Kinetics and reactor modeling of the conversion of n-pentane using HZSM-5 catalysts with different Si/Al ratios†
Abstract
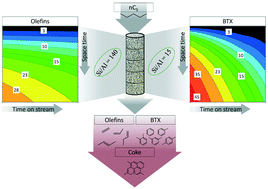
The production of olefins and aromatics from n-pentane has been modeled using the experimental results collected in an isothermal packed bed reactor with HZSM-5 zeolite catalysts with different Si/Al ratios (15 and 140) in the temperature range of 400–550 °C. In the first stage, a lump-based kinetic model has been established, evaluating the role of the Si/Al ratio in the kinetic parameters and therefore, in the conversion, product distribution and deactivation by coke. The effect of the catalyst acidity and the reaction conditions has been explained by analyzing the used catalysts by means of N2 and tert-butylamine adsorption–desorption, temperature-programmed oxidation and confocal fluorescence microscopy. In the second stage, the kinetic parameters extracted for both catalysts have been used in simulations of an isothermal packed bed reactor in order to study the evolution of the reaction with the space time and time on stream. Certain suitable conditions (550 °C and 3.5 gcat h molC−1) for maximizing the yield and selectivity to olefins (31 and 51%, respectively, using the zeolite with Si/Al = 140) and aromatics (yield and selectivity of 53%, using the one with Si/Al = 15) in the simulated range were found.


 Please wait while we load your content...
Please wait while we load your content...