Continuous flow processing as a tool for the generation of terpene-derived monomer libraries†
Abstract
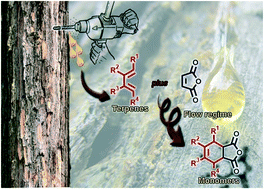
We report the development of a continuous flow approach for the preparation of two bio-derived monomer libraries. A small range of terpenes (ocimene, myrcene, α-terpinene, α-phellandrene, isoprene, and farnesene) have been used as the base set for the library, with the first library derived from a Diels–Alder reaction with the platform chemical maleic anhydride. The second library requires the derivatization of the first through a hydrogenation reaction. The potential for scale-up of both libraries has been demonstrated, with the Diels–Alder process delivering 10.5 grams of the product in 3 hours and the hydrogenation process delivering 10 grams of the material in 16 hours.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...