Control of phase separation behaviour of ionic liquid catalysts with reactants/products toward synthesis of long-chain wax esters at moderate temperatures†
Abstract
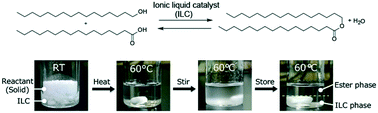
In the present study, the phase behaviour and reactivity of sulfonic acid-functionalised imidazolium- and phosphonium-based ionic liquid catalysts (ILCs) have been investigated for Fischer esterification reactions between alcohols and carboxylic acids with different alkyl chain lengths. Suitable design of ILCs enabled the control of phase behaviour with the reactants/products, and various wax esters derived from long-chain fatty acids and long-chain fatty alcohols underwent phase separation with the ILCs. Upon relating the phase behaviour and conversion (X), it has been revealed that higher X values were observed in the products that were phase-separated from the ILCs, as compared to the products that remained in the homogeneous phase with the ILCs. Furthermore, an excellent conversion of over 99% was achieved for production of cetyl palmitate, a typical wax ester, even at moderate temperature (60 °C) by removing water via bubbling dry N2 gas into the mixture or under reduced pressure.


 Please wait while we load your content...
Please wait while we load your content...