The development of Chinese undergraduate students’ competence of scientific writing in the context of an advanced organic chemistry experiment course
Yang
Deng
 *a,
Gregory J.
Kelly
*a,
Gregory J.
Kelly
 b and
Lishi
Xiao
a
b and
Lishi
Xiao
a
aInstitute of Chemistry Education, College of Chemistry, Central China Normal University, Wuhan, Hubei 430079, China. E-mail: yangdeng@mail.ccnu.edu.cn
bDepartment of Curriculum and Instruction, College of Education, Pennsylvania State University, State College, PA 16802-3206, USA
First published on 6th November 2018
Abstract
This study examines scientific practices associated with scientific writing in organic chemistry in China. Although there is rapidly growing literature on the features and strategies of scientific writing, further research in this area is needed to recognize and treat scientific writing as a social endeavor to evaluate it in a more comprehensive and detailed way in order to effectively convey scientific information to readers. This study shared these important premises and attempted to investigate the development of Chinese undergraduate students’ competence of scientific writing. Twenty-two undergraduate students majoring in chemistry participated in this study. They experienced a researcher-intervened Advanced Organic Chemistry Experiment course and were asked to write scientific articles on the six course experiments. Their scientific writings were analyzed based on normativity, objectivity, and logicality. These dimensions of the development of students’ competence in scientific writing during the course were portrayed. This study suggested that student's development in scientific writing can be divided into categories, demonstrating the importance and implications of teaching “learn to write” in science.
Introduction
A focus on engaging students in disciplinary practices has generated much interest in the contemporary education field (Reeves and Fox, 2008; Higgs, 2012; Kelly and Licona, 2018). In science education, some national and international policies (such as National Research Council, 2012) also highlight scientific practices. As a result, current science education reforms in many countries are encouraging students to “do science” in a science classroom or laboratory, rather than just learning about discrete science ideas (DeBoer, 1991; Berland et al., 2016).Scientific writing is a typical kind of scientific practice, which aims at communicating scientific ideas in a simple and concise yet comprehensive way. There are several reasons to emphasize the disciplinary values of scientific writing. First, from the perspective of philosophy of science, scientific writing is not just a simple tool for the storage, transmission, and communication of science knowledge; rather, it is an essential element of science, representing science itself to a broad range of readers. As Norris and Phillips (2003) mentioned, language elements are necessities in science, as science cannot be communicated without language. Second, from the sociocultural perspective of learning (Vygotsky, 1978), it is crucial that social interaction plays a key role in students’ science learning, including scientific knowledge construction, higher thinking skills acquisition, and emotional experience adaption. These processes particularly go through the mediation of language, including writing, which is definitely impossible without language. Bazerman (2006) put forward that to know the appropriate ways of analyzing the texts of students’ writing it is necessary to understand students’ writing abilities and their processes of learning. Finally, as argumentation is a process central to science and science education (Kuhn, 2012), such goals will not be realized until the argument is stated in a clear and effective expression. As Cavagnetto and Hand (2012) stated, “the production of any argument involves a learner/participant in negotiating publically and personally across a variety of settings. These include observations, written text (reading), oral text (debate), and the construction of written text (written report) (p. 45).”
There are two main educational research orientations paying attention to scientific writing. First, some previous scholars focused on “write to learn” approaches (Glynn and Muth, 1994; Hand and Prain, 2002; Hand et al., 2004; Dianovsky and Wink, 2012; Chen et al., 2013). A large number of studies drew from the Science Writing Heuristic approach (Martin and Hand, 2009; Choi et al., 2010; Hand and Choi, 2010). As a result, they treated scientific writing as an effective way of developing scientific understanding, competence, and ideas of nature of science. For example, Galbraith (1999) suggested that writing can be treated as a process that leads to the construction of knowledge. He and his colleagues appropriated the knowledge-transforming process and the knowledge-constituting process to illustrate the mechanism of “write to learn (knowledge)” (Galbraith, 1999; Galbraith et al., 2006). There were also other related studies, illustrating ways to facilitate students’ scientific writing (Guilford, 2001; Çetin and Eymur, 2017). However, some of them just considered scientific writing as a simple skill, which can be enhanced by practicing writing itself, similar to applying writing toolkits (Dirrigl and Noe, 2014) or the blogosphere context (Kramer and Kusurkar, 2017).
Second, some current science education research revealed that students’ competence in scientific writing is not well developed from the perspective of scientific argumentation. Sampson et al. (2013) summarized that students could not do well in constructing an argumentative text such as to communicate their ideas, support a claim with evidence, coordinate evidence and theory, or provide an adequate challenge to an alternative claim, consistent with a number of other studies of science (Kelly and Takao, 2002; Kelly and Bazerman, 2003; Kelly et al., 2008). Our prior study of Chinese students’ competence in written scientific argumentation reported that their written texts were fragmented without a formal form and definitely the writing was not up to the standard. Some Chinese students (both at the high school level and the college level) just wrote a few formulas to state their claims and the reasoning process (Deng and Wang, 2017). Some scholars in China represented the poor quality of Chinese students’ competence of scientific writing (Zhang and Zhang, 2009; Cai and Chen, 2010), as conclusions were just drawn from their personal teaching experiences, rather than systematic evaluation. As scientific writing itself is communicative as well as generative, attention should also be paid on “learn to write” approaches.
Focusing on a comprehensive and detailed analysis of students’ scientific writing through textual analysis is essential in order to facilitate students’ competence of scientific writing based on the criteria of what a sound scientific writing looks like. In order to deal with these issues, a study with twenty-two Chinese undergraduate students who attended the Advanced Organic Chemistry Experiment course was conducted. These students were expected to write scientific articles after finishing each course experiment on the synthesis of organic substances. While implementing these scientific writing tasks, these undergraduate students also engaged in researcher-intervened pedagogy, integrating reading, peer-evaluation, and discussion. In this article, the key point was focused on the development of Chinese undergraduate students’ competence of scientific writing to answer the following question:
How did students’ scientific writing develop while participating in the academic and epistemic practices of the Advanced Organic Chemistry Experiment course?
Theoretical framework
Scientific writing in science and science education
The theoretical framework of this study builds on the development from research on scientific writing. The key characteristics of a sound scientific writing are articulated based on the current knowledge base.Scientific writing in researchers’ current related works has some general criteria. Yore et al. (2002, 2004) defined some characteristics of scientific writing by asking questionnaires through semi-structured interviews. They emphasized that a good quality of scientific research should directly state the specific scientific problems, questions, or issues, provide data to describe and explain patterns of events in the natural world, and align these data with the scientific writing genres. To support specific elements of science writing, Berkenkotter and Huckin (2016) suggested that a strong instructional part in scientific writing should emphasize the practical importance of the topic to the society, the theoretical importance of the question, and reviewing differing prior claims about the question. With regard to students, Hand and Prain (2002) reported that students in scientific writing tasks should manipulate new content, clarify ideas and defend emerging understandings, rather than simply demonstrating what they already know. Dianovsky and Wink (2012) described a good chemical journal writing of students including some key parts, such as knowledge of a chemistry topic, the specific examples related to the topic and chemical substances involved, a connection between the topic and their own personal history, current experience, or future plans. As scientific writing could be treated as a type of written argumentation, many researchers tried to establish rubrics from the perspective of the pattern of scientific argumentation (Zohar and Nemet, 2002; Ryu and Sandoval, 2012; Lee et al., 2014). Scientific argument is basically evidence-based defense that provides reasons supporting a claim. In addition, consideration of possible counter arguments also often strengthens the claim. For example, Choi et al. (2013) asserted that in scientific writing, the argument must focus on the following components: the clarity of the claims, the sufficiency of evidence, and the relationship between questions and claims and between claims and evidence. Chen et al. (2013) pointed out six components of good scientific writing, highlighting the persuasive functions such as (a) the clarity of the claim, (b) the relationship between the question and the claim, (c) the relationship between the claim and the evidence, (d) the sufficiency of the evidence, (e) the overall cohesiveness, and (f) the text assessment.
As it was briefly mentioned, scientific writing is not only just a way to store ideas, leading to establish the proprietorship of intellectual property (Bazerman, 1988), but also a key method to communicate, share, review, investigate, and evaluate scientific ideas. Scientific writing is a social endeavor, reflecting the interactional, contextual, intertextual, and consequential nature of epistemic practice (Cunningham and Kelly, 2017). Therefore, while addressing “learn to write” approaches, it is necessary to understand the criteria embodied in good scientific writing, which reflects social characteristics. First, scientific writers should use disciplinary structures and expressions of the scientific discourse community to state their ideas to communicate the relevant meaning in disciplinary-specific norms. This view can be supported by the definition of disciplinary culture by Flowerdew and Miller (1995) as “Theories, concepts, norms, terms … of a particular academic discipline … perhaps the most obvious way to recognize a discipline is through its specialized vocabulary (p. 366).” Second, in scientific writing, the writer should avoid using vague words to describe content (Hyatt et al., 2017), indicating that the writer should clearly defend science claims by using currently accepted scientific concepts and principles and expressing the real process to make their research topic more understandable and reliable. Third, following Habermas (2001), the truth is always an articulated type of expression, and participants in discourse communities should evaluate the truth claims based on these expressions, and persuade the audience in conventional ways. By adhering to the scientific practices of the epistemic community and articulating the results properly and rhetorically in scientific writing, the meaning of the written discourse can be communicated so that perspective readers can evaluate and potentially accept the proposed ideas.
Social dimensions of scientific writing
The basic standards of sound scientific writing must be based on three social aspects: normativity, objectivity, and logicality.Normativity indicates that the scientific writing work should satisfy the norms of the scientific community and include two parts. First, the necessary components constituting the scientific writing must be normative, indicating that the scientific writing in the same genre should have some common key components. These ideas are based on the fact that each component has its own function to reflect different necessary stages of a scientific research practice and has relatively concentrated meaning. Similar to most physical science articles, components such as instruction, experimental/observation, results and discussion, and conclusion sections must be included. In the experimental section, the detailed operations and phenomena should be clearly stated. Second, some scholars mentioned that discourse genres (Florence and Yore, 2004), the multiple modes of representations of concepts (such as tables, graphs, diagrams, and symbols, diSessa (2004)), and the textual grammar (such as the vocabulary, syntax, spelling, punctuation, and word choice, Glynn and Muth (1994)) should also be considered as normative criteria of scientific writing because they reflect the basic features of the genre, even the traditions and conventions of a science discourse. The scientific ideas must be communicated to expand the knowledge of the scientific and social community. After thoroughly applying these normative language expressions and academic norms over a period of time, such practice may provide a much more convenient way to communicate in the specific discourse community.
In order to communicate well with other community members, and let others understand and believe the current work and argumentation as stated in the article, and to even make judgments, reaching the goal of objectivity is very important. Indeed, science could not be treated as a totally objective enterprise, as many philosophers may be against this to describe scientific practices. However, the adjective “objective” here just means to reach the global scope of temporary consensus currently in the majority of science community members and express the real process of scientific practices. As a result, the dimension of objectivity mainly concerns the content of scientific writing. Some researchers argued that the content is the core evaluating item in students’ scientific writing (Glynn and Muth, 1994; Hyatt et al., 2017). Keys (1999) also mentioned that expert scientific writers should pay attention to both the content and the discourse aspects of writing; however, the novice writers often become embroiled in the latter part and forget the former. Scientific articles should be written in correct and clear language and should articulate the real process, indicating that expressions should not only be aligned with the current confirmed scientific ideas without errors, but also comprehensively reveal actual research operations, phenomena, data, and conclusions correctly.
Conveying scientific information effectively through writing is critical for presenting novel claims, facts, and evidence to the broad readership in order to value your sincere efforts in science or any field of research. Therefore, the logicality of sound scientific writing should focus on two aspects. First, the scientific writing itself should be organized logically by considering the textual pattern. Previous research stressed that scientific writing should emphasize internal coherence (Klein, 1999), the synthesizing or rearrangement of information (Best, 1995), and structural representation (Barstow et al., 2017). Specifically, well-formulated scientific writing must consider how to weave the texts well in all different relations such as hierarchical organization, enumeration, exemplification, causality, contrast, and comparison. These relations can be reflected by the sequences of different components and may be expressed through relational words or headings. The second aspect of logicality is the logic of argumentation. The argumentation should be more reasonable and persuadable in diverse ways; however, some researchers reported that students could not do well in supporting claims by sufficient and relevant evidence (McNeill and Krajcik, 2007; McNeill, 2011), making a sound argument by connecting claims and evidence well (Erduran et al., 2004), and stating rebuttals strengthening the conclusion (Felton and Kuhn, 2001). To be rhetorical, persuasive arguments have to be well constructed in written scientific language, exhibiting a good logical organized relationship of questions, claims, evidence, warrants, and rebuttals, and strengthening the reasonability of the whole process of research.
Methods
Participants
The participants in this study were twenty-two Chinese undergraduate students (ten males and twelve females), all majoring in chemistry from a university located in Wuhan, China, which is a key comprehensive university directly under the administration of the Ministry of Education of China. In the future, some of those students may become potential graduate students to conduct chemistry or related disciplinary research, and some may work in the chemical industry or other jobs related to chemistry. The requirement of a bachelor's degree in chemistry is to conduct real chemistry research and write a research article.Yin (2014) mentioned that a single case study should embody five rationales, that is, having a critical, unusual, common, revelatory, or longitudinal case. The current single case study was designed in the context of an Advanced Organic Chemistry Experiment course. To design this course was to critically determine whether the theoretical framework mentioned was effective or should be more challenging, or even extended. As this course was an intervened research process, it was deviating from everyday occurrences, in which some target phenomena could not be observed and analyzed without intervening previously. The objective of this research and its data analysis was to capture the common circumstances and conditions of this course. During this course, different points of students’ competence of scientific writing could be diagnosed over time; as a result, it could be treated as a longitudinal case.
Students who chose to study this course must be 3rd year undergraduates and had already passed the basic courses such as elementary organic chemistry and elementary organic chemistry experiments to be equipped with basic organic chemistry knowledge and experimental skills. These twenty-two undergraduate students were chosen randomly and automatically from all the students eager to attend this course by an online course selection system equipped with a randomisation feature that was designed by the office of teaching affairs of this university, to fit the planned and available rooms of the course. That is, this randomisation feature is routinely used to select students randomly from enrolment applications that would exceed the capacity of the rooms where courses are to be taught.
All chosen students on the list were assigned numbers according to their position in the sequence of names provided by the randomisation procedure. Students were assigned to two groups supervised by two instructors in the department of chemistry of this university according to whether they were given an odd or even number in the sequence. Because the original sequence was a random ordering, which was then used to provide two groups according to the position on the list, the overall effect was to randomise the selected students to the two groups.
All the students expressed their willingness to participate in this study after knowing the research purposes. Also, all the students were told that their performances in scientific writing would just be treated as academic data and be secret.
The two instructors assigned to teach this course both majored in organic chemistry. Both of them received their doctorate degrees in this field, publishing articles in high impact chemistry journals. In addition, both of them had rich experiences in organic chemistry education and had instructed the Advanced Organic Chemistry Experiment course several times. They also expressed their willingness to allow us to conduct this study during the courses after being told about the research purposes.
Although the undergraduate students were divided into two groups and guided by two different instructors, all of them experienced the same interventions during this study and had equal access to use experimental equipment in the organic chemistry lab. Both instructors introduced the general content and intention of each experiment to students before conducting the experiment and walked around in the lab to assist students in implementing each experiment when they needed assistance; therefore, the differences between these two instructors would have little influence on the outcome of this study of developing scientific writing.
The researchers included the first and third authors of this article and one graduate student who majored in chemistry education. They did some work on organizing students’ participation in the course and collecting and analyzing data from students’ work in this study. Both of them were familiar with scientific argumentation and scientific writing, and they all understood the goals and the procedures of this study. Before each chemistry experiment, they conducted the preparation experiment first to understand the basic operations for clearly observing the target phenomenon.
Context
The Advanced Organic Chemistry Experiment course was aimed at developing undergraduate students’ comprehensive competence of conducting organic chemistry experiments and building a bridge to their future organic chemistry research. As all the experiments in this course focused on the synthesis of organic compounds, the prerequisite for this course was the basic knowledge of the subject and methods to design a route for synthesis and familiarity with basic experimental organic chemistry techniques such as distillation, fractional distillation, vacuum distillation, and thin-layer chromatography.Usually, in this course, the instructor asked students to read the set textbook about each experiment and complete a prelab report before entering the lab. During the class, the instructor first introduced the experimental lab materials and the course notifications to students, and checked their prelab reports. The next step involved students conducting the experiment individually or in pairs. During the experiment, students were instructed to take notes. If they had any questions, they were free to ask the instructor. After the experiment, students were required to finish writing their lab report by normally filling in the goals, principles, operations, phenomena, and conclusion of the experiment, even just a few key words of them, in a pre-designed report form, drawing pictures of the synthesis equipment in the assigned space, and answering certain questions related to the experiment, as posed by the instructor. In doing such works, students could just copy some words or sentences from the textbook as they were already well written and answered. The instructor then graded students’ performance based on the contents of the final report, paying close attention to the qualitative and quantitative descriptions of the synthesized chemical products.
In coordination with this study, certain course reforms were implemented in order to facilitate students’ scientific writing by implementing the strategy of integrating reading, peer evaluation, and discussion. This research was conducted from the beginning of September 2016 to the end of November 2016 during which the students were supposed to finish six course experiments on the synthesis of (1) benzoin, (2) succinic anhydride, (3) oxaprozin, (4) benzene pinacol and pinacolone, (5) benzil, and (6) benzilic acid. All students had learned the basic knowledge of these substances in the elementary organic chemistry course. These six experiments required students to prepare the target organic compounds from the chemical reagents provided by the instructors and characterize them by chemical characterization methods such as melting point measurement, infrared spectra, and thin-layer chromatography.
This course was organized based on some academic and epistemic practices. The researchers had already introduced some related textbooks and online resources to students before the course. At the beginning of this course, the students were instructed to go through four organic chemistry articles published by related organic chemistry researchers in order to familiarize them with basic aspects of scientific writing elements in organic chemistry reflecting the basic genre, structure, and content. These four papers were:
(1) Li H., Zhang Y., Xie X., Ma H., Zhao C., Zhao G. and She X., (2014), Bioinspired Total Synthesis of Gymnothelignan N, Org. Lett., 16, 4440–4443.
(2) Akiyama T., Morita H. and Fuchibe K., (2006), Chiral Brønsted Acid-Catalyzed Inverse Electron-Demand Aza Diels–Alder Reaction, J. Am. Chem. Soc., 128, 13070–13071.
(3) Xie M., Tang H., Zhang Y., Guo Z., Guo H. and Qu G., (2015), A New Method for the Synthesis of Alkyl-Substituted 1,2,3-Triazole Compounds, Chin. J. Org. Chem., 35, 2589–2594 (in Chinese).
(4) Li Q., Yang R., Ruan Z., Hu T., Ding H. and Xiao Q., (2013), Total Synthesis of Cordycepin, Chin. J. Org. Chem., 33, 1340–1344 (in Chinese).
These four papers were introduced by the two instructors because of the following reasons. First, they are all high in quality and reflected the basic genre, structure, content, and writing standards of an organic synthesis paper. Second, there are certain differences in writing styles between the four papers, and it was believed that there is sufficient variation and quality to prevent students from treating scientific writing in organic chemistry as having a fixed format. Given some concerns about the abilities of Chinese undergraduate students to read the two papers that were written in English, the latter were translated into Chinese. This translation was undertaken by a graduate student who had majored in organic chemistry, and was checked by the researchers of this study. Both language versions of each paper were handed out to the students.
A reading reflection guide (Fig. 1) was designed for the students to evaluate their basic understanding of these four articles. During reflection, the students were supposed to summarize the main content of each article, thinking “what should a good organic synthesis article be like?” This activity provided students with the opportunity to construct a general concept of what an organic synthesis paper should be like based on the reading task and the reflection that followed.
The students were instructed to take experimental notes sufficiently about the process and findings while conducting the experiments. The researchers would also take photos of their experimental notes after finishing each experiment, and wrote down some special events or phenomena that happened during the experiments as memos. After completing the experiment, every student was asked to write one science article to report each experiment after three days. The course was conducted on every Friday so that the students could have plenty of time to finish writing during the weekend and the next Monday. Specifically, they needed to illustrate the whole process of the scientific practice and their findings after completing each experiment. There was no fixed form to guide their writing, and they were supposed to present and organize their writing by themselves.
After handing over the written articles each time (Tuesday), the students were assigned into pairs to do peer-evaluation during Wednesday and Thursday. Every student received a scientific writing article from his (her) assigned peer (it was anonymous) and a brief rubric (Table 1) about the evaluation. At this stage, the students just saw the brief descriptions of the normativity, objectivity, and logicality of scientific writing and graded the peer's scientific writing in each dimension by scoring 1 to 4. After peer-evaluation, every student knew the evaluating results from his (her) partner. During the peer-evaluation period, the researchers always helped students’ dealing with the problems of understanding the normativity, objectivity, and logicality.
| Dimension | Descriptions | Use “✓” to score (high quality–low quality) | |||
|---|---|---|---|---|---|
| 4 | 3 | 2 | 1 | ||
| Normativity | Based on your opinion, the writing should contain all necessary components of an organic chemistry synthesis article to express the whole process of the experiment. In the writing, the words, sentences, paragraphs, graphs, tables, and literature are all properly written based on the scientific writing norm. | ||||
| Objectivity | Based on your opinion, the content of writing should align with the current confirmed scientific ideas without errors and reveal the actual experiment process, phenomena, data, and conclusion correctly. | ||||
| Logicality | Based on your opinion, the writing itself should be organized logically so that one can easily understand the relationships between different parts. The logic of the argumentation is also valid for one to believe the conclusions supported by the author. | ||||
Every Thursday night before the next experiment, a discussion was held online. The platform used in this study was the QQ group chatting room, which is a software designed by Tencent® Holdings Limited. Every student could give him(her) a name of their own while talking in the QQ group chatting room in order to get more freedom to speak and listen. The topics discussed in the QQ group chatting room mainly focused on students’ awareness about scientific writing and peer-evaluation. Their ideas of improving scientific writing and reflections were also an important part of the discussion. Here are two examples of episodes from the online discussion.
Example 1:
Jiang: What I want to say is… you need to list all of the phenomena observed in your paper.
Zhong: What's included?
Jiang: For example, when I reviewed another paper yesterday, I found that both of us talked about the color, the melting point, the mass, and the yield. But that paper did not mention that succinic anhydride is a columnar crystal.
Wang: And, the calculation process of the yield should also be shown.
(All names are pseudonyms so that actual participants remain anonymous in this paper.)
Example 2:
Researcher: Why should you calculate the yield?
Bao: This was the data I got during the experiment. I have to report it!
Researcher: OK, let's take an example. If the yield was 80%, what does that mean?
Bao: 80% of the reactants were changed to products.
Researcher: How about the other 20%?
Bao: This was the loss.
Liu: It could be a side reaction.
Researcher: Is there any relationship between this 20% and the conclusion?
Liu: No… but it is necessary to say that the conversion was 80%.
Bao: If you could point out the reason for the 20%… this would indicate that there is no relationship with the conclusion.
Researcher: Right! So you have to explain why the yield is not 100%, and make the reasons and conclusions separately! Similarly, if an unexpected phenomenon occurs, explanations for this are also needed.
These two examples illustrated some of the potential reasons for the improvements on students’ scientific writing. By doing peer-evaluation, students not only learned about others’ writing content, but also came to know how others presented this content. By comparing their own papers with others’, they were able to ascertain the advantages and disadvantages of each paper, including in comparison to their own writing. When sharing their experiences online, they could paste others’ writing sentences or discuss them directly to trigger off the topic on how to make progressions on scientific writing. In other cases, the researchers always joined their discussion to provide some problems and posted some comments when necessary. During the collective problem-solving period, the students and researchers needed to put forward their opinions to try to reach a consensus, step by step, by continuously querying, negotiating, and criticizing.
It should be mentioned that, during the discussion period, all the students would have access to join the talk or just be an onlooker. The discussion could help both the students who were talking and onlookers to get feedback of their problems in writing and doing reading, peer-evaluation, and discussion. Due to this reason, every student could get some feedback equally from the classmates and researchers regarding their weaknesses and problems in finishing scientific writing.
Data resource and analysis
Yin (2014) also mentioned four strategies to analyze the data from a case study, they were: “relying on theoretical propositions”, “working your data from the ‘ground up’”, “developing a case description”, and “examining plausible rival explanations”. The process of data collection and analysis of this study was conducted based on these four aspects. Theoretical considerations of sound scientific writing were applied in evaluating students’ scientific writing performances. The components and performances were all picked directly from the data set, and the three dimensions of normativity, objectivity, and logicality were used to classify the performances. After analyzing, all claims were tried to be summarized to describe students’ different situations of development. The plausible rival explanations were also considered.The data of this study mainly came from the students’ scientific writing after each course experiment, and they were analyzed in a detailed way to identify and describe the issues about the quality of students’ scientific writing by narrowing the focus on their normativity, objectivity, and logicality.
For the textual analysis (Fairclough, 2003), each article was evaluated separately at the beginning. First, the components in each article were identified. After summarizing the recognized components in all the articles and discussing with the instructors, the goals, background, principles, instruments and chemical reagents, operations, phenomena, results and discussion, conclusions, and references were treated as the basic and necessary components of writing a scientific article on organic synthesis.
Second, the poor performances related to the normativity, objectivity, and logicality in every article were pinpointed. As soon as all the concerned poor performances were detected in each article, they were classified into categories. A code was generated for each category for further analysis. Table 2 lists the codes for the poor performances related to each dimension.
| Normativity |
| Normativity 1. Incomplete or improper performances in each component |
| G: Goals: |
| G1: Missing the goal(s) of getting the product(s) of the target compound(s) |
| G2: Missing the goal(s) of confirming the principle(s) or the reaction mechanism(s) |
| G3: Missing the goal(s) of confirming the route(s) of the synthesis |
| G4: Stating other unrelated goal(s) of the experiment (such as learning something) |
| B: Background: |
| B1: Missing the basic introductions (such as the properties or functions) of the target compound(s) |
| B2: Missing the introduction of the current route(s) of the synthesis from the literature |
| B3: BRIEFLY introducing the background (e.g., just copying the sentences from the textbook DIRECTLY) |
| Pr: Principles |
| Pr1: Missing the chemical equation(s) of the synthetic reaction or the mechanism(s) of expressions based on the chemical equation(s) |
| Pr2: Missing sentences to describe the principles after the chemical equation(s) or the mechanism(s) of expressions |
| Pr3: BRIEFLY introducing the reaction(s) or mechanism(s) (e.g., just the name of the reaction) |
| I&C: Instruments & Chemical reagents |
| I&C1: Missing some necessary names of the instruments |
| I&C2: Missing some necessary specifications of the instruments (the specifications can be missed for some exceptions such as brush) |
| I&C3: Missing some necessary names of chemical reagents |
| I&C4: Missing some necessary information (such as amount, purity, …) of chemical reagents (some information can be missed, such as the amount of detergent) |
| O: Operations |
| O1: Missing some necessary operation(s) of the experiment |
| O2: Chaos in organizing the operations |
| O3: Chaos in presenting the operations |
| P&Res: Phenomena & Results |
| P&Res1: Missing some necessary phenomena that happened during the experiment |
| P&Res2: Missing some necessary quantitative data obtained during the experiment (the number and the unit) |
| P&Res3: Missing some necessary description of the necessary properties of the product(s) |
| P&Res4: Missing the presentation of the final result(s) of the yield(s) |
| C: Conclusions: |
| C1: Missing the conclusion(s) of getting the product(s) of the target compound(s) |
| C2: Missing the conclusion(s) of confirming the principle(s) or the reaction mechanism(s) |
| C3: Missing the conclusion(s) of confirming the route(s) for synthesis |
| C4: Stating other unrelated conclusion(s) of the experiment (such as learning something) |
| D: Discussion |
| D1: Missing the presentation of the reason(s) of the current phenomena & results |
| D2: Missing the presentation of the reason(s) of unexpected phenomena if they existed |
| D3: BRIEFLY presenting the discussion of the current phenomena & results |
| D4: BRIEFLY presenting the discussion of the unexpected phenomena if they existed |
| Ref: References |
| Ref1: No references |
| Ref2: Missing some references |
| Ref3: Errors in presenting some references |
| Normativity 2. Poor performances related to the language and academic norms |
| The codes related to language (L) |
| L1: Problems in writing words [misspellings; vague words (means not clear); ambiguous words (means various interpretations); redundant words] |
| L2: Problems in writing sentences (incomplete sentences; improper connections between sentences; improper mixture of sentences) |
| The codes related to academic norms (AN) |
| AN1: Problems in drawing figures (not in proper size or scale; no order number or description; unreadable picture) |
| AN2: Problems in drawing tables (unmatched content with headings; no order number or description) |
| Objectivity |
| Objectivity 1. For the components of instruments & chemical reagents, operations and phenomena & results, it focused on whether the descriptions fit the actual situations (AS) during the experiment |
| AS1: The description of the instruments & chemical reagents is inconsistent with the actual situation (Instruments & Chemical reagents) |
| AS2: The description of the operations or process of the experiment is inconsistent with the actual situation (Operations) |
| AS3: The description of the phenomena during the experiment is inconsistent with the actual situation (Phenomena & Results) |
| AS4: The qualitative description of the target substance(s) is inconsistent with the actual situation (Phenomena & Results) |
| AS5: The quantitative data of the target substance(s) is inconsistent with the actual situation (Phenomena & Results) |
| Objectivity 2. For the components of goals, background, principles, discussion and conclusions, it focused on whether the presentations fit the current confirmed scientific ideas (SIs) |
| SI1: Errors in presenting goal(s) |
| SI2: Errors in presenting background(s) |
| SI3: Errors in presenting principle(s) |
| SI4: Errors in presenting conclusion(s) |
| SI5: Errors in explaining result(s) |
| Logicality |
| Logicality 1. Poor performances, not fitting well with the logic of organizing different components (LO) |
| LO1: Disordered sequence(s) |
| LO2: Redundant part(s) |
| LO3: Overlap(s) in meaning |
| Logicality 2. Poor performances, which could not fit the logic of argumentation (LA) |
| LA1: Insufficient evidence |
| LA2: Irrelevant evidence (includes no connection between direct evidence and indirect evidence) |
| LA3: Contradictory evidence |
| LA4: No connection between the evidence and claim(s) |
| LA5: No (or unclear) explanations of the yield, which is not 100% |
| LA6: No (or unclear) explanations of the unexpected phenomena |
Based on the definition of normativity, the incomplete or improper performances in each component and the poor performances related to language and academic norms were detected. For each of the identified sections, the students’ articles were rated based on each component of writing as listed in Table 2. For sufficient adherence to the scientific genre, the components were rated as good. In instances where the students could not adhere to the norms of scientific writing, their performances evaluated from the codes in Table 2 were assigned to each component of the writing assignment. While dealing with their objectivity, the descriptions not fitting with the actual situations during the experiment and presentations, which did not fit the current confirmed scientific ideas, were identified. In this case, the researchers’ assessment of the writing included an evaluation of the scientific merit of synthesis including factors such as the fidelity of the description with the processes of the syntheses and consistency with the current scientific knowledge (see Table 2, under Objectivity). The final examination of the students’ writing focused on logicality, referring to the overall logical coherence of the students’ writing and organization. For this component, all the poor performances, which did not fit the logic of writing and argumentation, were noted. The logical flow of the scientific article was assessed by both the sequences of the writing components and the validity of the students’ argumentation including important dimensions of evidence use such as sufficiency, relevancy, consistency, the strength of the warrants, and the explanation of the unexpected results. These codes are also listed in Table 2 under logicality.
While applying the codes in each dimension, every student's article was reanalyzed through textual coding. All the codes of poor performances in each article were filled in, as listed in Table 3. Some points were also noted to describe the specific or notable findings during the coding.
| Key: G – goals; B – background; Pr – principles; I&C – instruments and chemical reagents; O – operations; P&Res – phenomena and results; C – conclusions; D – discussion; and R – references. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name: | Components | G | B | Pr | I&C | O | P&Res | C | D | Ref | Notes |
| …… | Normativity 1 | ||||||||||
| Experiment: | Normativity 2 | ||||||||||
| …… | Objectivity (AS/SI) | ||||||||||
| Logicality 1 | |||||||||||
| Logicality 2 | |||||||||||
After completely analyzing all the scientific articles in the six course experiments, the codes were counted to describe students’ improvement in scientific writing performance during this course. When generating the results of students’ development, not only the quantities of the codes but also the key episodes with positive correlations with changes were also treated as evidence.
All researchers first individually conducted the textual analysis work, and then met frequently to share the coding process, adjust the codes, and picked episodes, as they worked towards a consensus (Corbin and Strauss, 2008).
As Chinese was used during all of the instruction and intervention processes of this research, in order to report this research in an international journal, all course materials and data have been translated into English. To ensure the quality of translation, the translation was undertaken by a professional translator who is familiar with both English and Chemistry. After his translation, all translated sentences were checked by all the researchers who are able to read and write English articles.
Results
Developments of students’ performance in normativity
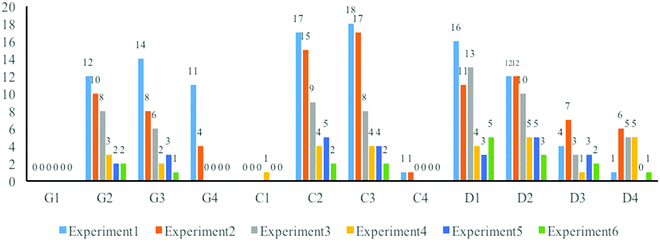
In general, the students’ scientific articles during these six experiments consisted of the necessary components mentioned above to describe the organic synthesis experiments except references, which will be discussed later. There were also some situations, which put different components into one relative centralized paragraph, such as some students (17 situations total) put background and goals together in the introduction part.Two main positive changes related to normativity were found during the course. First, some components in students’ scientific writing became more appropriate and sufficient. Fig. 2 illustrates the numbers of generated codes in the components of goals, discussion and conclusions.
 | ||
| Fig. 2 Numbers of poor performances related to the components of goals, conclusions, and discussion. | ||
The most distinct changes were observed in the components of goals and conclusions. In Fig. 2, the numbers of students’ missing the goal(s) and conclusion(s) of confirming the principle(s) or the reaction mechanism(s) (G2, C2) and confirming the route(s) of the synthesis (G3, C3) decreased.
For example, in the first and second writings, the goals written by some students were just a part of a sentence, while some information was missed, as shown in the following writing.
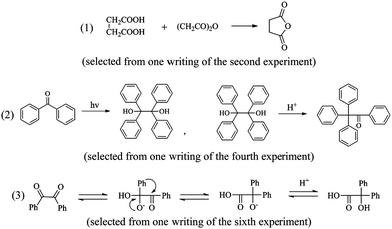
To finish the synthesis of succinic anhydride and get the target substance (selected from one writing of the second experiment)
Actually, this sentence expressed one key goal of conducting experiments in organic synthesis. However, as the goal leads to the conclusion, a larger number of students just drew a conclusion by changing the tense of the expressions of the goal to the perfect tense after they finished writing the whole article. One such example is as follows: “I have got succinic anhydride during the experiment.” As engaging in scientific practice would lead the process of constructing reliable knowledge claims (Ford, 2015), while obtaining the material objects, difficulties were observed in recognizing what knowledge the students constructed through the inquiry by just claiming that they prepared the target compounds.
The following sentence provided confirmatory evidence, supporting the positive changes:
In this experiment, the goal was to confirm the route of synthesis of benzilic acid by the rearrangement of benzil in surrounding alkali and the subsequent acidification. (selected from one writing of the sixth experiment)
Three elements were found through the analysis of this typical example. First, it indicated that the goal was to get the target compound (benzilic acid). Second, it stressed that the experiment would try to confirm whether the principles or the reaction mechanisms (rearrangement and acidification) could be effective to guide the synthesis. Third, it aimed at confirming whether the route for the synthesis (the sequence of rearrangement and acidification) was also effective in obtaining the target compound successfully. Through the textual analysis, it was confirmed that there were specific knowledge claims that needed to be supported, which were further confirmations of the principles (reaction mechanisms) and the routes for synthesis. Actually, preparing the target substance and characterization are just a necessary procedure of conducting organic chemistry experiments aimed at finding out evidence to support the principles and routes.
In the first two experiments, some students wrote the unrelated goal of the experiment (G4) as follows:
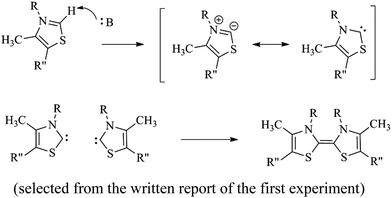
To learn how to synthesize benzoin. (selected from one writing of the first experiment)
The phrase “to learn” revealed that the goal was learning through experiment rather than the knowledge construction. It reflected the situations of “doing the lesson” (Jiménez-Aleixandre et al., 2000), indicating that the students just treated the task of experiment as a learning activity, guided directly by the teacher. Also, scientific community norms were not satisfied, because the goals and conclusions in a sound scientific writing should be related to the growth of common knowledge. Fortunately, this type of expression related to the goals (G4) and conclusions (C4) disappeared after the third experiment.
Other examples, which also supported the first positive change in normativity, were selected from the discussion component. There was a common situation during some experiments, where a few students could not get the desired yield of target substances or experienced unexpected phenomena. To explain these, some of them were unable to provide valid reasons (74 D1 and D2 codes were generated totally in the first three experiments) or just briefly accounted them to their improper operations (26 D3 and D4 codes were generated totally in the first three experiments) during the first three experiments, such as:
I did not deal well with the impurities so I could not get more yellow substance (oxaprozin). (selected from one writing of the third experiment)
The total numbers of generated codes from D1 to D4 changed to 12, 13, 6, and 6 during the last three experiments (showing a trend toward fewer errors) and could be treated as available evidence to suggest the change. Some students explained a lower yield by emphasizing improper operations by stressing the side reactions such as:
Sometimes the temperature for drying the final product was above 90 °C, which caused the heated decarboxylation reaction of benzilic acid. (selected from one writing of the sixth experiment)
Through the perspective of normativity, these changes indicated that the students could not provide valid reasons for the unsatisfactory results and unexpected phenomenon and tried to get away with putting forward very simple words such as “improper operations”, “side reactions”, and “inevitable loss” in the discussion component at the beginning. However, such explanations could not satisfy the scientific community norms, because they do not provide clear ideas of the reason of the unsatisfactory results and unexpected phenomenon, thus readers lose interest in reading the article. As shown in the second example, the student began to put forth some reasons by thinking deeply about the mechanisms of the reaction, the effects of the experimental factors, and even some ways to improve the yield. These types of appropriate and relevant discussion can not only make others feel comfortable with the unsatisfactory results and unexpected phenomenon, but also lead the person to enlarge his (her) horizons to a new field and conduct further research.
The second positive change in normativity was the suitable presentations of some components. Fig. 3 illustrates the change in students’ poor performances in the component of instruments & chemical reagents and operations, with considerably fewer errors in the later experiments.
 | ||
| Fig. 3 Numbers of poor performances related to components of instruments & chemical reagents and operations. | ||
The students’ presentation of instruments and chemical reagents in the later experiment was more suitable. Again, mostly similar to the goals, conclusions, and discussion sections, the number of student errors sharply decreased over the course of the six synthesis experiments. Further evidence supporting this idea was chosen from one article in the first experiment. This student just listed all the instruments and chemical reagents used in the experiment in the following way:
7 mL benzaldehyde (AR), 1.26 g vitamin B, ethyl alcohol, 3.5 mL distilled water, 3.5 mL 10% NaOH, zeolite, a few particles of activated carbon. (selected from one writing of the first experiment)
This was a very typical example, showing that students always forgot to write all the necessary elements such as instruments and chemical reagents when listing them one by one. In the case mentioned above, only the reactant “benzaldehyde” was written by stating the amount (7 mL) and the purity (AR). Some of the other reagents were not followed by the clarification of the purity. As ethyl alcohol was used twice in this experiment, the student did not mention the amount clearly.
The numbers of missing necessary instruments (I&C1) and chemical reagents (I&C3) and their information (I&C2 and I&C4) decreased in the latter part of the six experiments. The reasons for this change may partly be due to the presentation of this component. For example, some students presented the instruments and chemical reagents in tables. One example from a scientific writing of the third experiment is shown below. This type of table always had several columns of basic information such as the specifications; instrument materials; and the density, melting point, boiling point, purity, mass, relative molecular mass, and color of the chemical reagents. The students indicated all necessary instruments or the chemical reagents’ information in a tabulated form. Moreover, the codes of academic norms indicate that there were few students having problems in drawing tables, such as forgetting the order number and the description. The AN2 codes were generated as 0, 4, 2, 0, 0, and 0 during the six experiments. Nobody drew a table for mentioning the instruments and reagents in the first article writing. Nevertheless, after the first assignment (where tables were generally omitted), a few students at first had difficulty in referring to data tables. By the fourth assignment, there were no instances of missing or incomplete references to data tables in the students’ descriptive paragraphs in the main texts, clearly indicating their improvement.
However, all the situations cannot be expressed in a tabulated form, and words and sentences must be used to explain and state the facts. Fig. 3 also shows the decrease in student's confusion in organizing (O2) and presenting (O3) the operations during the second half of the course. The example shown below was chosen from one student's scientific article of the third experiment. Generally speaking, chaos between the instruments, reactants, and operations were easily observed in the flow chart, because this student attempted to put all information together without any good distinction. Sometimes this student used a symbol such as “↓” to mean the operation of “drop”, sometimes the words were applied to describe the concrete operations (such as “insert”, “heat”, “stir”, …). In addition, operations were indicated by arrows pointing upward or downward, making the sequences of different operations difficult to understand.
Writing the operations in narrative words is definitely a better way. In the scientific articles of the second half of the experiments, most students changed their ideas to describe the operations in words rather than other forms. The narrative genre presented the procedure of the experiment like a well-organized story, providing a good description of the operations and phenomena. As Fina and King (2011) mentioned, “narrative discourse is also a form of arguments (p. 166);” therefore, the narrator (student) could do well in stating the operations, letting others understand the whole experimental process in a better way.
Not all the performance exhibited a positive change. For example, a few students (less than 5) refused to describe the principles in words, or just briefly introduced them in every writing. Also, some of the students often faced problems in writing words and sentences.
A noticeable situation, which should be clarified here, was the component of the background. Most students stated the basic introduction of the target compound(s) (B1). In the writings of the first two experiments, about half of the students (9, 11) stated the other routes for the synthesis of benzoin and succinic anhydride. Here is an example from one writing of the second experiment:
Succinic anhydride is widely used in different chemical industries, such as the production of succinic acid, alkyd resin, ion-exchange resin, or some kinds of plastics. The melting point is 119.6 °C, the boiling point is 261 °C, the sublimation point is 90 °C (133 Pa), and the density is 1.572 g cm −3 . In this experiment, the succinic acid was used to synthesize succinic anhydride. The MTBE was also used to purify the product (someone also mentioned that chloroform could also be used, see http://muchong.com/html/201310/6530494.html). (selected from one writing of the second experiment)
However, in the third time of the synthesis of oxaprozin, only one student talked about the other background routes for the synthesis of oxaprozin. In the rest three times of the writing tasks, about 2/3rd of the students (13, 14, and 16) briefly wrote a few sentences about the background, or even did not mention the background. As we know, scientists will try to search for a lot of literature to review, and come up with their ideas of the synthesis of unknown substances based on current studies. However, we do not hope students to write the component of the background of a totally new substance like scientists. It was obvious that the target compounds mentioned in this experiment course were common enough, and the students had already learned these substances in the elementary organic chemistry course. In addition, there was easy access to get plenty of information on them (the researchers had already introduced some related textbooks and online resources to students before the course). As a result, it was not as difficult as scientists’ works to include background and references in students’ writing by referring to ready references. Through data analysis, it was disappointing that these students did not present all the relative information about the target substance(s) through the process of the literature review. As they did not refer to other literature studies, except the main textbook while preparing the experiments or writing the articles, they just preferred to write what had already been mentioned in the main textbook rather than searching for much related information by referring to other related textbooks and online or library resourses. A few students attempted to search on the Internet to get more information at first; however, they gave up soon as they would not like to find the descriptions of the other routes, such as the synthesis of oxaprozin. Therefore, only a few students (mostly less than 5) who found some additional information added a reference part at the end of their writing each time.
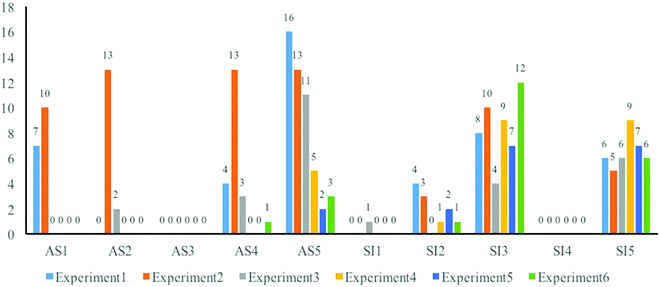
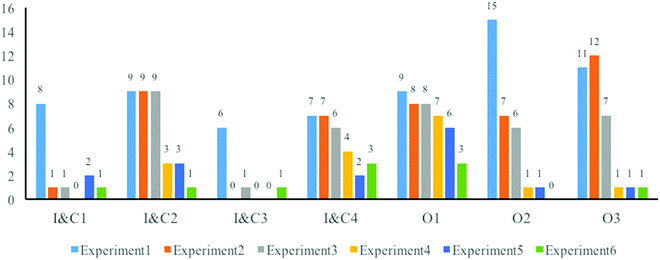
Developments of students’ performance in objectivity
Fig. 4 shows that the majority of students always performed well in describing the actual phenomena they observed (AS3) and avoided errors in presenting goal(s), background(s), and conclusion(s) (SI1, SI2 and SI4) during the whole course. Nevertheless, some changes in poor performances related to objectivity during the course were also observed.Two positive changes were noticed pertaining to students’ presenting the actual situations of the experiments. In the first one, the students changed to presenting the actual process of the experiment in some aspects (AS1, AS2, and AS4). At the beginning of this research, comparing students’ writings with the textbook reveals that some students just copied the experimental process of the experiment from the textbook. Even though the instructors modified some unnecessary operations according to the real conditions in the lab and the actual time cost in the real experiment context, the students ignored the changes and still copied the words from the textbook, once again not fitting the real situations. For example, in the second experiment, the instructors did not provide methods to dry the target product, but half of the students’ mentioned drying oven in the component of instruments and the drying process in the operations, as shown below:
Dry and get the crude product. Wash it by using 2 mL ethyl ether, suction filtrate, and dry. The weight of the final white crystal of succinic anhydride was 0.78 g. (selected from one writing of the second experiment)
Together with this additional information, this student also forgot to emphasize the exact amount of the final product. Obviously, the quantity (0.78 g) included the mass of succinic anhydride and water, because the final product was definitely not dried.
The second positive change was that more students began to be more conscious of the correctness of numbers (AS5). There were mistakes of the significant figures in the measurement and the calculated numbers in the first half of the writings. Therefore, one student's writing of chemical reagents of the third experiment as a part of the normativity was reanalyzed (see Table 4). The table shows all the amounts of the solid used in this experiment up to one digit after the decimal point (such as 1.2 g and 1.8 g). These numbers were not accurate, because the scale used in this experiment could measure data with accuracy up to two digits after the decimal points. In contrast, some students always reported the results in three digits after the decimal point (such as 79.334%) in calculating the final yield, once again not conforming with proper measurement practices. Such mistakes still happened in calculating the theoretical yield, as students always either had wrong molecular masses or ignored the stoichiometric numbers in chemical equations during the first two experiments.
| Name | Density (g cm−3) | Relative molecular mass | Amount |
|---|---|---|---|
| (Selected from one writing of the third experiment). | |||
| Succinic anhydride | — | 100 | 1.2 g (12 mmol) |
| Benzoin | — | 212 | 1.8 g (8.5 mmol) |
| Pyridine | 0.9819 | 79.1 | 1 mL (13 mmol) |
| Ammonium acetate | — | 77.08 | 1.2 g (15.5 mmol) |
| Glacial acetic acid | 1.050 | 60.05 | 40 mL (0.07 mol) |
Although focusing on whether the writings could fit the current confirmed scientific ideas, some types of incorrectness, probably serious in organic synthesis article writing, were distributed in the whole period of the course. First, it seemed fair to suggest that students’ writings of the principles or mechanisms of the experiments, especially writing the chemical equations, always had some easily noticeable faults (SI3). Here are the examples:
The first two cases reflect students’ carelessness in writing the chemical equations of the experiment. The first chemical equation missed the 2 moles of “CH3COOH”, and thus did not obey the conservation of mass. In the second chemical equation, the student forgot to add the stoichiometric number “2” before benzophenone, and H+, as the necessary reactant in the first one, then missed the “H2O” as one of the resulting products in the following equation. Such carelessness might lead to incorrect theoretical yield and the ignorance of the state of the final products.
The third case mentioned above was with regard to students’ misconceptions of specific chemical mechanisms. At first, the main step is the only reversible process of the whole process of rearrangement, indicating that the rest of the reaction steps should be irreversible and thus must be marked with “→”. Second, the third intermediate is less basic than the hydroxyl anion, and therefore the proton transfer would take place to form an intermediate “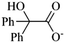 ” which can be protonated in the acidic work up to the final product. Obviously, the student did not show the necessary proton shift.
” which can be protonated in the acidic work up to the final product. Obviously, the student did not show the necessary proton shift.
The incorrectness also happened frequently in the discussion component (SI5). When trying to explain unexpected phenomena, they not only attributed them to improper operations, but also presented errors, which could not correspond to the current confirmed scientific ideas. One representative example was found in the writings of the fifth experiment, during which most students observed an unexpected phenomenon about a new oily substance floating on the liquid after injecting 40 mL of water. Some of them explained this phenomenon like:
It was necessary to be slow when adding 40 mLof water in the three-necked bottle, or else an oily substance would appear. (selected from one writing of the fifth experiment)
Actually, the students were either confused or could not understand the real reason for this problem, which definitely is not related to the speed of injecting water. In this reaction, some by-products, which could not dissolve in water, were formed. For proper explanations, the students should point out that these typical by-products might not be soluble in water, instead of accounting for the operations.
Developments of students’ performance in logicality
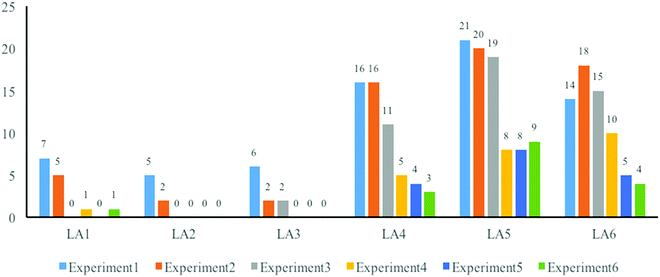
Most students did well in organizing the article logically. They understood the sequence of linking different components together. There were just 5 and 2 LO2 codes generated to indicate the redundant cases in the first and third experiments, as students separated the components of operations and phenomena apart and repeated the operations again while narrating the phenomena.Statistics of LA1 to LA6 codes (in Fig. 5) suggested some positive changes in the students’ performance in the logic of argumentation.
The first aspect was about evidence (LA1, LA2, and LA3). They should clarify all related evidence obtained during the experiment in order to justify the conclusion. However, 18 (7 + 5 + 6) and 9 (5 + 2 + 2) students failed to present all the evidence in a proper way in the first two writings. First, students always forgot some key evidence such as the description of the color and appearance of the final product, or their melting points, which had already been measured. Second, a lot of students could not reveal the process of linking indirect evidence, which could be reasoned or calculated from direct evidence. One typical case in many students’ writings of the final yield is as follows:
The mass of benzoin was 6.0 g, the yield would be 64.3%. (selected from one writing of the first experiment)
The yield should be treated as an important indicator of the outcome of the synthesis reaction. In the example mentioned above, 6.0 g was direct evidence, as it was obtained by measuring the mass of the final product (benzoin). However, this absolute quantity cannot be used as evidence to support the outcome of the synthesis, and the percentage yield must be given as indirect evidence. The percentage yield should be calculated by dividing the yield obtained by the theoretical yield, to make more sense. In this case, the necessary reasoning process of calculating the percentage yield including figuring out the theoretical yield was missing.
The third problem about the evidence was contradictory. In some circumstances, students could not get enough final product after the experiment, because of several personal and unavoidable factors. The little amount decreased the percentage yield to a significant extent. However, some students still put this quantitative data with other qualitative descriptions of the final product together as usual without providing some explanations. This mixture of conflicting evidence made the situation more contradictory and thus could not well support the specific conclusion.
These three problems almost disappeared from the third experiment, indicating that students had a fair idea of putting all effective evidence and the process of reasoning direct evidence to indirect evidence. When facing contradictions, they provided a logical explanation in their articles.
The next positive change related to the logic of argumentation was the relationship of evidence and conclusion (LA4). During the first half of this study, students always put evidence and the conclusions in different components and seldom linked them together. Evidence was shown in the component of the phenomenon, and the conclusions were just at the end of the article. These situations would be more understandable after analyzing the following two examples.
(1)
![[T with combining low line]](https://www.rsc.org/images/entities/b_char_0054_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/b_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/b_char_006d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/b_char_006c_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/b_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/b_char_006e_0332.gif)
![[g with combining low line]](https://www.rsc.org/images/entities/b_char_0067_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/b_char_0070_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/b_char_006f_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/b_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/b_char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/b_char_0077_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/b_char_0073_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[8 with combining low line]](https://www.rsc.org/images/entities/b_char_0038_0332.gif) –
–![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/b_char_0032_0332.gif) 0 °
0 °![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) , so
, so ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[f with combining low line]](https://www.rsc.org/images/entities/i_char_0066_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/i_char_0077_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) . (selected from one writing of the second experiment)
. (selected from one writing of the second experiment)
(2) The infrared spectra of the production showed some spectral peaks. First, ![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/b_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/b_char_0070_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[k with combining low line]](https://www.rsc.org/images/entities/b_char_006b_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/b_char_0033_0332.gif)
![[4 with combining low line]](https://www.rsc.org/images/entities/b_char_0034_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/b_char_0030_0332.gif) 0
0 ![[c with combining low line]](https://www.rsc.org/images/entities/b_char_0063_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/b_char_006d_0332.gif) −1
−1 ![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif) –
–![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif)
![[b with combining low line]](https://www.rsc.org/images/entities/i_char_0062_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif) becausethe O–H bond presented in the acid is always below 3400 cm−1. Second,
becausethe O–H bond presented in the acid is always below 3400 cm−1. Second, ![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/b_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/b_char_0070_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[k with combining low line]](https://www.rsc.org/images/entities/b_char_006b_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/b_char_0032_0332.gif)
![[9 with combining low line]](https://www.rsc.org/images/entities/b_char_0039_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/b_char_0035_0332.gif) 0
0 ![[c with combining low line]](https://www.rsc.org/images/entities/b_char_0063_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/b_char_006d_0332.gif) −1
−1 ![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[x with combining low line]](https://www.rsc.org/images/entities/i_char_0078_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[f with combining low line]](https://www.rsc.org/images/entities/i_char_0066_0332.gif) –
–![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif)
![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) asit is always found between 2500 and 3300 cm−1. Third,
asit is always found between 2500 and 3300 cm−1. Third, ![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/b_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/b_char_0070_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[k with combining low line]](https://www.rsc.org/images/entities/b_char_006b_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[7 with combining low line]](https://www.rsc.org/images/entities/b_char_0037_0332.gif)
![[2 with combining low line]](https://www.rsc.org/images/entities/b_char_0032_0332.gif) 0
0 ![[c with combining low line]](https://www.rsc.org/images/entities/b_char_0063_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/b_char_006d_0332.gif) −1 was
−1 was ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[k with combining low line]](https://www.rsc.org/images/entities/i_char_006b_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[f with combining low line]](https://www.rsc.org/images/entities/i_char_0066_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif)
![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[b with combining low line]](https://www.rsc.org/images/entities/i_char_0062_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[x with combining low line]](https://www.rsc.org/images/entities/i_char_0078_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif)
![[g with combining low line]](https://www.rsc.org/images/entities/i_char_0067_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/i_char_006f_0332.gif)
![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) asit always appears between 1690 and 1740 cm−1. Finally, the
asit always appears between 1690 and 1740 cm−1. Finally, the![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/b_char_0077_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/b_char_006f_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/b_char_0070_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/b_char_0065_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[k with combining low line]](https://www.rsc.org/images/entities/b_char_006b_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/b_char_0073_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/b_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/b_char_0074_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[5 with combining low line]](https://www.rsc.org/images/entities/b_char_0035_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/b_char_0030_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/b_char_0030_0332.gif) –
–![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[6 with combining low line]](https://www.rsc.org/images/entities/b_char_0036_0332.gif)
![[0 with combining low line]](https://www.rsc.org/images/entities/b_char_0030_0332.gif) 0
0 ![[c with combining low line]](https://www.rsc.org/images/entities/b_char_0063_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/b_char_006d_0332.gif) −
−![[1 with combining low line]](https://www.rsc.org/images/entities/b_char_0031_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/i_char_006d_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[g with combining low line]](https://www.rsc.org/images/entities/i_char_0067_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/i_char_0064_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/i_char_0063_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/i_char_0061_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/i_char_0073_0332.gif) . (selected from one writing of the sixth experiment)
. (selected from one writing of the sixth experiment)
Different symbols were used to distinguish different parts of argumentation as shown in these two examples. The sentences in bold and underlined indicate evidence, which are the data, directly obtained in the experiment. The sentences under solid lines are conclusions. The words in bold are necessary sentences (warrants), which could be regarded as the bridges between the evidence and conclusions, and always came from concrete knowledge. For instance, the first sentence in bold in the second example indicates that although there are two O–H bonds in benzilic acid, the peak at 3400 cm−1 in the infrared spectra could be attributed to the alcoholic hydroxyl group, because the O–H bond of the acid would show a peak below 3400 cm−1.
In the first example, there are no words related to the reasoning process. As the sentence just stated two facts (the melting point and the final product), it was difficult to clearly judge that the melting point data of the target compound measured during the experiment could confirm the final product, unless the melting point of succinic anhydride was presented by citing from the handbook of chemical products or other authoritative resources. Although the structural elucidation of phenyls is not sufficient in the second example (there are some other data such as “C–H stretching, C–H out-of-plane bending, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching occurring in the range of 3050–3010 cm−1, 690–900 cm−1, and 1600–1475 cm−1, respectively.” In addition, monosubstituted rings exhibiting strong absorptions at 690 and 750 cm−1 demonstrate the presence of phenyls), the logic of argumentation obviously increased as some of the elements of argumentation were clarified in detail.
C stretching occurring in the range of 3050–3010 cm−1, 690–900 cm−1, and 1600–1475 cm−1, respectively.” In addition, monosubstituted rings exhibiting strong absorptions at 690 and 750 cm−1 demonstrate the presence of phenyls), the logic of argumentation obviously increased as some of the elements of argumentation were clarified in detail.
The third positive change was the explanation of the lower yield and the unexpected phenomenon (LA5 and LA6). As the side reactions could not be avoided in most of the synthetic reactions in organic chemistry, the target product could not always be obtained in 100% yield, and the side reaction may lead to some unexpected phenomenon. In the case of very low yield of the desired product and unexpected phenomena, the synthetic process and routes leading to the desired product may not be considered viable. With this in mind, a sound logic of drawing conclusions should explain the low yield and the unexpected phenomenon more clearly by exonerating the routes of synthesis themselves. The clarification of these exceptions would strengthen the conclusion as mentioned by Szu and Osborne (2012) by explicitly pointing out a possible explanatory framework, and the likelihood of the data supporting the target hypothesis over the rival hypothesis increases. For example:
(1) The yield was 39% because there were side reactions. (selected from one writing of the first experiment)
(2) The yield was 59% because the adjustment of the pH was not well done (the pH was above 10). This led to a side reaction which consumed the reactant:
Although the percentage yields in these two examples were low, and thus the conclusion was difficult to support, the second explanation could be easily accepted, because the improper operation and the specific side reactions that happened were illustrated. This indicates that the side reactions were totally different from the routes for the synthesis of benzoin, and it could be inferred that they might not happen or might not become the main reactions in the solution if the pH was well adjusted. Consequently, the catalyst would promote the target reaction rather than being consumed. However, this was the only case where the student explained the lower yield in the writing of the first experiment. Fortunately, over the course of the experiments, the students provided the reason of the unsatisfactory results in a positive way.
The data from the dimension of logicality also showed that the biggest challenge in students’ argumentation was related to warrants and rebuttals, indicating that the students could not provide links between evidence and claims and explaining the unexpected results. Most students exhibited poor performances related to these issues at first. Even in the last one or two writings, a few students, even though they changed gradually, still had some problems. These results were supported by our previous findings on students’ written scientific argumentation, illustrating that the students could put forward claims and evidence more easily than warrants and rebuttals (Deng and Wang, 2017). Others studies also revealed the same weakness of students’ argumentation (Sandoval, 2003; Erduran et al., 2004; McNeill and Krajcik, 2007).
Conclusion
In general, the implementation of the Advanced Organic Chemistry Experiment course revealed that some aspects of the scientific writing of Chinese undergraduate students were always well developed, while other aspects had several positive changes. Some poor performances remained unchanged. Table 5 illustrates a summary of the range of writing performances for all the situations of the development by categorizing them into the related dimensions of normativity, objectivity, and logicality.| Always performed well | Normativity | Students’ scientific writings always comprised several necessary components to describe the organic synthesis experiment. |
| Objectivity | Majority of students always did well in describing the actual phenomena they observed, and avoided errors in presenting goals, backgrounds, and conclusions. | |
| Logicality | Most students did well in the logical organization of the article. | |
| Positive changes | Normativity | The presentations of the goals, conclusions, and discussions became more appropriate and sufficient. |
| The presentations of instruments & chemical reagents and operations became more appropriate. | ||
| Objectivity | Students changed to present the actual process of the experiment. | |
| Students changed to be more conscious about the correctness of numbers. | ||
| Logicality | The evidence presented became more sufficient, reasonable, and relevant. | |
| Students changed to pay more attention to the relationship of evidence and conclusions. | ||
| Students explained the yield and the unexpected phenomenon in a better way in the later part of the course. | ||
| Poor performance without changes | Normativity | Students did not refer to others in writing the background and added references in the end. |
| Objectivity | Students’ writings of the principles or mechanisms of the experiments, especially writing the chemical equations always had some faults. | |
| Students could not always be correct in writing the discussion component. | ||
| Logicality | No situations related to this dimension. |
This study provides a unique perspective of evaluating students’ scientific writing through textual analysis. A comprehensive and detailed method was constructed by summarizing the three dimensions, normativity, objectivity, and logicality, of sound scientific writing through a literature review. These three dimensions are considered as the basic requirements of sound scientific writing, because they reflect the nature of scientific writing as a type of social practice. From this point of view, scientific writing is not only a modality of presenting scientific knowledge, ideas, principles, and theories, but also a linguistic way of sharing, understanding, persuading, and presenting arguments to defend claims in a scientific community. It is an important way to be acculturated in the scientific community through writing. These writing practices can be treated as communicational ways to make sense of chemical ideas and reach a consensus while writing about the objective facts and real processes of scientific practices and to make the claims and conclusions in a more reliable and persuasive way when organizing and arguing logically. Bereiter and Scardamalia (1987) found that novices always tell their knowledge through writing, while experts use writing to construct, build, and transform knowledge. Galbraith et al. (2006) further developed the knowledge-constituting process on Beretier's idea to illustrate the process of knowledge creation by emphasizing the tension between the explicit text and the implicit disposition toward a topic. The data analysis revealed that the undergraduate students’ poor performances in writing articles on organic chemistry experiments may not have a positive effect on their social understanding and communication. For example, as mentioned before, students’ brief discussion of unexpected phenomena of the experiment may not be sufficient for the potential readers to reach the target conclusion, and the readers may lose further interest or ideas in future research. As a result, the science teachers should treat and evaluate students’ scientific writing from the perspective of social practice instead of their knowledge acquirement or writing mistakes. At the same time, instructors need to facilitate students to recognize the social criteria of scientific writing and apply these criteria to judge between effective and non-effective scientific writing.
In this study, the development of undergraduate students’ scientific writing was divided into three categories. First, the students always presented the majority of the necessary components of a scientific article and organized different components in a proper way, probably attributed to their prior experience of reading and writing scientific articles, or perhaps even the reading task in the intervention of the course. Second, positive changes in some of the students’ writing may push us to treat the intervention of the course as an approach to teach students “learn to write.” Actually, during the study, these undergraduate students definitely learned to present some of the components in more appropriate, sufficient, and suitable ways, and understood the necessity of reflecting the real context, process, phenomena, and experimental data based on scientific knowledge. In addition, they also acquired the basic scientific argumentation skills such as presenting evidence, linking evidence and claims, and constructing rebuttals. In general, these positive changes should be treated as the concrete contents of “how to write” from the perspectives of social considerations of scientific writing, and this study could also be regarded as guiding students to scientific writing in a more detailed way, differing from other researchers who just paid attention to some skills in writing. Third, the students continuously faced problems in presenting the principles of the experiment and discussing the results in non-objective and unscientific ways. This could be related to the misconceptions and misunderstandings of specific knowledge in organic chemistry, or even to students’ lack of recognition of the importance of written communication in scientific practices.
Understanding the students’ struggles and specific difficulties with scientific writing may be beneficial to science teachers in teaching scientific writing to students in the future, stressing the fact that they should pay more attention to the knowledge, social practice, and affective dimensions of writing. Also, the results provide further implications on complete interventions, which can help students “learn to write” by integrating scientific knowledge with genre-specific practices into scientific writing tasks, allowing greater contributions to the development of scientific writing literacy.
This study was considered as a single case study, held in the Advanced Organic Chemistry Experiment course at one Chinese university. The importance and results of teaching “learn to write” in science are clearly demonstrated by the conclusion. The role of the intervention process will be illustrated sufficiently in our later paper. Although this single case study did not make any comparisons between students’ writing in the normal way and the current way as they are totally different, it could be inferred that students’ participating in the current paper-written tasks would benefit from their familiarity with the normativity, objectivity, and logicality of sound scientific writing, as they could consider how to express the ideas after reflecting the basic norms and requirements of the intended readers and how to describe the process of scientific practice in a detailed, well-organized and persuasive way, instead of finishing the normal lab report as just filling some blanks by copying some words or sentences from textbooks. It was believed that the potential value of the specific intervention process on the results of development can be used as standards for follow-up study. Also, future studies should also pay more attention to scientific writings in different themes or subjects, because scientific writing is a type of social practice for the flourishing of science and its development. The key factors such as the nature, characteristics, and criteria of scientific writing are determined by different contents and contexts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the support of the Youth Project Supported by the Ministry of Education (MOE) of China of Humanities and Social Sciences (Grant No. 17YJC880016) and the financial support by the Self-Determined Research Funds of CCNU from the Colleges’ Basic Research and Operation of MOE (Grant No. CCNU16GD008).References
- Barstow B., Fazio L., Schunn C. and Ashley K., (2017), Experimental evidence for diagramming benefits in science writing, Instruct. Sci., 45, 537–556.
- Bazerman C., (1988), Shaping Written Knowledge, Madison, WI: University of Wisconsin Press.
- Bazerman C., (2006), Analyzing the multidimensionality of texts in education, in Green J. L., Camilli G., Elmore P. B., Skukauskaiti A. and Grace E. (ed.), Handbook of Complementary Methods in Education Research, Washington, DC: American Educational Research Association.
- Bereiter C. and Scardamalia M., (1987), The psychology of written composition, Hillsdale, NJ: Erlbaum.
- Berkenkotter C. and Huckin T. N., (2016), Genre Knowledge in Disciplinary Communication: Cognition/Culture/Power, Routledge.
- Berland L. K., Schwarz C. V., Krist C., Kenyon L., Lo A. S. and Reiser B. J., (2016), Epistemologies in practice: making scientific practices meaningful for students, J. Res. Sci. Teach., 53, 1082–1112.
- Best L., (1995), A critique of cognitive research on writing from three critical perspectives: Theoretical, methodological, and practical, Resources in Education (ERIC Document Reproduction Service No. ED 377516).
- Cai T. Q. and Chen L. H., (2010), Scientific writing in science education, Global Educ., 39(4), 85–89 (Chinese Journal).
- Cavagnetto A. and Hand B., (2012), The importance of embedding argument within science classrooms, in Khine M. S. (ed.), Perspectives on Scientific Argumentation: Theory, Practice and Research, Dordrecht: Springer.
- Çetin P. S. and Eymur G., (2017), Developing students’ scientific writing and presentation skills through argument driven inquiry: an exploratory study, J. Chem. Educ., 94, 837–843.
- Chen Y.-C., Hand B. and McDowell L., (2013), The effects of writing-to-learn activities on elementary students’ conceptual understanding: learning about force and motion through writing to older peers, Sci. Educ., 97, 745–771.
- Choi A., Notebaert A., Diaz J. and Hand B., (2010), Examining arguments generated by year 5, 7, and 10 students in science classrooms, Res. Sci. Educ., 40, 149–169.
- Choi A., Hand B. and Greenbowe T., (2013), Students’ written arguments in general chemistry laboratory investigations, Res. Sci. Educ., 43, 1763–1783.
- Corbin J. M. and Strauss A. L., (2008), Basics of Qualitative Research, London: Sage.
- Cunningham C. M. and Kelly G. J., (2017), Epistemic practices of engineering for education, Sci. Educ., 101, 486–505.
- DeBoer G. E., (1991), A History of Ideas in Science Education: Implications for Practice, New York, NY: Teachers College Press.
- Deng Y. and Wang H., (2017), Research on evaluation of Chinese students' competence in written scientific argumentation in the context of chemistry, Chem. Educ. Res. Pract., 18, 127–150.
- Dianovsky M. T. and Wink D. J., (2012), Student learning through journal writing in a general education chemistry course for pre-elementary education majors, Sci. Educ., 96, 543–565.
- Dirrigl Jr. F. J. and Noe M., (2014), The student writing toolkit: enhancing undergraduate teaching of scientific writing in the biological sciences, J. Biol. Educ., 48(3), 163–171.
- diSessa A. A., (2004), Metarepresentation: native competence and targets for instruction, Cognit. Instruct., 22(3), 293–331.
- Erduran S., Simon S. and Osborne J., (2004), TAPping into argumentation: developments in the application of Toulmin's argument pattern for studying science discourse, Sci. Educ., 88, 915–933.
- Fairclough N., (2003), Analysing discourse: textual analysis for social research, New York: Routledge.
- Felton M. and Kuhn D., (2001), The development of argumentative discourse skill, Discourse Process., 32(2&3), 135–153.
- Fina A. D. and King K. A., (2011), Language problem or language conflict? Narratives of immigrant women's experiences in the US, Discourse Stud., 13(2), 163–188.
- Florence M. K. and Yore L. D., (2004), Learning to write like a scientist: coauthoring as an enculturation task, J. Res. Sci. Teach., 41, 637–668.
- Flowerdew J. and Miller L., (1995), On the notion of culture in L2 lectures, TESOL Quart., 29, 345–373.
- Ford M. J., (2015), Educational implications of choosing “practice” to describe science in the Next Generation Science Standards, Sci. Educ., 99, 1041–1048.
- Galbraith D., (1999), Writing as a knowledge-constituting process, in Galbraith D. and Torrance M. (ed.), Knowing what to write: conceptual processes in text production, Amsterdam: Amsterdam University Press, pp. 139–159.
- Galbraith D., Torrance M. and Hallam J., (2006), Effects of writing on conceptual coherence, in Sun R. and Miyake N. (ed.), Proceedings of the 28th Annual Conference of the Cognitive Science Society, Mahwah, NJ: Erlbaum.
- Glynn S. M. and Muth K. D., (1994), Reading and writing to learn science: achieving scientific literacy, J. Res. Sci. Teach., 31, 1057–1073.
- Guilford W. H., (2001), Teaching peer review and the process of scientific writing, Adv. Physiol. Educ., 25, 167–175.
- Habermas J., (2001), Postmetaphysical Thinking, Cao W. D., et al., trans., Nanjing: Yilin Press, (original work published 1988).
- Hand B. and Choi A., (2010), Examining the impact of student use of multiple modal representations in constructing arguments in organic chemistry laboratory classes, Res. Sci. Educ., 40, 29–44.
- Hand B. and Prain V., (2002), Teachers implementing writing-to-learn strategies in junior secondary science: a case study, Sci. Educ., 86, 737–755.
- Hand B., Hohenshell L. and Prain V., (2004), Exploring students’ responses to conceptual questions when engaged with planned writing experiences: a study with year 10 science students, J. Res. Sci. Teach., 41, 186–210.
- Higgs J., (2012), Practice-based education pedagogy, in Higgs J., Barnett R., Billett S., Hutchings M. and Trede F. (ed.), Practice-based Education: Perspectives and Strategies, Rotterdam: Sense Publishers.
- Hyatt J. K., Bienenstock E. J. and Tilan J. U., (2017), A student guide to proofreading and writing in science, Adv. Physiol. Educ., 41(3), 324–331.
- Jiménez-Aleixandre M. P., Bugallo Rodríguez A. and Duschl R. A., (2000), “Doing the lesson” or “doing science”: argument in high school genetics, Sci. Educ., 84, 757–792.
- Kelly G. J. and Bazerman C., (2003), How students argue scientific claims: a rhetorical-semantic analysis, Appl. Linguist., 24(1), 28–55.
- Kelly G. J. and Licona P., (2018), Epistemic Practices and Science Education, in Matthews M. (ed.), History, philosophy and science teaching: new research perspectives, Springer: Dordrecht, pp. 139–165.
- Kelly G. J. and Takao A., (2002), Epistemic levels in argument: an analysis of university oceanography students’ use of evidence in writing, Sci. Educ., 86(3), 314–342.
- Kelly G. J., Regev J. and Prothero W., (2008), Analysis of lines of reasoning in written argumentation, in Erduran S. and Jimenez-Aleixandre M. (ed.), Argumentation in Science Education: Perspectives from Classroom-Based Research, Dordrecht: Springer.
- Keys C. W., (1999), Revitalizing instruction in scientific genres: connecting knowledge production in the writing to learn in science, Sci. Educ., 83, 115–130.
- Klein P. D., (1999), Reopening inquiry into cognitive processes in writing-to-learn, Educ. Psychol. Rev., 11, 203–270.
- Kramer I. M. and Kusurkar R. A., (2017), Science-writing in the blogosphere as a tool to promote autonomous motivation in education, Internet High. Educ., 35, 48–62.
- Kuhn D., (2012), Foreword, in Khine M. S. (ed.), Perspectives on Scientific Argumentation: Theory, Practice and Research, Dordrecht: Springer.
- Lee H. S., Liu O. L., Pallant A., Roohr K. C., Pryputniewicz S. and Buck Z. E., (2014), Assessment of uncertainty-infused scientific argumentation, J. Res. Sci. Teach., 51(5), 581–605.
- Martin A. M. and Hand B., (2009), Factors affecting the implementation of argument in the elementary science classroom: a longitudinal case study, Res. Sci. Educ., 39, 17–38.
- McNeill K. L., (2011), Elementary students’ views of explanation, argumentation, and evidence, and their abilities to construct arguments over the school year, J. Res. Sci. Teach., 48(7), 793–823.
- McNeill K. L. and Krajcik J., (2007), Middle school students’ use of appropriate and inappropriate evidence in writing scientific explanations, in Lovett M. C. and Shah P. (ed.), Thinking with Data: The Proceedings of the 33rd Carnegie Symposium on Cognition, Mahwah, NJ: Erlbaum.
- National Research Council, (2012), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas, Washington, DC: National Academies Press.
- Norris S. P. and Phillips L. M., (2003), How literacy in its fundamental sense is central to scientific literacy, Sci. Educ., 87, 224–240.
- Reeves J. and Fox A., (ed.), (2008), Practice based Learning: Developing Excellence in Teaching, Edinburgh: Dunedin Academic Press.
- Ryu S. and Sandoval W. A., (2012), Improvements to elementary children's epistemic understanding from sustained argumentation, Sci. Educ., 96, 448–526.
- Sampson V., Enderle P., GroomS J. and Witte S., (2013), Writing to learn by learning to write during the school science laboratory: helping middle and high school students develop argumentative writing skills as they learn core ideas, Sci. Educ., 97, 643–670.
- Sandoval W. A., (2003), Conceptual and epistemic aspects of students’ scientific explanations, J. Learn. Sci., 12(1), 5–51.
- Szu E. and Osborne J., (2012), Scientific reasoning and argumentation from a bayesian perspective, in Khine M. S. (ed.), Perspectives on Scientific Argumentation: Theory, Practice and Research, Dordrecht: Springer.
- Vygotsky L. S., (1978), Mind in Society: The Development of Higher Psychological Processes, Cambridge, MA: Harvard University Press.
- Yin R. K., (2014), Case study: research design and methods, 5th edn, Thousand Oaks, CA: Sage.
- Yore L. D., Hand B. and Prain V., (2002), Scientists as writers, Sci. Educ., 86, 672–692.
- Yore L. D., Hand B. and Florence M. K., (2004), Scientists’ views of science, models of writing, and science writing practices, J. Res. Sci. Teach., 41, 338–369.
- Zhang H. Y. and Zhang H. D., (2009), Scientific writing: a considerable domain in science education, Primary and Secondary Schooling Aboard, 2, 246–251 (Chinese Journal).
- Zohar A. and Nemet F., (2002), Fostering students’ knowledge and argumentation skills through dilemmas in human genetics, J. Res. Sci. Teach., 39(1), 35–62.
| This journal is © The Royal Society of Chemistry 2019 |