer studies on chemistry textbooks analysis that relates to the nature of science (Upahi et al., 2018), and classification of end-of-chapter questions (Upahi and Jimoh, 2015). It is evident that researchers and science educators do not seem to be paying sufficient attention to this important area of research. To the best of our knowledge, there is no research on the analysis of how chemical phenomena are depicted in chemistry textbooks used in Nigeria. Therefore, the study discussed in this paper examined how chemistry textbooks can contribute to helping students develop mental images of chemical concepts through the different levels of representations.
Following the development and application of the rubric by Gkitzia and colleagues, preceding paragraphs have reported studies that applied the same rubric to analyze how the use of representations in chemistry textbooks in different cultural contexts contribute to students’ learning. Such studies are important because of the possible influences of cultural factors in different contexts (Han and Roth, 2006). The authors believe that this study could be relevant to a wider readership given that, the results could provide valuable information and reference for other researchers, for comparison with findings from international studies on how chemical phenomena are represented in chemistry textbooks. Research on chemistry textbooks is especially significant for developing countries, as findings could inform revision of textbooks and curriculum documents – that will pay adequate attention to the formation of mental images of chemical phenomena which are often underestimated, and sometimes neglected by teachers and textbook authors. With this study, we hope to contribute towards improving the coherence of chemical representations in textbooks.
Analytical framework
The earlier section presented a brief review of literature on a few studies that have adopted and applied the rubric developed by Gkitzia et al. (2011) to evaluate images/visual representations in chemistry textbooks. In this section, we describe each criterion contained in the rubric that was adopted as an analytical framework for the study. The framework is based on Johnstone's initial levels of representations (macroscopic, sub-microscopic and the symbolic). We understand that there are different adaptations and reinterpretation of this framework within the community of chemistry education researchers. However, the notion that all three levels of macro, sub-micro and symbolic can be used together to describe chemical phenomena informed three additional levels of hybrid, multiple and mixed representations by Gkitzia et al. (2011). It is these six levels/types of representations that formed the first criterion in the rubric for our analysis.1st criterion (C1): types of representations
In the first criterion, types of representations fall under six sub-categories: macroscopic, sub-microscopic, symbolic, multiple, hybrid and mixed (Gkitzia et al., 2011). The macroscopic, sub-microscopic and symbolic are based on Johnstone's (1991) original levels of chemical representations. The multiple representations portray a chemical phenomenon at two or three levels by combining two or three different types of representation simultaneously, usually indicating their complementary roles. In a hybrid representation, the characteristics of two or three levels/representations coexist to complement each other to form one representation. There is only a slight difference between multiple and hybrid representations. While a multiple representation depicts a phenomenon at two or three levels by combining two or three representations, hybrid representation combines the characteristics of two or three levels to form one representation. On the other hand, mixed representations could have one, two or the three types of representations (macro, sub-micro, and symbolic) and another type of visual depiction, for instance, an analogy co-existing together (Gkitzia et al., 2011). The mixed representations also serve as a bridge to understand the symbolic representations by using pictorial images (Ainsworth, 2008).2nd criterion (C2): relatedness to text
This criterion on relatedness of representation (image/visual) to text is used to investigate the extent to which a representation is comprehensible, related and linked to the text. In other words, this criterion is used not only to investigate the relationship between the images and the text, but also to analyze whether there is any link in the text that directs readers to the representations. Pozzer and Roth (2003) suggested the placement of important concepts and information in the text content with appropriate reference to the representation that could help the reader establish a link and thereby make sense of the phenomenon under investigation. Similarly, Cheng and Gilbert (2015) noted that students are only able to make sense of science concepts when they read texts and diagrams that are well-linked.The criterion has five sub-categories that characterize the representations: (i) completely related and linked, (ii) completely related and unlinked, (iii) partially related and linked, (iv) partially related and unlinked, (v) unrelated. According to Gkitzia et al. (2011), a representation is labeled completely related when it represents the exact text content. If the representation depicts a similar subject to the text but not the exact one, it is tagged partially related. In contrast, a representation is labeled unrelated when it is not connected to the text content. In addition, a representation is considered linked or unlinked when the text refers to it by using a direct link or not.
3rd criterion (C3): properties of captions
This criterion is used to investigate properties of captions to determine whether they are appropriate or problematic in relation to the representations. The caption attached to a representation is an important part of the representation, intended to inform the reader of what to lookout for in an image, and how to read and understand it (Pozzer and Roth, 2003). Appropriate captions play both complementary and constraining roles in interpretation. They complement what an image represents or portrays, and if it is appropriate, the image makes it easier for the reader to understand the text (Gkitzia et al., 2011). Captions can also constrain or limit the meaning readers could infer from an image, within the context of use. In this criterion, the properties of the captions are categorized into three sub-categories which include: suitable captions, problematic captions and no caption. The caption of an image is labeled ‘suitable’, if the caption explains exactly what the visual represents or portrays. In other words, for representations with suitable captions, the captions should ideally be “brief, explicit and comprehensible” (Gkitzia et al., 2011, p. 11). Conversely, if the caption does not accurately describe what the visual/image represents, it is considered as ‘problematic’. An image without a caption is tagged ‘no caption’.Research methods
The present study employed a qualitative research design to understand a specific phenomenon (Creswell, 2005), in this case, how chemical phenomena are represented in chemistry textbooks. The study used a qualitative content analysis of three chemistry textbooks recommended for use by the NERDC. Mouton (2008) described content analysis as a systematic, rigorous approach used to analyze documents by analyzing units such as paragraphs, activities, worked examples, figures with captions, tables with captions, charts with captions and marginal comments. Krippendorff (2004) stated that content analysis enables content analyst to better understand textual information and the “context of their use” (p. 18). In this study, this approach is considered appropriate for examining representations of chemical phenomena in textbooks for effective understanding of chemical concepts. Chemical phenomena in textbooks were coded and converted into numerical values such as frequency counts and simple percentages. The quantitative data allowed the researchers to make judgements linked to the research questions.Scoring rubrics and analysis
Varied and diverse rubrics have been used to analyze science textbooks for different purposes. As already stated, this study adopted the rubric developed by Gkitzia et al. (2011). The rubric and the typology for each of the criteria in relation to representations have been described in the previous section. As noted earlier, we understand that there a lot of issue involved in the chemistry triplet framework proposed by Johnstone. Several scholars (e.g.Talanquer, 2011; Taber, 2013) have criticized this framework. However, studies that adopted teaching approaches from these three levels of chemistry have shown a positive effect on students’ conceptual understanding of chemical concepts (e.g. Jaber and BouJaoude, 2012). Researchers have equally suggested that textbook authors need to take cognizance of these chemistry triplet (macro, sub-micro and symbolic) in designing textbooks (Treagust et al., 2003; Talanquer, 2011). Shehab and BouJaoude (2017) argued that while textbooks may not necessarily help students to reflect on chemical phenomenon at these levels, the design of textbooks using Gkitzia et al. (2011) rubric can be a useful step in helping students to become aware, think and describe chemical phenomena at these levels.This rubric has been successfully applied to evaluate chemical representations or visuals in textbooks in different contexts (e.g.Nyachwaya and Wood, 2014; Kapici and Acikalin-SavaŞci, 2015; Demirdöğen, 2017). Its usefulness lies in the fact that it can be used to investigate chemistry textbooks and can serve as a guide to authors and publishers to effectively integrate chemical representations in the design of textbooks. Table 1 presents three criteria from the rubric and their related typology, specifically designed for chemical representations in textbooks.
| Criterion | Typology for each criterion |
|---|---|
| C1: types of representations | i. Macroscopic |
| ii. Sub-microscopic | |
| iii. Symbolic | |
| iv. Multiple | |
| v. Hybrid | |
| vi. Mixed | |
| C2: relatedness to text | i. Completely related and linked |
| ii. Completely related and unlinked | |
| iii. Partially related and linked | |
| iv. Partially related and unlinked | |
| v. Unrelated | |
| C3: properties of caption | i. Suitable caption |
| ii. Problematic caption | |
| iii. No caption | |
The chemistry textbooks as sources of data used in this study are the textbooks recommended by the Nigerian Education and Research and Development Council (NERDC), adopted and approved for use in schools by the Federal Ministry of Education. Three chemistry textbooks that have 75% of Nigeria's textbook market share and are widely used among senior school chemistry students and teachers were selected. Unlike in other countries where textbooks are written or designed for each grade level, the textbooks we refer to in this study are used throughout the Senior School (SS) phase of education in Nigeria for SS 1–3, which is equivalent to grades 10–12 in countries that use a grade system. The textbooks coded and analyzed in this study are: the Essential Chemistry by Odesina (2015), Comprehensive Chemistry by Ezechukwu (2010) and New School Chemistry by Ababio (2012).
Before applying the rubric to code representations in the textbooks, we agreed on what should constitute our units of analysis. For a convenience analysis, all the images of scientists, natural scenes, of chemical reactions, separation techniques, diagrams of chemical equations, graphs, structures of organic compounds, figures of chemical reactions, experimental setups were selected as units of analysis. These representations in the three textbooks were coded and analyzed, except for the representations in the revision, summary and exercise sections. The textbooks were coded and analyzed for three criteria and the typology of each criterion described in Table 1. A deductive approach was employed to analyze the visuals and texts in accordance with the scoring rubric for types of representations, relation of visuals to texts and properties of captions. Since it was not our intention to compare the representations across the different textbooks, at the point of analysis, the representations from the different textbooks were merged together and analyzed using the rubric developed by Gkitzia et al. (2011). The total number of representations extracted from the three textbooks analyzed is 5774 visual representations, which are distributed as, 1085 for Textbook 1, 1914 for Textbook 2 and 2775 for Textbook 3.
To promote reliability of the study, 10% of the representations analyzed were rated independently by two science educators who have vast experience in textbook analysis. The values of kappa's measure of agreement was calculated based on the classification of the raters for each of the types of representations, their relatedness to text and properties of captions. The kappa-values for the types of representations, relatedness of representation to text and properties of captions were 0.95, 0.76 and 0.79 respectively. The significant kappa-value for each of the classifications showed a good measure of agreement between the two raters, which indicates the reliability of the study.
Results
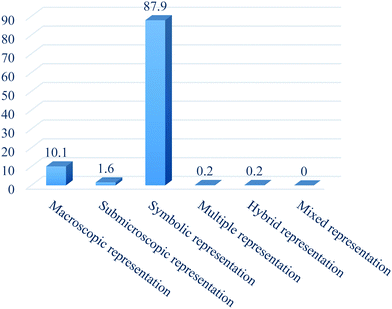
The results of our analysis are presented in the order in which research questions were raised to guide the study. The first research question states, (1) What types of representations are featured in the Nigerian chemistry textbooks? From Fig. 1, it can be observed that the most frequent representations in the textbooks are the symbolic representations (87.9%), which are largely equations, graphs, charts, signs etc. The next are the macroscopic representations (10.1%), followed by sub-microscopic (1.6%), then hybrid representation (0.2%) and multiple representation (0.2%) (see examples in Appendix 1). In all the textbooks, there is no evidence of mixed representation. Although there is a wide disparity between the symbolic and macroscopic representations in the textbooks based on the findings, we can deduce that representations in the textbooks were more aligned towards symbolic-macroscopic representations.(2) To what extent do the chemical representations relate to the texts?
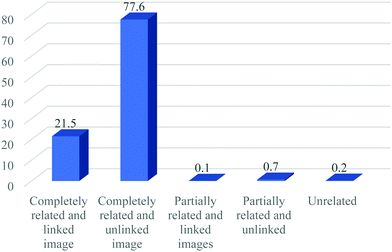
Fig. 2 presents the results of the relatedness of representations in the textbooks to text content. About 77.6% of the chemical representations were completely related but unlinked to specific text in the textbooks. Image representations that were completely related and linked to chemical concepts and phenomena are approximately 21.5% of the total. Only 0.7% of the representations are partially related but unlinked to the text. Image representations that were unrelated to their text are approximately 0.2%, while 0.1% of the representations were partially related and linked to the text content. In relation to Fig. 2, the result indicated that a larger percentage of representations were completely related but unlinked to the actual text, followed by visuals/images that are completely related and linked.
The examples of representations embedded in Fig. 3a and b were drawn from Textbook 3, chapters 14 and 5 respectively. In Fig. 3a, the preceding text on the composition of air does not completely described the percentage composition of air by volume as intended and indicated in the caption of the figure. The text content only made a partial reference to the composition of air as a mixture but does not direct readers to the figure in order to establish a link between the text and the figure. Fig. 3b explains the nature of (hydrogen and chlorine) molecules in a reaction that results in the formation of hydrogen chloride gas. The hybrid representation that depicts the formation of hydrogen chloride, is completely related to text, but the text is not directly linked to the figure. In this case, the reader is also left to establish a relationship between the text and the figure.
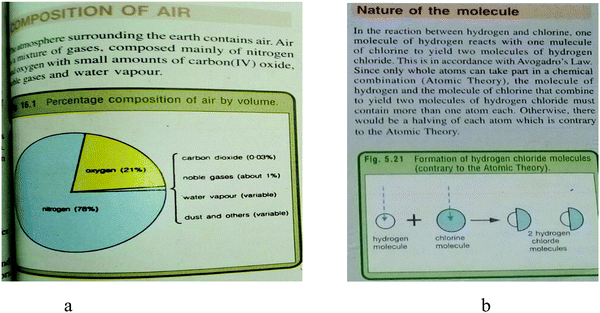 | ||
| Fig. 3 Example of a symbolic, partially related and unlinked (a), hybrid (submicroscopic and symbolic), completely related and unlinked representation (b). | ||
(3) How suitable are the properties of captions?
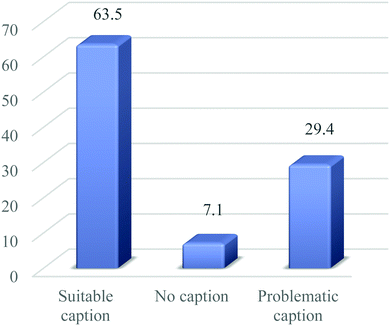
In response to our examination of whether a caption associated with a visual/image representation is suitable or problematic, Fig. 4 shows that more than half (63.5%) of the image representations had suitable captions, which are brief, explicit and comprehensive. While (29.4%) of the visual representations were problematic, about (7.1%) of the representations had no caption. Suitable captions make the understanding of the text content explicit and reduce difficulties that may arise in trying to interpret the representations in relation to texts (see examples in Appendix 1).
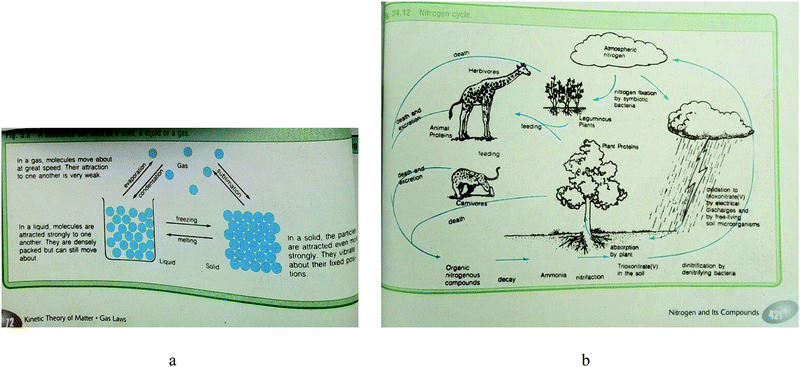
Fig. 5a is an example of a representation included in Textbook 3, which has a suitable caption because the image demonstrates the exact transition between three phases of matter as a multiple representation. A closer examination of Fig. 5a shows the use of arrows that depict state changes symbolically. The ‘lattice structures’ and representation of a ‘beaker’ depict sub-microscopic and macroscopic representations respectively. Fig. 5b has a problematic caption. The representation is hybrid in nature and is intended to describe how nitrogen-fixing microorganisms capture atmospheric nitrogen, and convert it to ammonia (NH3), which can be taken up by plants and used to make organic molecules. The nitrogen-containing molecules are passed to animals when plants are eaten. They may become incorporated into the animal's body or are broken down and excreted as waste, such as the urea found in urine or in dead materials. However, the caption may not enable the reader to understand the image easily. The caption is too brief, and neither explicit nor comprehensive. In this case, the reader is left to make sense of the content of the representation.
Discussion and implications
This study investigated representations of chemical phenomena in chemistry textbooks used in Nigeria. The overarching aim of the study was to determine the extent to which the representations depicted in chemistry textbooks can facilitate meaningful and conceptual understanding of chemical phenomena.In relation to types of representations, symbolic representations were dominant. When compared to macroscopic representations, the proportion of symbolic to macroscopic representations in the textbooks was in a ratio 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this regard, chemistry textbooks focus heavily on symbolic levels, most of which are equations, formulas, symbols and mathematical derivations. One concern raised by the dominance of symbolic representations is that learners may be restricted from seeing chemical phenomena at different representational levels. As a result, students may encounter difficulties in developing conceptual understanding that is fundamental to certain chemical concepts and may experience cognitive overload and a waning motivation to learn chemistry (Talanquer, 2011; Dangur et al., 2014). This is consistent with the findings of (Nyachwaya and Wood, 2014; Shehab and BouJaoude, 2017) and particularly Gkitzia et al. (2011), who reported “a bias towards a macro-symbolic orientation in the textbook” (p. 12).
1. In this regard, chemistry textbooks focus heavily on symbolic levels, most of which are equations, formulas, symbols and mathematical derivations. One concern raised by the dominance of symbolic representations is that learners may be restricted from seeing chemical phenomena at different representational levels. As a result, students may encounter difficulties in developing conceptual understanding that is fundamental to certain chemical concepts and may experience cognitive overload and a waning motivation to learn chemistry (Talanquer, 2011; Dangur et al., 2014). This is consistent with the findings of (Nyachwaya and Wood, 2014; Shehab and BouJaoude, 2017) and particularly Gkitzia et al. (2011), who reported “a bias towards a macro-symbolic orientation in the textbook” (p. 12).
In terms of dominance, after the symbolic were macroscopic representations. In the introductory chapters, it is expected that textbooks macroscopically depict chemical phenomena, as this level of representation has potential to attract, stimulate and sustain students’ interest in chemistry. It is also important to note that, this is not to suggest an emphasis on depiction of representations at the macro level, but that textbook designers should rather make visual or image representations that are abstract, real and observable to students. The results of Nyachwaya and Wood's (2014) study contradicts this finding, with a low indication of macroscopic representations. We assume that what accounts for this difference is that Nyachwaya and Wood evaluated advance physical chemistry textbooks, which predominantly featured symbolic representations.
In addition, the results of our study indicated a very small proportion of sub-microscopic representations in the textbooks and this fail to address the important point that many chemical phenomena are understood at the particulate level (Gilbert and Treagust, 2009). Having established the critical role played by the sub-microscopic level in helping students develop mental images of intangible concepts, it becomes equally important that textbook authors and publishers give adequate attention to this form of representation. Similarly, in a study on how visuals that focus on the particulate nature of matter are used in middle school science textbooks, Kapici and Acikalin-Savasci (2015) also reported a less frequent use of the sub-microscopic levels in chemical explanations.
The use of integrated representations in textbooks, such as the multiple and hybrid were infrequent. While multiple and hybrid representations were the fewest, there was not a single mixed representation. The depiction of the same phenomena at multiple levels in textbooks, hold potential for readers to move between the different levels (macro and sub-micro/symbolic, Chittleborough and Treagust, 2007; Chittleborough and Treagust, 2008). Students’ use of such textbooks under the teacher's guidance during classroom discourse could be quite significant, as the teachers attempt to make clear to students, multiple representations that depicts chemical phenomena. However, our results indicate a low prevalence of multiple representations, but rather higher prevalence of the discrete levels, which Treagust et al. (2003) considered to promote fragmented knowledge when students experience chemical phenomena at one level, i.e., independent of the other levels. Given the role played by these representations in aiding conceptual understanding of chemical concepts, it is a cause for concern that authors, and publishers have not responded to the request for substantial integration of sub-microscopic, multiple, hybrid and mixed representations in the textbooks (NERDC, 2009).
The application of the second criterion on relatedness of representations to text content reveals significant findings. They are significant because, even though, there are representations that were completely related to the text content, the representations were not linked. Only about 21.5% of the representations were completely related and linked. This indicates that authors included representations in textbooks without paying sufficient attention to how these representations could best be linked to the text content. The importance of relatedness of text to representations in terms of completeness and linkage is that readers do not have to try to establish a link and interpret the image in relation to the text by themselves (Pozzer-Ardenghi and Roth, 2004). Once there is a cognitive overload occasioned by partial or unlinked representations, students could become discouraged and eventually shutdown. It is not impossible that students could also make incorrect interpretations of images that are not related or linked to the text content (Kapici and Acikalin-Savasci, 2015). For a ‘visual science’ like chemistry, it is essential that representations in chemistry textbooks are completely related and closely linked to the text. The proximity should be such that they are within the same page. As Wu and Shah (2004) suggested, text and the accompanying representations should be presented close together, so that students can easily understand the association between them.
The germaneness of image captions in textbooks relates to the explanation these give to the entire representation and the understanding they convey to readers. When Kapici and Acikalin-Savasci (2015) compared the surface features of representations with captions, they pointed out that while images clarify the labelled parts of a representation, suitable “captions explain the entire representation” (p. 526). Our findings indicated that more than half of the visuals had suitable captions. However, there were also images with problematic captions that did not clearly portray the understanding the visuals were meant to convey. The existence of images without captions was minimal.
Conclusion
The study of chemistry largely involves chemical phenomena that are not open to direct observation. Chemistry as a visual science, therefore, requires the integration of chemical representations to depict these phenomena in textbooks and during classroom instructions, for effective teaching and learning of chemical concepts to take place. Where the teacher may be limited in portraying these phenomena in two-or three-dimensional representations during instruction, the textbook becomes a vital and indispensable tool for conveying an adequate understanding of the underlying concepts and principles of a chemical phenomenon under investigation.The overarching goal of this study was to investigate how chemical phenomena are represented in chemistry textbooks, through the types and dominant representations, the relatedness and linkage of images or visuals to the text content and the suitability of captions that link images to texts. The findings that have emerged from this study, particularly the dominance of symbolic representations over the other representations, the comparatively fewer examples of hybrid and multiple representations; and the non-existence of mixed representations should raise serious concerns for chemistry educators within the geographical context of this study. Our results showed the prevalence of symbolic representations and insufficient integration of two or more levels of chemical representations to depict phenomena. Since research has documented students’ difficulties with symbolic representations (e.g.Salta and Tzougraki, 2011), we can conclude that, students who use these textbooks may experience similar difficulties in trying to make sense of the chemical concepts depicted by symbolic representations.
Given that these results are very similar to those of other international studies (Gkitzia et al., 2011; Kapici and Acikalin-Savasci, 2015), it is imperative that concerted efforts are made to communicate and interpret these research outcomes for authors and publishers, so that they can pay attention to them. In textbook designing and writing, textbook authors and publishers need to recognize that if the different levels of chemistry are well-integrated in textbooks, they have the potential to reduce students’ difficulties and promote their conceptual learning of the subject. In this way, teachers who understand the role of chemical representations may also be able to use textbooks to scaffold meaningful learning at each level, particularly at the sub-microscopic level, and then assist students to understand the different levels of chemistry.
It is equally important that curriculum developers and textbook writers affirm these representations in the chemistry curriculum and provide teachers with the necessary support for them to reflect on the representations and emphasize chemical concepts at the different levels in chemistry classes. For instance, if teachers present the concept of kinetic molecular theory of matter to students and use the combination of macroscopic, sub-microscopic and symbolic representations, students can observe the phenomenon under investigation, develop conceptual understanding from the explanation of the phenomenon, and be able to symbolize the phenomenon effectively. Teachers should explore other alternative resources such as conceptual models or computer-based (2D or 3D) models, and the internet to provide rich experiences for students to develop mental images of intangible phenomena which can foster meaningful understanding of chemistry.
Conflicts of interest
There are no conflicts of interest to declare.Appendix 1
Examples of representations for criterion analyzed and coded in this study.1st criterion: types of representations
An example of a macroscopic representation taken from the New School Chemistry textbook. The image was used to represent how salt is produce from sea water by evaporation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) –
–![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) →). It depicts the reaction between hydrogen and chlorine gases to form hydrogen chloride gas at symbolic and sub-microscopic levels. The symbolic and sub-microscopic representations are also placed in parallel so that students can understand their links.
→). It depicts the reaction between hydrogen and chlorine gases to form hydrogen chloride gas at symbolic and sub-microscopic levels. The symbolic and sub-microscopic representations are also placed in parallel so that students can understand their links.
2nd criterion: relatedness to text
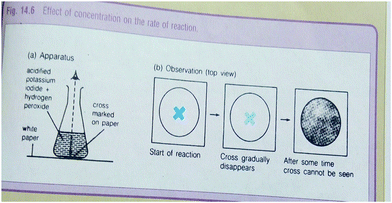
An example of a representation taken from New School Chemistry textbook that is completely related and linked to the text. The pictures illustrate that as time goes, on the concentration of the solution decreases. Therefore, it is completely related.
3rd criterion: properties of captions
An example of a representation that has an appropriate or a suitable caption. The caption indicates that alloys of copper can be used to make sculptures and steel are in high demand for building and construction of bridges.
Acknowledgements
The authors would like to thank Prof. Yvonne Reed, University of Witwatersrand for providing valuable comments and proofreading the manuscript. We equally appreciate the First Africana Publishers for their permission to reuse images from the textbooks sampled for this research.References
- Ababio O. Y., (2012), New school chemistry, Onitsha: Africana-FEP.
- Abd-El-Khalick F., Waters M. and An-Phong L., (2008), Representations of nature of science in high school chemistry textbooks over the past four decades, J. Res. Sci. Teach., 45(7), 835–855.
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33, 131–152.
- Ainsworth S., (2006), DeFT: a conceptual framework for considering learning with multiple representations, Learn. Instruct., 16(3), 183–198.
- Ainsworth S., (2008), The educational value of multiple-representations when learning complex scientific concepts, in Gilbert J. K., Reiner M. and Nakhleh M. (ed.), Visualization: theory and practice in science education, Netherlands: Springer, pp. 191–208.
- Bergqvist A., Drechsler M., De Jong O. and Rundgren S. N. C., (2013), Representations of chemical bonding models in school textbooks–help or hindrance for understanding? Chem. Educ. Res. Pract., 14(4), 589–606.
- Bunce D. M. and Gabel D., (2002), Differential effects on the achievement of males and females of teaching the particulate nature of chemistry, J. Res. Sci. Teach., 39(10), 911–927.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students’ ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2008), An evaluation of a teaching intervention to promote students’ ability to use multiple levels of representation when describing and explaining chemical reactions, Res. Sci. Educ., 38, 237–248.
- Cheng M. M. W. and Gilbert J. K., (2015), Students’ visualization of diagrams representing the human circulatory system: the use of spatial isomorphism and representational conventions, Int. J. Sci. Educ., 37(1), 136–161.
- Chiappetta E. L. and Fillman D. A., (2007), Analysis of five high school biology textbooks used in the United States for inclusion of the nature of science, Int. J. Sci. Educ., 29(15), 1847–1868.
- Chiappetta E. L. and Koballa T. R., (2002), Science instruction in the middle and secondary schools, 5th edn, Upper Saddle River, NY: Pearson Education.
- Chiappetta E. L., Sethna G. H. and Fillman D. A., (1991), A quantitative analysis of high school chemistry textbooks for scientific literacy themes and expository learning aids, J. Res. Sci. Teach., 28(10), 939–951.
- Childs P. E. and Sheehan M., (2009), What's difficult about chemistry? An Irish perspective, Chem. Educ. Res. Pract., 10(3), 204–218.
- Chittleborough G. and Treagust D., (2007), The modeling ability of non-major chemistry students and their understanding of the sub-microscopic level, Chem. Educ. Res. Pract., 8(3), 274–292.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38, 463–482.
- Creswell J. W., (2005), Educational research: planning, conducting and evaluating quantitative and qualitative research, 2nd edn, New Jersey: Pearson Merrill Prentice-Hall.
- Dangur V., Avargil S., Peskin U. and Dori J. Y., (2014), Learning quantum chemistry via visual-conceptual approach: students’ bidirectional textual and visual understanding, Chem. Educ. Res. Pract., 15, 297–310.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: diagrams, in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Netherlands: Springer, pp. 169–191.
- Demirdöğen B., (2017), Examination of chemical representations in Turkish high school chemistry textbooks, Journal of Baltic Science Education, 16(4), 472–499.
- Dori Y. J. and Hameiri M., (2003), Multidimensional analysis system for quantitative chemistry problems: symbol, macro, micro, and process aspects, J. Res. Sci. Teach., 40(3), 278–302.
- Ezechukwu J., (2010), Comprehensive chemistry for senior secondary schools, Onitsha: Africana-FEP.
- Gabel D. L., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76(4), 548–554.
- Gilbert J. K. and Treagust, D., (2009), Introduction: macro, sub-micro and symbolic representations and the relationship between them: key models in chemical education, in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Dordrecht: Springer, pp. 1–8.
- Gkitzia V., Salta K. and Tzougraki C., (2011), Development and application of suitable criteria for the evaluation of chemical representations in school textbooks, Chem. Educ. Res. Pract., 12, 5–14.
- Han J. and Roth W. M., (2006), Chemical inscriptions in Korean textbooks: Semiotics of macro and microworld, Sci. Educ., 90(2), 173–201.
- Harrison A. G., (2001), How do teachers and textbook writers model scientific ideas for students? Res. Sci. Educ., 31(3), 401–435.
- Jaber L. Z. and BouJaoude S., (2012), A macro-micro-symbolic teaching to promote relational understanding of chemical reactions, Int. J. Sci. Educ., 34(7), 973–998.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput.-Assist. Learn., 7, 75–83.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705.
- Johnstone A. H., (2000a), Chemical education research: where from Here? University Chemistry Education, 4(1), 34–38.
- Johnstone A. H., (2000b), Teaching of chemistry: logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15.
- Kapici H. and Acikalin-Savasci F., (2015), Examination of visuals about the particulate nature of matter in Turkish middle school science textbooks, Chem. Educ. Res. Pract., 16, 518–536.
- Kern A. L., Wood N. B., Roehrig G. H. and Nyachwaya J. M., (2010), A qualitative report of the ways high school chemistry students attempt to represent a chemical reaction at the atomic/molecular level, Chem. Educ. Res. Pract., 11, 165–172.
- Kozma R. and Russell J., (2005), Students becoming chemists: developing representational competence, in Gilbert J. K. (ed.), Visualization in science education, Netherlands: Springer, pp. 121–145.
- Krippendorff K., (2004), Content analysis: an introduction to its methodology, 2nd edn, Thousand Oaks, CA: Sage.
- Mathewson J. H., (2005), The visual core of science: definitions and applications to education, Int. J. Sci. Educ., 27, 529–548.
- Mohammed F. R. and Kumari R., (2007), Effective use of textbooks: a neglected aspect of Education in Pakistan, Journal of Education for International Development, 3(1), 1–11.
- Mouton J., (2008), How to succeed in your master's and doctoral studies. A South African guide and resource book, Pretoria: Van Schaik.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13, 186–194.
- Nigerian Educational Research and Development Council [NERDC], (2009), Senior secondary education curriculum. Chemistry for SS1-3, Lagos: NERDC Press.
- Nyachwaya J. M. and Gillaspie M., (2016), Features of representations in general chemistry textbooks: a peek through the lens of the cognitive load theory, Chem. Educ. Res. Pract., 17, 58–71.
- Nyachwaya J. M. and Wood N. B., (2014), Evaluation of chemical representations in physical chemistry textbooks, Chem. Educ. Res. Pract., 15(4), 720–728.
- Nyachwaya J. M., Mohamed A., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students’ understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- O’Dwyer A. and Childs P., (2014), Organic chemistry in action! Developing an intervention program for introductory organic chemistry to improve learners’ understanding, interest, and attitudes, J. Chem. Educ., 91(7), 987–993.
- Odesina I. A., (2015), Essential chemistry for senior secondary schools, Onitsha: Africana-FEP.
- Özalp D. and Kahveci A., (2011), Development of two-tier diagnostic items based on ontology in the topic of the particulate nature of matter, Journal of National Education, 40(191), 135–156.
- Özmen H., (2011), Turkish primary students’ conceptions about the particulate nature of matter, Int. J. Environ. Sci. Educ., 6(1), 99–121.
- Pedrosa M. A. and Dias M. H., (2000), Chemistry textbook approaches to chemical equilibrium and student alternative conceptions, Chem. Educ. Res. Pract., 1(2), 227–236.
- Pozzer L. L. and Roth W., (2003), Prevalence, function and structure of photographs in high school biology textbooks, J. Res. Sci. Teach., 40(10), 1089–1114.
- Pozzer-Ardenghi L. and Roth W., (2004), Students’ interpretation of photographs in high school biology textbooks, Annual Meeting of the National Association for Research in Science Teaching, Vancouver, BC, April 1–4.
- Ramnarain U. and Joseph A., (2012), Learning difficulties experienced by grade 12 South African students in the chemical representation of phenomena, Chem. Educ. Res. Pract., 13, 462–470.
- Salta K. and Tzougraki C., (2011), Conceptual versus algorithmic problem-solving: focusing on problems dealing with conservation of matter in chemistry, Res. Sci. Educ., 41(4), 587–609.
- Sanger M. J. and Greenbowe T. J., (1999), An analysis of college chemistry textbooks as sources of misconceptions and errors in electrochemistry, J. Chem. Educ., 76, 853–86.
- Shehab S. S. and BouJaoude S., (2017), Analysis of the chemical representations in secondary Lebanese chemistry textbooks, Int. J. Sci. Math. Educ., 15(5), 797–816.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, sub-micro, and symbolic: the many faces of the chemistry triplet, Int. J. Sci. Educ., 33(2), 179–195.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of sub-microscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- Upahi J. E. and Jimoh M. A., (2015), Classification of end-of-chapter questions in senior school chemistry textbooks used in Nigeria, Electronic Journal of Science Education, 19(7), 1–16.
- Upahi J. E., Ramnarain U. and Ismail I. S., (2018), The nature of science as represented in chemistry textbooks used in Nigeria, Res. Sci. Educ., DOI:10.1007/s11165-018-9734-7.
- Uttal D. H. and O’Doherty K., (2008), Comprehending and learning from visualization: a developmental perspective, in Gilbert J. K., Reiner M. and Nakhleh M. (ed.), Visualization: theory and practice in science education, Netherlands: Springer, pp. 53–72.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
| This journal is © The Royal Society of Chemistry 2019 |