Open Access Article
Open Access ArticleExploiting a multicomponent domino reaction strategy for the tailoring of versatile environmentally sensitive fluorophore-based nicotinonitriles incorporating pyrene and fluorene moieties†
Essam M. Hussein ab,
Nizar El Guesmiac and
Saleh A. Ahmed
ab,
Nizar El Guesmiac and
Saleh A. Ahmed *ab
*ab
aChemistry Department, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. E-mail: saahmed@uqu.edu.sa
bDepartment of Chemistry, Faculty of Science, Assiut University, 71516 Assiut, Egypt. E-mail: saleh.a.ahmed@aun.edu.eg; saleh_63@hotmail.com
cDépartement de Chimie, Faculté des Sciences de Monastir, Avenue de L’Environnement, 5019 Monastir, Tunisia
First published on 3rd December 2019
Abstract
A simplistic and highly effective protocol for the synthesis of a new class of poly-functionalized innovative nicotinonitriles incorporating pyrene and/or fluorene moieties has been developed through the domino four-component condensation reaction of 1-(pyren-1-yl)ethanone/1-(9H-fluoren-2-yl)ethanone, numerous aromatic aldehydes, and 3-oxo-3-(pyren-1-yl)propanenitrile/3-(9H-fluoren-2-yl)-3-oxopropanenitrile and ammonium acetate in acetic acid as a reaction medium. The advantages of this approach are the short reaction time, excellent yield, and the easy experimental workup that affords substrate diversity and operative competence under metal-free reaction conditions for the formation of C–C and C–N bonds. The substituent effects on the photophysical property-based absorption and the emission of the synthesized compounds in dichloromethane have been well-investigated. Strong absorption quenching of around 100 nm was observed when substitution of the benzene ring at the C4-position of the pyridine moiety occurred with an electron-donating (–N(CH3)2) group. All of the newly synthesized nicotinonitrile derivatives showed strong blue-green fluorescence emission with maxima in the range between 420–630 nm. These highly pronounced emission spectra will help this family of compounds to find application in many areas and the field of materials science.
Introduction
Carbon–carbon and carbon–nitrogen bond-forming reactions are amongst the most important transformations in organic synthesis.1 Dissimilar conventional multistep reactions, enhanced efficacy, higher reaction yields, atom economy, shorter reaction times, ecologically benign reactions, amended selectivity, and lower costs can be accomplished using multicomponent reactions (MCRs), which are influential and helpful tools in modern medicinal chemistry, providing easy access to an enormous number of structurally connected drug-like heterocyclic compounds.2–5 Poly-functionalized nitrogen-containing heterocycles are essential structural components in numerous natural products and synthetic drugs. They have great applications in drug discovery and are beneficial and useful materials.6–8 Pyridine derivatives substituted at the 2, 4, and 6 positions have an extensive range of applications, most of which are based upon their unique photophysical properties. The applications of these compounds comprise photographic acid-mediated imaging media,9 thermal recording materials,10 photo-curable assembly for stereolithography, laser dyes11 and ion probes.12,13 In the last few decades, design and synthesis of materials with light-emitting properties has emerged as an exciting topic of research in both academic and industrial applications.14 Furthermore, pyrene is a π-extensive conjugated polynuclear aromatic hydrocarbon, which was recently considered as being one of the most extensively studied organic fragments in the field of photochemistry and photophysics. The extremely fluorescence properties of pyrene mean it is the first choice fluorophore in both fundamental and applied photochemical and photophysical research.15–21 As its monomer emission typically appears at 370–420 nm and it is a distinguishing violet color, the pyrene moiety is considered to be one of the most beneficial assembly moieties for the construction of fluorogenic chemosensors that are frequently used for necessary chemical applications.22 Pyrene derivatives have been extensively used in many applications as fluorescent probes23 and fluorescent sensors.24,25 On the other hand, fluorene derivatives, which are less important than pyrene, are adaptable moieties which are used in a wide range of synthetic purposes.26 Amongst the fluorophores, fluorene derivatives have reasonable quantum yields and are less bulky than other frequently used fluorophores, such as fluorescein and cyanine dyes.27 Nevertheless, fluorene-based polymers and copolymers are brilliant candidates for optical and electrical applications because they display extraordinary chemical/thermal stability, outstanding fluorescence quantum yields and consequently are commonly used in organic light-emitting diodes, flat panel displays and in solar cells.28–30 In the last decay, numerous approaches have been developed by many researchers for the synthesis of 2,4,6-trisubstituted nicotinonitriles.31–37 However, no reports were found of the synthesis of a single molecular structure with these three units (pyrene, nicotinonitrile and fluorene). Based on the above described findings, and in continuation of our ongoing research interest which deals with the synthesis of novel environment-sensitive fluorophores38,39 and the development of efficient, low-cost and simple new methodologies for the synthesis of nitrogen-containing heterocyclic compounds through MCRs,40–44 a simple, straight-forward and efficient procedure for the synthesis of novel highly functionalized nicotinonitriles incorporating pyrene and/or fluorene moieties in good to excellent yields has been achieved. Yields of up to 98% via a one-pot four-component condensation reaction of 1-(pyren-1-yl)ethanone/1-(9H-fluoren-2-yl)ethanone, various aromatic aldehydes, 3-(9H-fluoren-2-yl)-3-oxopropanenitrile/3-oxo-3-(pyren-1-yl)propanenitrile and ammonium acetate in refluxing acetic acid were successfully attained. The aim behind this work is to develop convenient approaches to numerous novel fluorophores based on nicotinonitrile as fluorescent type molecules containing pyrene and/or fluorene moieties and study their emission spectral characteristics owing to the promising physiological and fluorescent properties of these kind of molecules.Results and discussion
Synthetic strategy
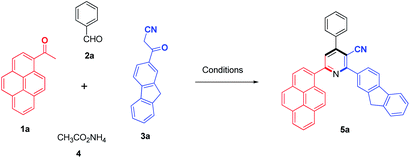
The choice of appropriate reaction conditions was of key importance for effectual synthesis. Initially, we began our present investigation of the four-component one-pot reaction with 1-acetylpyrene (1a), benzaldehyde (2a), 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a), and ammonium acetate (4) which were selected as a model reaction (Scheme 1).To optimize the reaction conditions, a series of experiments were implemented and the effects of different catalysts and solvents at the same reflux temperature on the yield of the model reaction was investigated (Table 1). First of all, it should be mentioned that the model reaction in the absence of a catalyst was accomplished in different solvents such as methanol (MeOH), ethanol (EtOH), 2-propanol, dimethylformamide (DMF) and dioxane, but it failed to afford the desired product 5a even after increasing the reaction time to 24 h (Table 1, entries 1–5). Then, the model reaction was carried out in the presence of basic catalysts such as potassium carbonate (K2CO3) and piperidine in ethanol as a solvent, which led to the formation of product 5a in a low yield (12 and 16%), respectively (Table 1, entries 6, 7).
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1-acetylpyrene (1a, 1.0 mmol), benzaldehyde (2a, 1.0 mmol), 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a, 1.0 mmol), ammonium acetate (4, 3.0 mmol), solvent (10 mL)/reflux. | ||||
| 1 | — | MeOH | 24 | — |
| 2 | — | EtOH | 24 | — |
| 3 | — | 2-Propanol | 24 | — |
| 4 | — | DMF | 24 | — |
| 5 | — | Dioxane | 24 | — |
| 6 | K2CO3 (30) | EtOH | 15 | 12 |
| 7 | Piperidine (30) | EtOH | 15 | 16 |
| 8 | ZnCl2 (30) | EtOH | 15 | 21 |
| 9 | p-TsOH (30) | EtOH | 10 | 35 |
| 10 | HCO2H (30) | EtOH | 12 | 34 |
| 11 | AcOH (30) | EtOH | 7 | 43 |
| 12 | AcOH (30) | MeOH | 7 | 38 |
| 13 | AcOH (30) | 2-Propanol | 8 | 37 |
| 14 | AcOH (30) | DMF | 7 | 39 |
| 15 | AcOH (30) | Dioxane | 10 | 19 |
| 16 | AcOH (50) | EtOH | 7 | 50 |
| 17 | AcOH (100) | EtOH | 7 | 66 |
| 18 | — | EtOH/AcOH (1/1) | 6 | 70 |
| 19 | — | EtOH/AcOH (2/3) | 6 | 79 |
| 20 | — | EtOH/AcOH (1/4) | 5 | 86 |
| 21 | — | AcOH | 4 | 94 |
However, the yield was enhanced to 21–43% when acidic catalysts such as zinc chloride (ZnCl2), p-toluenesulfonic acid (p-TsOH), formic acid (HCO2H) and acetic acid (AcOH) were used, as presented in Table 1 (entries 8–11). Amongst all of the acids used, acetic acid was found to be the best catalyst with regard to the isolated yield (Table 1, entry 11). These observations encouraged us to explore the catalytic proficiency of AcOH in other solvents such as MeOH, 2-propanol, DMF, and dioxane, but no improvement in the yield of the target product 5a was observed (Table 1, entries 12–15). Furthermore, we observe that the yields of 5a were distinctly affected by the amount of AcOH (Table 1, entries 16–21). It was found that when the amount of acetic acid was increased, the desired product 5a was isolated in a significantly higher yield. Excitingly, we observed that acetic acid also operated as the best reaction medium compared with the other solvents (Table 1, entry 21) and the reaction proceeded smoothly affording the target product 5a in an excellent yield (94%) in a short reaction time (4 h). According to the results obtained, it is clear that the reaction proceeds very smoothly with a high product yield in refluxing acetic acid as the reaction media.
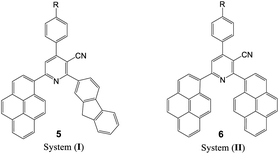
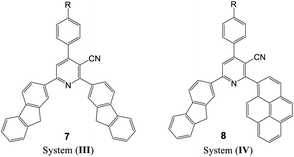
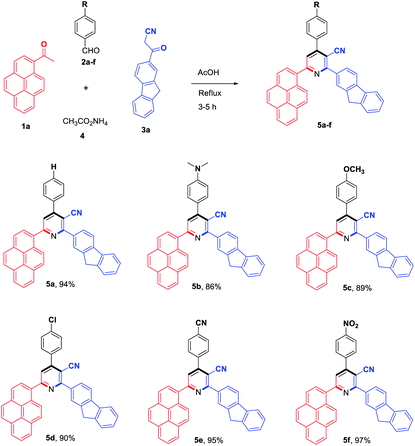
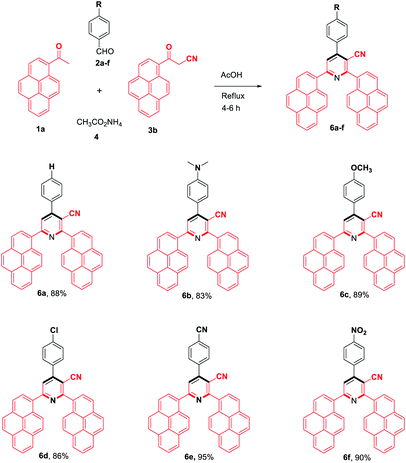
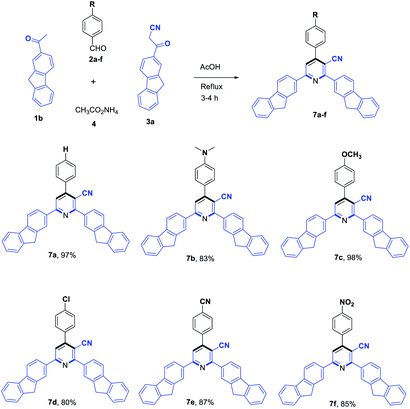
To evaluate the scope and generality of the optimized reaction conditions, a wide range of aromatic aldehydes ketones 2a–2f were selected to undergo this four-component cyclocondensation reaction with acyl derivatives such as 1-acetylpyrene (1a) and 2-acetylfluorene (1b). Additionally, 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a) and 3-oxo-3-(pyren-1-yl)propanenitrile (3b) were found to be compatible under the optimized reaction conditions, leading to the formation of novel highly functionalized nicotinonitriles incorporating pyrene and/or fluorene moieties (Scheme 2).
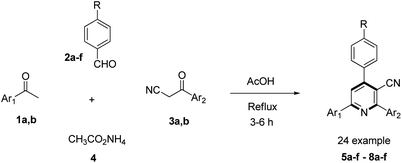 | ||
| Scheme 2 The synthesis of novel poly-functionalized nicotinonitriles containing pyrene and/or fluorene moieties 5a–f–8a–f. | ||
All reactions proceeded smoothly to afford the target products 5a–f–8a–f in good to excellent yields (75–98%) and the obtained results are summarized in Tables 2–5. The aromatic aldehydes used in this study were deliberately selected to tolerate either electron-donating substituents (such as N(CH3)2 and OCH3) or electron-withdrawing substituents (such as Cl, CN and NO2) to give noteworthy changes in their photophysical properties. Generally, the reactions were sufficiently clean and no side products were detected (TLC-controlled). In all cases, the reactions proceeded competently in acetic acid as a reaction media in refluxing conditions.
| a Reaction conditions: 1-acetylpyrene (1a, 1.0 mmol), aldehyde (2a–f, 1.0 mmol), 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a, 1.0 mmol), ammonium acetate (4, 3.0 mmol), AcOH (10 mL)/reflux. |
|---|
 |
| a Reaction conditions: 1-acetylpyrene (1a, 1.0 mmol), aldehyde (2a–f, 1.0 mmol), 3-oxo-3-(pyren-1-yl)propanenitrile (3b, 1.0 mmol), ammonium acetate (4, 3.0 mmol), AcOH (10 mL)/reflux. |
|---|
 |
| a Reaction conditions: 2-acetylfluorene (1b, 1.0 mmol), aldehyde (2a–f, 1.0 mmol), 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a, 1.0 mmol), ammonium acetate (4, 3.0 mmol), AcOH (10 mL)/reflux. |
|---|
 |
| a Reaction conditions: 2-acetylfluorene (1b, 1.0 mmol), aldehyde (2a–f, 1.0 mmol), 3-oxo-3-(pyren-1-yl)propanenitrile (3b, 1.0 mmol), ammonium acetate (4, 3.0 mmol), AcOH (10 mL)/reflux. |
|---|
 |
The chemical structures of all of the novel synthesized compounds 5a–f–8a–f were well-confirmed by means of spectroscopic techniques such as Fourier transform infrared spectroscopy (FT-IR), 1H NMR, 13C NMR, and 13C-distortionless enhancement by polarization transfer (DEPT)-135 data (c.f. Experimental section and ESI†). The FT-IR spectra of compounds 5a–f–8a–f showed the presence of characteristic absorption bands at 2218–2199 and 1615–1610 cm−1 corresponding to the cyano and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups, respectively. Furthermore, to fully confirm the chemical structures of the products, intensive 1D (1H, 13C, and DEPT-135) NMR were conducted in CDCl3. Surprisingly, the 1H NMR spectra of the fluorene-containing nicotinonitrile derivatives 5a–f and 7a–f revealed that the sp3 CH2 protons of the fluorene moiety showed an “AB” spin pattern at δH 4.06–3.88 and 4.09–3.95 ppm, respectively. It should be emphasized that the coupling constants (2JHH) in the CH2 group (ranging from −21.5 to −35.5 and −19.0 to −24.0 Hz, respectively) show some variations and unusually higher absolute values. This observation may be concisely elucidated based on the following two facts: (a) the 2JHH coupling constant becomes more negative when a CH2 group is attached to a π-acceptor such as carbonyl, imino, or cyano group or conjugated with aryl, alkene and alkyne substituents, as the H–C–H angle is decreased and 2JHH coupling constant becomes more negative (larger);45 and (b) the negative mesomeric effect of the cyano group attached to the pyridine ring decreases the electron density especially at the 2-position, and sequentially, it may act as a π-acceptor group.
N groups, respectively. Furthermore, to fully confirm the chemical structures of the products, intensive 1D (1H, 13C, and DEPT-135) NMR were conducted in CDCl3. Surprisingly, the 1H NMR spectra of the fluorene-containing nicotinonitrile derivatives 5a–f and 7a–f revealed that the sp3 CH2 protons of the fluorene moiety showed an “AB” spin pattern at δH 4.06–3.88 and 4.09–3.95 ppm, respectively. It should be emphasized that the coupling constants (2JHH) in the CH2 group (ranging from −21.5 to −35.5 and −19.0 to −24.0 Hz, respectively) show some variations and unusually higher absolute values. This observation may be concisely elucidated based on the following two facts: (a) the 2JHH coupling constant becomes more negative when a CH2 group is attached to a π-acceptor such as carbonyl, imino, or cyano group or conjugated with aryl, alkene and alkyne substituents, as the H–C–H angle is decreased and 2JHH coupling constant becomes more negative (larger);45 and (b) the negative mesomeric effect of the cyano group attached to the pyridine ring decreases the electron density especially at the 2-position, and sequentially, it may act as a π-acceptor group.
For example, analysis of the 13C and 13C-DEPT-135 NMR spectra of 5a in CDCl3 indicated the presence of 38 signals (22 aromatic CH signals, 14 aromatic quaternary carbons, one cyano carbon, and one methylene carbon). The characteristic signals at δ 162.9, 104.2, and 36.9 ppm correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, cyano, and methylene carbons, respectively. In the 1H NMR spectrum, the proton signals were revealed, a doublet signal at δ 8.58 ppm (3J = 6.5 Hz) for the C2–H of the pyrene moiety, this shows the presence of methylene protons as a doublet (2JHH = 35.5 Hz) at 4.00 ppm, and the remaining 21 aromatic protons appear in the expected region at 8.33–7.38 ppm. On the other hand, the 13C and 13C-DEPT-135 NMR spectra of 6a in CDCl3 revealed the presence of 29 signals in the region at 164.0–117.7 ppm (22 aromatic CH signals and 7 aromatic quaternary carbons) and the cyano carbon resonated at δ 107.5 ppm. The 1H NMR spectrum showed the signals corresponded to 24 aromatic protons in the region at 8.59–7.15 ppm.
N, cyano, and methylene carbons, respectively. In the 1H NMR spectrum, the proton signals were revealed, a doublet signal at δ 8.58 ppm (3J = 6.5 Hz) for the C2–H of the pyrene moiety, this shows the presence of methylene protons as a doublet (2JHH = 35.5 Hz) at 4.00 ppm, and the remaining 21 aromatic protons appear in the expected region at 8.33–7.38 ppm. On the other hand, the 13C and 13C-DEPT-135 NMR spectra of 6a in CDCl3 revealed the presence of 29 signals in the region at 164.0–117.7 ppm (22 aromatic CH signals and 7 aromatic quaternary carbons) and the cyano carbon resonated at δ 107.5 ppm. The 1H NMR spectrum showed the signals corresponded to 24 aromatic protons in the region at 8.59–7.15 ppm.
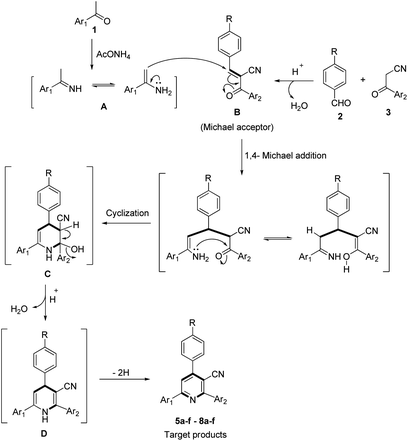
The postulated mechanism for the formation of the desired products 5a–f–8a–f is proposed in Scheme 3. Acetyl compound 1 condensed with ammonium acetate to form the corresponding imine intermediate A, a Knoevenagel condensation reaction between nitrile derivative 3 and aromatic aldehyde 2 afforded the intermediate B which can act as a Michael receptor. Then, a 1,4-Michael addition reaction between intermediates A and B followed by intramolecular cyclization via nucleophilic attack of the amino group to the carbonyl group gave the dihydro intermediate C. Dehydration of the intermediate C afforded the corresponding intermediate D, which could finally undergo dehydrogenation to give the desired products 5–8.
The proposed mechanistic pathway shown in Scheme 3 was experimentally supported by the stepwise synthesis of 2-(9H-fluoren-2-yl)-4-phenyl-6-(pyren-1-yl)nicotinonitrile (5a) under the optimized conditions via the reaction of benzaldehyde (2a) and 3-(9H-fluoren-2-yl)-3-oxopropanenitrile (3a) to afford the corresponding 2-(9H-fluorene-2-carbonyl)-3-phenylacrylonitrile (Michael acceptor), which was isolated in a quantitative yield, and this was then allowed to react with 1-acetylpyrene (1a) and ammonium acetate (4) to afford the target product 5a.
Substituent effect on absorption and emission spectra of the synthesized nicotinonitrile derivatives 5a–f–8a–f
The UV-visible absorption spectra of the synthesized nicotinonitrile derivatives 5a–f–8a–f were recorded in dichloromethane (CH2Cl2) solution at a concentration of 1 × 10−5 M. The UV-visible absorption spectra of the 5a–f–8a–f series in CH2Cl2 are shown in Fig. 1 and the results are summarized in Table 6. All of the 5a–f–8a–f compounds exhibited a strong absorption (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε > 4.00) (Table 6). The photophysical properties of 7a–f with two fluorene fragments in the solution state displayed only one absorption maximum in the range of 313–316 nm and a substantial shift (∼20 nm) in the absorption band of 7b corresponding to the N(CH3)2 substituent was observed, in which the absorption band appeared at 333 nm (Fig. 1, system (III)). Moreover, products 5a–f with one fluorene (on C2-position) and the pyrene (on C6-position) fragment of the pyridine moiety showed the absorption maximum with a different energy absorption in the range of 311–370 nm (Fig. 1, system (I)). However, 6a–f with two pyrene fragments and 8a–f with one pyrene (on C2-position) and fluorene (on C6-position) fragment of pyridine are fairly exceptional in this series, as they showed a conspicuously red shifted absorption band at 448 nm relative to the electron donating group (–N(CH3)2) substituent presents on the benzene ring at the C4-position of pyridine (Fig. 1, systems (II) and (IV)). Based on these findings, it can be concluded that owing to the presence of the pyrene fragment on the C2-position of the nicotinonitrile moiety, substantial changes in absorption properties are observed. The broadened band and the pronounced bathochromic shift absorption of around 100 nm can be explained by the transitions having a mixed character of n–π* and π–π*.
ε > 4.00) (Table 6). The photophysical properties of 7a–f with two fluorene fragments in the solution state displayed only one absorption maximum in the range of 313–316 nm and a substantial shift (∼20 nm) in the absorption band of 7b corresponding to the N(CH3)2 substituent was observed, in which the absorption band appeared at 333 nm (Fig. 1, system (III)). Moreover, products 5a–f with one fluorene (on C2-position) and the pyrene (on C6-position) fragment of the pyridine moiety showed the absorption maximum with a different energy absorption in the range of 311–370 nm (Fig. 1, system (I)). However, 6a–f with two pyrene fragments and 8a–f with one pyrene (on C2-position) and fluorene (on C6-position) fragment of pyridine are fairly exceptional in this series, as they showed a conspicuously red shifted absorption band at 448 nm relative to the electron donating group (–N(CH3)2) substituent presents on the benzene ring at the C4-position of pyridine (Fig. 1, systems (II) and (IV)). Based on these findings, it can be concluded that owing to the presence of the pyrene fragment on the C2-position of the nicotinonitrile moiety, substantial changes in absorption properties are observed. The broadened band and the pronounced bathochromic shift absorption of around 100 nm can be explained by the transitions having a mixed character of n–π* and π–π*.
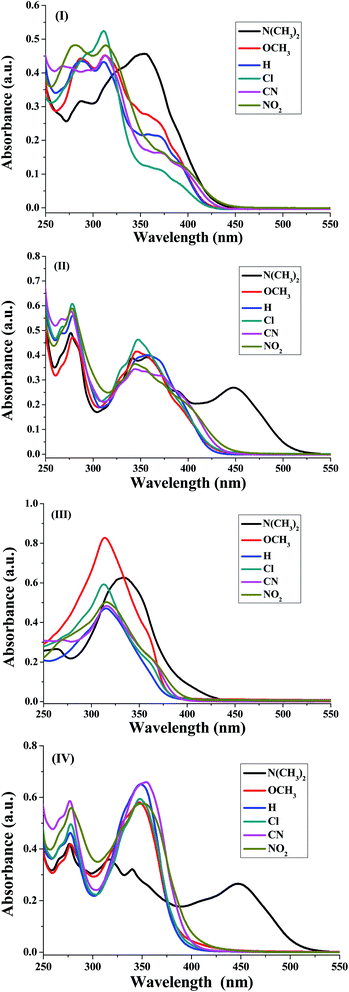 | ||
| Fig. 1 Absorption spectra of the four synthesized systems (I)–(IV) corresponding to 5a–f, 6a–f, 7a–f and 8a–f, respectively, in CH2Cl2 (1 × 10−5 M). | ||
| R | λabs (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λem (nm) | λabs (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λem (nm) |
|---|---|---|---|---|---|---|
| a; H | 288 | 279 | ||||
| 311 | 4.63 | 459 | 357 | 4.60 | 455 | |
| 357 | 4.34 | 585 | 593 | |||
| b; N(CH3)2 | 288 | 277 | ||||
| 354 | 4.66 | 449 | 356 | 4.59 | 542 | |
| 633 | 448 | 4.43 | 590 | |||
| c; OCH3 | 288 | 279 | ||||
| 313 | 4.65 | 449 | 346 | 4.62 | 435 | |
| 359 | 4.44 | 580 | 554 | |||
| d; Cl | 311 | 4.72 | 469 | 279 | 474 | |
| 360 | 4.08 | 574 | 348 | 4.66 | 583 | |
| e; CN | 268 | 277 | ||||
| 313 | 4.65 | 482 | 345 | 4.53 | 493 | |
| 370 | 4.21 | 618 | 616 | |||
| f; NO2 | 283 | 278 | ||||
| 313 | 4.68 | 495 | 345 | 4.56 | 500 | |
| 370 | 4.22 | |||||
| R | λabs (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λem (nm) | λabs (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λem (nm) |
|---|---|---|---|---|---|---|
| a; H | 316 | 4.67 | 427 | 278 | 483 | |
| 490 | 349 | 4.81 | 544 | |||
| b; N(CH3)2 | 333 | 4.79 | 455 | 277 | ||
| 340 | 4.50 | 526 | ||||
| 615 | 447 | 4.42 | 584 | |||
| c; OCH3 | 314 | 4.92 | 412 | 278 | 475 | |
| 505 | 347 | 4.76 | 543 | |||
| d; Cl | 313 | 4.77 | 435 | 278 | ||
| 499 | 348 | 4.77 | 513 | |||
| e; CN | 316 | 4.68 | 453 | 277 | ||
| 534 | 354 | 4.82 | 548 | |||
| f; NO2 | 316 | 4.70 | 477 | 279 | 454 | |
| 506 | 350 | 4.76 | 457 | |||
The fluorescence emissions of the 5a–f–8a–f series with various substituents were recorded in CH2Cl2 solution at the concentration of 1 × 10−5 M (Fig. 2). The emission spectra are shown in Fig. 3 and the obtained results are summarized in Table 6. In all systems, the fluorescent spectra of the 5a–f–8a–f compounds showed emissions bands at around 450 ± 30 and 550 ± 30 nm unlike the emissions of 5e and 6e (Fig. 2). Amongst them, the nicotinonitrile derivative 5b, which contains a (–N(CH3)2) group, exhibited the clearest red-shifted emissions bands at 529 and 633 nm. This kind of strong emission is characteristic of compounds containing electron donor and acceptor groups constituting of a conjugated π-electron system.
 | ||
| Fig. 2 Effects of the substituents on the fluorescence emission of (I)–(IV) in CH2Cl2 solution (substituents from left to right are: N(CH3)2, OCH3, H, Cl, and CN, NO2). | ||
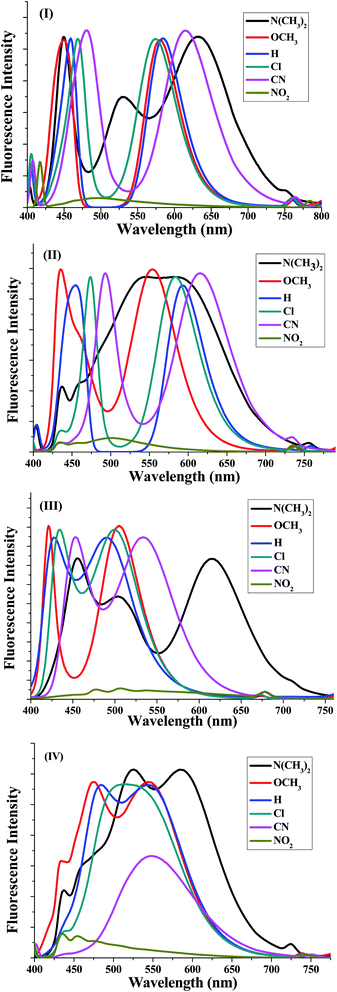 | ||
| Fig. 3 Fluorescence spectra of the four synthesized systems (I)–(IV) corresponding to 5a–f, 6a–f, 7a–f and 8a–f, respectively, in dichloromethane (1 × 10−5 M). | ||
In all systems of the 5a–f–8a–f compounds, a large red shifted emission band was detected within the wavelength range 495–633 nm for compounds 5a–f, 500–616 nm for compounds 6a–f, 490–615 nm for compounds 7a–f and 475–584 nm for 8a–f in addition to the short wavelength band (Table 6). A red shifted broad absorption band indicated that the allowed transition is π–π* with a charge transfer character. A noteworthy red shift of 100 nm was observed for nicotinonitriles containing fluorene and pyrene moieties on moving from 7a to 6a, whereas the shift was 50 nm upon moving from 7a to 8a; suggesting the involvement of a photo induced intramolecular charge transfer (ICT).
It is noticeable that the solutions of most compounds 5a–f–8a–f showed a strong fluorescence except for 5f, 6f, 7f and 8f, these compounds present a weak fluorescence in the blue region with maxima in the range of 475–500 nm. This low intensity was initially explained by the existence of –NO2 group. The observed data also corroborates an assumption about the essential role of the cyano group in the pyridine ring causing the high fluorescence of the pyridine derivatives and allows it to act as an excellent fluorescent core with good electron-transporting properties.46–52 It can be easily observed from the study that our nicotinonitrile-based fluorescent type molecules containing pyrene and/or fluorene moieties have been proven to be promising fluorophores for application as chemo- and biosensors. Moreover, this type of structure with a highly electron deficient system could be of interest to many researchers working on the development of n-type organic semiconductors and for the design of new liquid crystal materials.
Conclusions
In conclusion, we have developed a simple and efficient protocol for the synthesis of a novel series of pyrene and/or fluorene-containing nicotinonitrile derivatives bearing electron-donating and electro-withdrawing groups via one-pot four-component condensation reactions of 1-(pyren-1-yl)ethanone/1-(9H-fluoren-2-yl)ethanone, different aromatic aldehydes, 3-oxo-3-(pyren-1-yl)propanenitrile/3-(9H-fluoren-2-yl)-3-oxopropanenitrile, and ammonium acetate in acetic acid as a reaction media under reflux conditions. This procedure offers several distinguished advantages, such as, a relatively short reaction time, excellent yields, and easy reaction work up (with no need for chromatographic separation). The photophysical properties of the synthesized nicotinonitriles were investigated using UV-visible absorption and fluorescence emission spectrophotometers, using CH2Cl2 as the solvent, and significant results were obtained. Moreover, the optical results revealed that the λabs values are in the range of 311–447 nm (solution state) while the λem values are in the range of 421–493 and 543–633 nm. The absorption and emission at long wavelengths are a result of an increase in the push–pull system generated by the two pyrene/fluorene rings and the cyano (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) group attached to the pyridine ring. More details relating to fluorescence emission, time-resolved spectroscopy, solvatochromism and theoretical studies of these newly synthesized materials will be discussed in depth in a forthcoming paper.
N) group attached to the pyridine ring. More details relating to fluorescence emission, time-resolved spectroscopy, solvatochromism and theoretical studies of these newly synthesized materials will be discussed in depth in a forthcoming paper.
Experimental
Materials and methods
All solvents used purchased from Sigma-Aldrich were of spectroscopic grade and were used without further purification. The FT-IR spectra were recorded on a Shimadzu IR-3600 FT-IR spectrometer in KBr pellets. The NMR spectra were acquired on a Bruker Avance 500 instrument (at 500 MHz for 1H, 125 MHz for 13C) in CDCl3 solutions, using residual solvent signals as the internal standards. Melting points were determined on a Stuart SMP3 melting point apparatus and are uncorrected. The electronic absorption spectra were recorded using a UV/VIS/NIR spectrophotometer in a concentration of 1 × 10−5 mol dm−3. Fluorescence spectra were performed using a Horiba Spectrofluorometer (model FluoroMax-4).1-Acetylpyrene (1a) and 2-acetyl-9H-fluorene (1b) were synthesized from pyrene and fluorene, respectively, using the classical Friedel–Crafts reaction in accordance with the previously reported procedure.53,54 3-(9H-Fluoren-2-yl)-3-oxopropanenitrile (3a) and 3-oxo-3-(pyren-1-yl)propanenitrile (3b) were synthesized from 1-acetylpyrene and 2-acetyl-9H-fluorene, respectively, according to the previously reported procedures.55,56
Synthetic procedures
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.58 (d, J = 6.5 Hz, 1H), 8.34–8.32 (m, 2H), 8.28–8.26 (m, 2H), 8.20–8.16 (m, 3H), 8.10–8.08 (m, 1H), 8.00 (s, 1H), 7.89–7.83 (m, 4H), 7.67–7.61 (m, 4H), 7.52 (d, J = 11.5 Hz, 1H), 7.44–7.38 (m, 3H), 4.00 (d, 2J = 35.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.9 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.58 (d, J = 6.5 Hz, 1H), 8.34–8.32 (m, 2H), 8.28–8.26 (m, 2H), 8.20–8.16 (m, 3H), 8.10–8.08 (m, 1H), 8.00 (s, 1H), 7.89–7.83 (m, 4H), 7.67–7.61 (m, 4H), 7.52 (d, J = 11.5 Hz, 1H), 7.44–7.38 (m, 3H), 4.00 (d, 2J = 35.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.8 (C), 155.1 (C), 145.0 (C), 144.1 (C), 144.0 (C), 143.7 (C), 143.5 (C), 140.9 (C), 136.5 (C), 136.0 (C), 133.8 (C), 132.3 (C), 130.1 (CH), 129.1 (CH), 128.8 (CH), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.0 (CH), 127.8 (CH), 127.7 (CH), 127.4 (CH), 127.3 (CH), 126.9 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.5 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 124.0 (CH), 120.5 (CH), 119.9 (CH), 117.9 (C), 104.2 (CN), 36.9 (CH2) ppm. Elemental analysis: calcd for C41H24N2: C, 90.42; H, 4.44; N, 5.14; found: C, 90.19; H, 4.25; N, 4.90.
N), 161.8 (C), 155.1 (C), 145.0 (C), 144.1 (C), 144.0 (C), 143.7 (C), 143.5 (C), 140.9 (C), 136.5 (C), 136.0 (C), 133.8 (C), 132.3 (C), 130.1 (CH), 129.1 (CH), 128.8 (CH), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.0 (CH), 127.8 (CH), 127.7 (CH), 127.4 (CH), 127.3 (CH), 126.9 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.5 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 124.0 (CH), 120.5 (CH), 119.9 (CH), 117.9 (C), 104.2 (CN), 36.9 (CH2) ppm. Elemental analysis: calcd for C41H24N2: C, 90.42; H, 4.44; N, 5.14; found: C, 90.19; H, 4.25; N, 4.90.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.59 (d, J = 8.0 Hz, 1H), 8.40 (d, J = 5.5 Hz, 1H), 8.35–8.30 (m, 5H), 8.18–8.16 (m, 4H), 8.08 (d, J = 6.0 Hz, 2H), 7.97 (s, 1H), 7.88–7.85 (m, 2H), 7.78 (d, J = 6.5 Hz, 1H), 7.61–7.59 (m, 1H), 7.42–7.38 (m, 3H), 3.98 (d, 2J = 35.5 Hz, 2H, CH2), 3.08 (s, 3H, CH3), 2.92 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 163.2 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.59 (d, J = 8.0 Hz, 1H), 8.40 (d, J = 5.5 Hz, 1H), 8.35–8.30 (m, 5H), 8.18–8.16 (m, 4H), 8.08 (d, J = 6.0 Hz, 2H), 7.97 (s, 1H), 7.88–7.85 (m, 2H), 7.78 (d, J = 6.5 Hz, 1H), 7.61–7.59 (m, 1H), 7.42–7.38 (m, 3H), 3.98 (d, 2J = 35.5 Hz, 2H, CH2), 3.08 (s, 3H, CH3), 2.92 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 163.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.4 (C), 147.8 (C), 145.2 (C), 144.2 (C), 144.0 (C), 143.8 (C), 143.4 (C), 141.1 (C), 140.5 (C), 134.2 (C), 134.0 (C), 131.9 (C), 131.3 (C), 131.0 (C), 130.8 (C), 130.5 (C), 130.1 (CH), 129.7 (CH), 129.6 (CH), 129.5 (CH), 128.4 (CH), 128.0 (CH), 127.2 (CH), 127.1 (CH), 126.4 (CH), 126.3 (CH), 126.2 (CH), 126.1 (CH), 125.9 (CH), 125.2 (CH), 124.9 (CH), 124.0 (CH), 123.2 (CH), 120.8 (CH), 120.5 (CH), 120.2 (CH), 119.8 (CH), 116.6 (C), 104.4 (CN), 37.0 (CH2), 30.5 (CH3) ppm. Elemental analysis: calcd for C43H29N3: C, 87.88; H, 4.97; N, 7.15; found: C, 87.65; H, 4.81; N, 6.88.
N), 162.4 (C), 147.8 (C), 145.2 (C), 144.2 (C), 144.0 (C), 143.8 (C), 143.4 (C), 141.1 (C), 140.5 (C), 134.2 (C), 134.0 (C), 131.9 (C), 131.3 (C), 131.0 (C), 130.8 (C), 130.5 (C), 130.1 (CH), 129.7 (CH), 129.6 (CH), 129.5 (CH), 128.4 (CH), 128.0 (CH), 127.2 (CH), 127.1 (CH), 126.4 (CH), 126.3 (CH), 126.2 (CH), 126.1 (CH), 125.9 (CH), 125.2 (CH), 124.9 (CH), 124.0 (CH), 123.2 (CH), 120.8 (CH), 120.5 (CH), 120.2 (CH), 119.8 (CH), 116.6 (C), 104.4 (CN), 37.0 (CH2), 30.5 (CH3) ppm. Elemental analysis: calcd for C43H29N3: C, 87.88; H, 4.97; N, 7.15; found: C, 87.65; H, 4.81; N, 6.88.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.44 (s, 1H), 8.40 (d, J = 14.0 Hz, 2H), 8.24–8.18 (m, 5H), 8.10–8.04 (m, 3H), 7.90–7.82 (m, 4H), 7.64–7.58 (m, 1H), 7.43–7.38 (m, 3H), 7.15–7.13 (m, 2H), 3.97 (d, 2J = 21.5 Hz, 2H, CH2), 3.93 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 163.6 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.44 (s, 1H), 8.40 (d, J = 14.0 Hz, 2H), 8.24–8.18 (m, 5H), 8.10–8.04 (m, 3H), 7.90–7.82 (m, 4H), 7.64–7.58 (m, 1H), 7.43–7.38 (m, 3H), 7.15–7.13 (m, 2H), 3.97 (d, 2J = 21.5 Hz, 2H, CH2), 3.93 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 163.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.2 (C), 159.1 (C), 154.5 (C), 144.2 (C), 144.1 (C), 144.0 (C), 140.8 (C), 132.4 (C), 131.3 (C), 130.3 (CH), 129.4 (CH), 128.5 (CH), 128.4 (CH), 127.5 (CH), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.7 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.4 (CH), 120.5 (CH), 120.2 (CH), 114.6 (CH), 107.0 (CN), 55.5 (CH3), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H26N2O: C, 87.78; H, 4.56; N, 4.87; found: C, 87.60; H, 4.45; N, 4.69.
N), 161.2 (C), 159.1 (C), 154.5 (C), 144.2 (C), 144.1 (C), 144.0 (C), 140.8 (C), 132.4 (C), 131.3 (C), 130.3 (CH), 129.4 (CH), 128.5 (CH), 128.4 (CH), 127.5 (CH), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.7 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.4 (CH), 120.5 (CH), 120.2 (CH), 114.6 (CH), 107.0 (CN), 55.5 (CH3), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H26N2O: C, 87.78; H, 4.56; N, 4.87; found: C, 87.60; H, 4.45; N, 4.69.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.56 (s, 1H), 8.32 (s, 1H), 8.28–8.26 (m, 3H), 8.20–8.16 (m, 3H), 8.10–8.08 (m, 1H), 8.00 (s, 1H), 7.90–7.82 (m, 3H), 7.76–7.74 (m, 2H), 7.64–7.60 (m, 3H), 7.48–7.39 (m, 3H), 4.01 (d, 2J = 32.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.9 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.56 (s, 1H), 8.32 (s, 1H), 8.28–8.26 (m, 3H), 8.20–8.16 (m, 3H), 8.10–8.08 (m, 1H), 8.00 (s, 1H), 7.90–7.82 (m, 3H), 7.76–7.74 (m, 2H), 7.64–7.60 (m, 3H), 7.48–7.39 (m, 3H), 4.01 (d, 2J = 32.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.1 (C), 153.9 (C), 144.2 (C), 144.0 (C), 143.9 (C), 143.7 (C), 143.6 (C), 140.9 (C), 140.2 (C), 136.5 (C), 135.8 (C), 134.9 (C), 133.6 (C), 132.3 (C), 131.3 (C), 130.7 (C), 130.2 (CH), 129.6 (CH), 129.4 (CH), 129.1 (CH), 128.8 (CH), 128.7 (CH), 127.5 (CH), 127.3 (CH), 127.1 (CH), 126.9 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.6 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 120.5 (CH), 119.9 (CH), 117.7 (C), 104.0 (CN), 37.1 (CH2) ppm. Elemental analysis: calcd for C41H23ClN2: C, 85.04; H, 4.00; N, 4.84; Cl, 6.12; found: C, 84.76; H, 3.79; N, 4.68; Cl, 5.90.
N), 162.1 (C), 153.9 (C), 144.2 (C), 144.0 (C), 143.9 (C), 143.7 (C), 143.6 (C), 140.9 (C), 140.2 (C), 136.5 (C), 135.8 (C), 134.9 (C), 133.6 (C), 132.3 (C), 131.3 (C), 130.7 (C), 130.2 (CH), 129.6 (CH), 129.4 (CH), 129.1 (CH), 128.8 (CH), 128.7 (CH), 127.5 (CH), 127.3 (CH), 127.1 (CH), 126.9 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.6 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 120.5 (CH), 119.9 (CH), 117.7 (C), 104.0 (CN), 37.1 (CH2) ppm. Elemental analysis: calcd for C41H23ClN2: C, 85.04; H, 4.00; N, 4.84; Cl, 6.12; found: C, 84.76; H, 3.79; N, 4.68; Cl, 5.90.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.45 (d, J = 7.5 Hz, 1H), 8.21 (s, 1H), 8.18–8.16 (m, 3H), 8.10–8.05 (m, 3H), 8.00–7.98 (m, 1H), 7.90–7.88 (m, 1H), 7.79–7.73 (m, 6H), 7.54–7.50 (m, 2H), 7.34–7.31 (m, 2H), 7.18 (s, 1H), 3.91 (d, 2J = 30.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.0 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.45 (d, J = 7.5 Hz, 1H), 8.21 (s, 1H), 8.18–8.16 (m, 3H), 8.10–8.05 (m, 3H), 8.00–7.98 (m, 1H), 7.90–7.88 (m, 1H), 7.79–7.73 (m, 6H), 7.54–7.50 (m, 2H), 7.34–7.31 (m, 2H), 7.18 (s, 1H), 3.91 (d, 2J = 30.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.5 (C), 152.9 (C), 144.0 (C), 143.6 (C), 140.9 (C), 140.8 (C), 135.7 (C), 133.4 (C), 133.2 (C), 132.8 (CH), 132.4 (C), 131.3 (C), 130.7 (C), 129.6 (CH), 128.9 (CH), 128.8 (CH), 128.5 (CH), 127.7 (CH), 127.6 (CH), 127.3 (CH), 127.0 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.6 (CH), 125.2 (CH), 124.9 (CH), 124.6 (C), 124.0 (CH), 123.4 (CH), 120.6 (CH), 120.0 (CH), 118.1 (C), 117.4 (C), 113.9 (C), 103.6 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H23N3: C, 88.55; H, 4.07; N, 7.38; found: C, 88.30; H, 3.92; N, 7.10.
N), 162.5 (C), 152.9 (C), 144.0 (C), 143.6 (C), 140.9 (C), 140.8 (C), 135.7 (C), 133.4 (C), 133.2 (C), 132.8 (CH), 132.4 (C), 131.3 (C), 130.7 (C), 129.6 (CH), 128.9 (CH), 128.8 (CH), 128.5 (CH), 127.7 (CH), 127.6 (CH), 127.3 (CH), 127.0 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.6 (CH), 125.2 (CH), 124.9 (CH), 124.6 (C), 124.0 (CH), 123.4 (CH), 120.6 (CH), 120.0 (CH), 118.1 (C), 117.4 (C), 113.9 (C), 103.6 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H23N3: C, 88.55; H, 4.07; N, 7.38; found: C, 88.30; H, 3.92; N, 7.10.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.56 (d, J = 6.5 Hz, 1H), 8.45 (s, 1H), 8.37–8.33 (m, 3H), 8.27–8.16 (m, 4H), 8.10–8.08 (m, 1H), 8.00–7.95 (m, 2H), 7.89–7.86 (m, 2H), 7.81 (s, 1H), 7.72–7.70 (m, 1H), 7.64–7.60 (m, 2H), 7.44–7.40 (m, 3H), 4.01 (d, 2J = 33.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.0 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.56 (d, J = 6.5 Hz, 1H), 8.45 (s, 1H), 8.37–8.33 (m, 3H), 8.27–8.16 (m, 4H), 8.10–8.08 (m, 1H), 8.00–7.95 (m, 2H), 7.89–7.86 (m, 2H), 7.81 (s, 1H), 7.72–7.70 (m, 1H), 7.64–7.60 (m, 2H), 7.44–7.40 (m, 3H), 4.01 (d, 2J = 33.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.4 (C), 152.5 (C), 148.7 (C), 148.6 (C), 147.8 (C), 145.4 (C), 144.2 (C), 142.7 (C), 140.8 (C), 139.7 (C), 135.6 (C), 132.3 (C), 131.2 (C), 130.7 (C), 130.0 (CH), 129.0 (CH), 128.8 (CH), 128.7 (CH), 127.3 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.3 (CH), 125.6 (CH), 125.3 (CH), 125.2 (CH), 124.9 (CH), 124.7 (CH), 124.2 (CH), 123.4 (CH), 120.7 (CH), 120.0 (CH), 118.6 (C), 107.2 (CN), 37.1 (CH2) ppm. Elemental analysis: calcd for C41H23N3O2: C, 83.52; H, 3.93; N, 7.13; found: C, 83.33; H, 3.69; N, 7.00.
N), 162.4 (C), 152.5 (C), 148.7 (C), 148.6 (C), 147.8 (C), 145.4 (C), 144.2 (C), 142.7 (C), 140.8 (C), 139.7 (C), 135.6 (C), 132.3 (C), 131.2 (C), 130.7 (C), 130.0 (CH), 129.0 (CH), 128.8 (CH), 128.7 (CH), 127.3 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.3 (CH), 125.6 (CH), 125.3 (CH), 125.2 (CH), 124.9 (CH), 124.7 (CH), 124.2 (CH), 123.4 (CH), 120.7 (CH), 120.0 (CH), 118.6 (C), 107.2 (CN), 37.1 (CH2) ppm. Elemental analysis: calcd for C41H23N3O2: C, 83.52; H, 3.93; N, 7.13; found: C, 83.33; H, 3.69; N, 7.00.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.59 (d, J = 7.5 Hz, 1H), 8.28–8.25 (m, 3H), 8.18 (d, J = 6.5 Hz, 2H), 8.14–8.11 (m, 4H), 8.07–8.01 (m, 5H), 7.96–7.94 (m, 3H), 7.78 (d, J = 6.5 Hz, 2H), 7.52–7.49 (m, 3H), 7.16–7.14 (m, 1H) ppm. 13C NMR (CDCl3): δ = 164.0 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.59 (d, J = 7.5 Hz, 1H), 8.28–8.25 (m, 3H), 8.18 (d, J = 6.5 Hz, 2H), 8.14–8.11 (m, 4H), 8.07–8.01 (m, 5H), 7.96–7.94 (m, 3H), 7.78 (d, J = 6.5 Hz, 2H), 7.52–7.49 (m, 3H), 7.16–7.14 (m, 1H) ppm. 13C NMR (CDCl3): δ = 164.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.9 (C), 154.1 (C), 136.3 (C), 131.3 (C), 130.2 (CH), 129.2 (CH), 129.0 (CH), 128.9 (CH), 128.8 (C), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.0 (CH), 127.6 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.8 (CH), 125.6 (CH), 125.5 (CH), 124.9 (CH), 124.7 (CH), 124.4 (CH), 124.3 (CH), 124.2 (CH), 117.1 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C44H24N2: C, 91.01; H, 4.17; N, 4.82; found: C, 90.80; H, 4.00; N, 4.69.
N), 161.9 (C), 154.1 (C), 136.3 (C), 131.3 (C), 130.2 (CH), 129.2 (CH), 129.0 (CH), 128.9 (CH), 128.8 (C), 128.7 (CH), 128.6 (CH), 128.5 (CH), 128.0 (CH), 127.6 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 125.9 (CH), 125.8 (CH), 125.6 (CH), 125.5 (CH), 124.9 (CH), 124.7 (CH), 124.4 (CH), 124.3 (CH), 124.2 (CH), 117.1 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C44H24N2: C, 91.01; H, 4.17; N, 4.82; found: C, 90.80; H, 4.00; N, 4.69.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 9.07 (d, J = 8.5 Hz, 1H), 8.38–8.34 (m, 2H), 8.28–8.22 (m, 6H), 8.16–8.12 (m, 5H), 8.10–8.05 (m, 5H), 7.89 (d, J = 6.5 Hz, 2H, Ph-H), 6.61 (d, J = 6.5 Hz, 2H, Ph-H), 3.05 (s, 3H, CH3), 2.91 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 164.6 (C
N) cm−1. 1H NMR (CDCl3): δ = 9.07 (d, J = 8.5 Hz, 1H), 8.38–8.34 (m, 2H), 8.28–8.22 (m, 6H), 8.16–8.12 (m, 5H), 8.10–8.05 (m, 5H), 7.89 (d, J = 6.5 Hz, 2H, Ph-H), 6.61 (d, J = 6.5 Hz, 2H, Ph-H), 3.05 (s, 3H, CH3), 2.91 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 164.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1 (C), 153.9 (C), 134.7 (C), 134.0 (C), 130.0 (C), 132.3 (C) 131.8 (C), 131.1 (C), 131.0 (C), 130.6 (C), 130.4 (CH), 129.7 (CH), 129.6 (CH), 129.4 (CH), 129.2 (CH), 129.1 (CH), 129.0 (CH), 127.2 (CH), 127.1 (CH), 127.0 (CH), 126.4 (CH), 126.3 (CH), 126.1 (CH), 125.9 (CH), 125.7 (CH), 124.9 (CH), 124.7 (CH), 124.3 (CH), 124.0 (CH), 119.4 (CH), 118.6 (C), 111.6 (CH), 104.3 (CN), 40.0 (CH3) ppm. Elemental analysis: calcd for C46H29N3: C, 88.58; H, 4.69; N, 6.74; found: C, 88.34; H, 4.40; N, 6.60.
N), 156.1 (C), 153.9 (C), 134.7 (C), 134.0 (C), 130.0 (C), 132.3 (C) 131.8 (C), 131.1 (C), 131.0 (C), 130.6 (C), 130.4 (CH), 129.7 (CH), 129.6 (CH), 129.4 (CH), 129.2 (CH), 129.1 (CH), 129.0 (CH), 127.2 (CH), 127.1 (CH), 127.0 (CH), 126.4 (CH), 126.3 (CH), 126.1 (CH), 125.9 (CH), 125.7 (CH), 124.9 (CH), 124.7 (CH), 124.3 (CH), 124.0 (CH), 119.4 (CH), 118.6 (C), 111.6 (CH), 104.3 (CN), 40.0 (CH3) ppm. Elemental analysis: calcd for C46H29N3: C, 88.58; H, 4.69; N, 6.74; found: C, 88.34; H, 4.40; N, 6.60.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, J = 9.5 Hz, 1H), 8.65 (d, J = 8.0 Hz, 1H), 8.39 (s, 1H), 8.37–8.31 (m, 2H), 8.28–8.05 (m, 14H), 7.87 (d, J = 8.0 Hz, 2H, Ph–H), 7.13 (d, J = 8.0 Hz, 2H, Ph-H), 3.89 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 164.1 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, J = 9.5 Hz, 1H), 8.65 (d, J = 8.0 Hz, 1H), 8.39 (s, 1H), 8.37–8.31 (m, 2H), 8.28–8.05 (m, 14H), 7.87 (d, J = 8.0 Hz, 2H, Ph–H), 7.13 (d, J = 8.0 Hz, 2H, Ph-H), 3.89 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 164.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.8 (C), 153.7 (C), 133.8 (C), 132.9 (C), 132.3 (C), 132.2 (C), 131.8 (C) 131.4 (C), 131.3 (C), 130.9 (C), 130.8 (C), 130.7 (C), 130.6 (C), 130.5 (C), 130.4 (CH), 128.7 (CH), 128.6 (CH), 128.3 (CH), 128.1 (CH), 127.5 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 126.1 (CH), 125.6 (CH), 125.5 (CH), 125.2 (CH), 124.9 (CH), 124.8 (CH), 124.7 (CH), 117.4 (C), 114.8 (CH), 114.6 (CH), 114.4 (CH), 114.3 (CH), 107.2 (CN), 55.5 (CH3) ppm. Elemental analysis: calcd for C45H26N2O: C, 88.50; H, 4.29; N, 4.59; found: C, 88.22; H, 4.31; N, 4.37.
N), 161.8 (C), 153.7 (C), 133.8 (C), 132.9 (C), 132.3 (C), 132.2 (C), 131.8 (C) 131.4 (C), 131.3 (C), 130.9 (C), 130.8 (C), 130.7 (C), 130.6 (C), 130.5 (C), 130.4 (CH), 128.7 (CH), 128.6 (CH), 128.3 (CH), 128.1 (CH), 127.5 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 126.1 (CH), 125.6 (CH), 125.5 (CH), 125.2 (CH), 124.9 (CH), 124.8 (CH), 124.7 (CH), 117.4 (C), 114.8 (CH), 114.6 (CH), 114.4 (CH), 114.3 (CH), 107.2 (CN), 55.5 (CH3) ppm. Elemental analysis: calcd for C45H26N2O: C, 88.50; H, 4.29; N, 4.59; found: C, 88.22; H, 4.31; N, 4.37.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.67 (s, 1H), 8.41–8.37 (m, 3H), 8.32 (m, 1H), 8.27–8.24 (m, 6H), 8.18–8.14 (m, 4H), 8.08–8.05 (m, 4H), 7.84 (d, J = 7.5 Hz, 2H), 7.62 (d, J = 7.5 Hz, 2H) ppm. 13C NMR (CDCl3): δ = 164.0 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.67 (s, 1H), 8.41–8.37 (m, 3H), 8.32 (m, 1H), 8.27–8.24 (m, 6H), 8.18–8.14 (m, 4H), 8.08–8.05 (m, 4H), 7.84 (d, J = 7.5 Hz, 2H), 7.62 (d, J = 7.5 Hz, 2H) ppm. 13C NMR (CDCl3): δ = 164.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.0 (C), 153.1 (C), 136.8 (C), 134.5 (C), 132.5 (C), 131.3 (C), 130.8 (C), 130.7 (C), 130.2 (CH), 129.7 (CH), 129.6 (CH), 129.5 (CH), 129.3 (CH), 129.0 (CH), 128.8 (CH), 128.7 (CH), 128.6 (CH), 128.1 (CH), 127.6 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 126.0 (CH), 125.9 (CH), 125.7 (CH), 125.6 (CH), 124.9 (CH), 124.7 (CH), 124.3 (C), 124.0 (C), 116.7 (C), 106.0 (CN) ppm. Elemental analysis: calcd for C44H23ClN2: C, 85.91; H, 3.77; N, 4.55; Cl, 5.76; found: C, 85.66; H, 3.70; N, 4.42; Cl, 5.59.
N), 162.0 (C), 153.1 (C), 136.8 (C), 134.5 (C), 132.5 (C), 131.3 (C), 130.8 (C), 130.7 (C), 130.2 (CH), 129.7 (CH), 129.6 (CH), 129.5 (CH), 129.3 (CH), 129.0 (CH), 128.8 (CH), 128.7 (CH), 128.6 (CH), 128.1 (CH), 127.6 (CH), 127.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 126.0 (CH), 125.9 (CH), 125.7 (CH), 125.6 (CH), 124.9 (CH), 124.7 (CH), 124.3 (C), 124.0 (C), 116.7 (C), 106.0 (CN) ppm. Elemental analysis: calcd for C44H23ClN2: C, 85.91; H, 3.77; N, 4.55; Cl, 5.76; found: C, 85.66; H, 3.70; N, 4.42; Cl, 5.59.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, J = 9.0 Hz, 1H), 8.41–8.38 (m, 3H), 8.32 (d, J = 9.0 Hz, 1H), 8.30–8.27 (m, 4H), 8.25 (d, J = 7.5 Hz, 2H), 8.22–8.19 (m, 4H), 8.15 (d, J = 9.0 Hz, 1H), 8.11 (d, J = 7.5 Hz, 1H), 8.07 (d, J = 7.5 Hz, 2H), 8.01 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.0 Hz, 2H) ppm. 13C NMR (CDCl3): δ = 164.3 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, J = 9.0 Hz, 1H), 8.41–8.38 (m, 3H), 8.32 (d, J = 9.0 Hz, 1H), 8.30–8.27 (m, 4H), 8.25 (d, J = 7.5 Hz, 2H), 8.22–8.19 (m, 4H), 8.15 (d, J = 9.0 Hz, 1H), 8.11 (d, J = 7.5 Hz, 1H), 8.07 (d, J = 7.5 Hz, 2H), 8.01 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.0 Hz, 2H) ppm. 13C NMR (CDCl3): δ = 164.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.9 (C), 152.3 (C), 141.0 (C), 136.8 (C), 133.2 (C), 132.9 (C), 132.5 (C), 131.6 (C), 131.1 (C), 130.0 (CH), 129.8 (CH), 129.2 (CH), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.1 (CH), 127.9 (CH), 127.5 (CH), 127.4 (CH), 126.5 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.9 (CH), 125.8 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 124.1 (CH), 124.0 (CH), 118.4 (C), 118.3 (C), 116.8 (C), 114.4 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C45H23N3: C, 89.23; H, 3.83; N, 6.94; found: C, 89.00; H, 3.69; N, 6.80.
N), 162.9 (C), 152.3 (C), 141.0 (C), 136.8 (C), 133.2 (C), 132.9 (C), 132.5 (C), 131.6 (C), 131.1 (C), 130.0 (CH), 129.8 (CH), 129.2 (CH), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.1 (CH), 127.9 (CH), 127.5 (CH), 127.4 (CH), 126.5 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.9 (CH), 125.8 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 124.1 (CH), 124.0 (CH), 118.4 (C), 118.3 (C), 116.8 (C), 114.4 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C45H23N3: C, 89.23; H, 3.83; N, 6.94; found: C, 89.00; H, 3.69; N, 6.80.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, 1H, J = 9.0 Hz, 1H), 8.50 (d, J = 8.5 Hz, 2H), 8.42–8.39 (m, 3H), 8.33 (d, J = 8.5 Hz, 1H), 8.30–8.25 (m, 7H), 8.21–8.17 (m, 4H), 8.15 (d, J = 9.0 Hz, 1H), 8.11–8.06 (m, 4H) ppm. 13C NMR (CDCl3): δ = 164.6 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.69 (d, 1H, J = 9.0 Hz, 1H), 8.50 (d, J = 8.5 Hz, 2H), 8.42–8.39 (m, 3H), 8.33 (d, J = 8.5 Hz, 1H), 8.30–8.25 (m, 7H), 8.21–8.17 (m, 4H), 8.15 (d, J = 9.0 Hz, 1H), 8.11–8.06 (m, 4H) ppm. 13C NMR (CDCl3): δ = 164.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.9 (C), 152.0 (C), 149.1 (C), 142.7 (C), 132.9 (C), 131.6 (C), 131.1 (C), 130.4 (C), 130.2 (CH), 129.3 (CH), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.2 (CH), 127.7 (CH), 127.5 (CH), 127.4 (CH), 126.5 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.8 (CH), 125.7 (CH), 125.1 (CH), 124.9 (CH), 124.5 (CH), 124.2 (CH), 124.1 (CH), 124.0 (CH), 116.7 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C44H23N3O2: C, 84.46; H, 3.71; N, 6.72; found: C, 84.29; H, 3.58; N, 6.63.
N), 162.9 (C), 152.0 (C), 149.1 (C), 142.7 (C), 132.9 (C), 131.6 (C), 131.1 (C), 130.4 (C), 130.2 (CH), 129.3 (CH), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.2 (CH), 127.7 (CH), 127.5 (CH), 127.4 (CH), 126.5 (CH), 126.4 (CH), 126.2 (CH), 126.1 (CH), 125.8 (CH), 125.7 (CH), 125.1 (CH), 124.9 (CH), 124.5 (CH), 124.2 (CH), 124.1 (CH), 124.0 (CH), 116.7 (C), 107.5 (CN) ppm. Elemental analysis: calcd for C44H23N3O2: C, 84.46; H, 3.71; N, 6.72; found: C, 84.29; H, 3.58; N, 6.63.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.27–8.23 (m, 1H), 8.14–8.09 (m, 2H), 8.01–7.99 (m, 2H), 7.93–7.82 (m, 4H), 7.75 (s, 1H), 7.68 (d, J = 15.0 Hz, 2H), 7.62–7.57 (m, 2H), 7.45–7.41 (m, 5H), 4.06 (d, 2J = 21.0 Hz, 2H, CH2), 3.96 (d, 2J = 19.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.6 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.27–8.23 (m, 1H), 8.14–8.09 (m, 2H), 8.01–7.99 (m, 2H), 7.93–7.82 (m, 4H), 7.75 (s, 1H), 7.68 (d, J = 15.0 Hz, 2H), 7.62–7.57 (m, 2H), 7.45–7.41 (m, 5H), 4.06 (d, 2J = 21.0 Hz, 2H, CH2), 3.96 (d, 2J = 19.5 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.3 (C), 155.4 (C), 146.7 (C), 144.5 (C), 144.4 (C), 144.0 (C), 143.7 (C), 143.4 (C), 140.9 (C), 136.7 (C), 135.0 (C), 132.4 (C), 130.5 (CH), 129.3 (CH), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.7 (CH), 128.4 (CH), 128.0 (CH), 127.9 (CH), 127.1 (CH), 125.3 (CH), 125.2 (CH), 125.1 (CH), 122.2 (CH), 120.9 (CH), 120.6 (CH), 119.7 (CH), 104.0 (CN), 37.0 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H24N2: C, 89.74; H, 4.76; N, 5.51; found: C, 89.50; H, 4.58; N, 5.39.
N), 159.3 (C), 155.4 (C), 146.7 (C), 144.5 (C), 144.4 (C), 144.0 (C), 143.7 (C), 143.4 (C), 140.9 (C), 136.7 (C), 135.0 (C), 132.4 (C), 130.5 (CH), 129.3 (CH), 129.1 (CH), 129.0 (CH), 128.9 (CH), 128.8 (CH), 128.7 (CH), 128.4 (CH), 128.0 (CH), 127.9 (CH), 127.1 (CH), 125.3 (CH), 125.2 (CH), 125.1 (CH), 122.2 (CH), 120.9 (CH), 120.6 (CH), 119.7 (CH), 104.0 (CN), 37.0 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H24N2: C, 89.74; H, 4.76; N, 5.51; found: C, 89.50; H, 4.58; N, 5.39.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.43 (d, 1H, J = 10 Hz, 1H), 8.32 (s, 1H), 8.25 (s, 1H), 8.19 (d, J = 10.5 Hz, 2H), 8.11 (s, 1H), 7.99–7.97 (m, 2H), 7.93–7.84 (m, 2H), 7.76–7.74 (m, 2H), 7.63–7.61 (m, 3H), 7.44–7.39 (m, 4H), 4.06 (s, 2H, CH2), 4.00 (d, 2J = 19.0 Hz, 2H, CH2), 3.14 (s, 6H, 2CH3) ppm. 13C NMR (CDCl3): δ = 162.9 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.43 (d, 1H, J = 10 Hz, 1H), 8.32 (s, 1H), 8.25 (s, 1H), 8.19 (d, J = 10.5 Hz, 2H), 8.11 (s, 1H), 7.99–7.97 (m, 2H), 7.93–7.84 (m, 2H), 7.76–7.74 (m, 2H), 7.63–7.61 (m, 3H), 7.44–7.39 (m, 4H), 4.06 (s, 2H, CH2), 4.00 (d, 2J = 19.0 Hz, 2H, CH2), 3.14 (s, 6H, 2CH3) ppm. 13C NMR (CDCl3): δ = 162.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 162.5 (C), 159.2 (C), 147.8 (C), 144.2 (C), 144.1 (C), 144.0 (C), 143.8 (C), 141.0 (C), 140.9 (C), 140.5 (C), 134.2 (C), 130.2 (CH), 128.5 (CH), 128.4 (CH), 128.0 (CH), 126.9 (CH), 126.1 (CH), 125.9 (CH), 125.3 (CH), 125.2 (CH), 125.1 (CH), 120.9 (CH), 120.8 (CH), 120.5 (CH), 120.4 (CH), 120.2 (CH), 119.7 (CH), 117.8 (CH), 116.6 (C), 104.4 (CN), 43.6 (CH3), 37.1 (CH2), 37.0 (CH2) ppm. Elemental analysis: calcd for C40H29N3: C, 87.08; H, 5.30; N, 7.62; found: C, 86.80; H, 5.11; N, 7.50.
N), 162.5 (C), 159.2 (C), 147.8 (C), 144.2 (C), 144.1 (C), 144.0 (C), 143.8 (C), 141.0 (C), 140.9 (C), 140.5 (C), 134.2 (C), 130.2 (CH), 128.5 (CH), 128.4 (CH), 128.0 (CH), 126.9 (CH), 126.1 (CH), 125.9 (CH), 125.3 (CH), 125.2 (CH), 125.1 (CH), 120.9 (CH), 120.8 (CH), 120.5 (CH), 120.4 (CH), 120.2 (CH), 119.7 (CH), 117.8 (CH), 116.6 (C), 104.4 (CN), 43.6 (CH3), 37.1 (CH2), 37.0 (CH2) ppm. Elemental analysis: calcd for C40H29N3: C, 87.08; H, 5.30; N, 7.62; found: C, 86.80; H, 5.11; N, 7.50.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.43 (s, 1H), 8.25 (d, J = 8.5 Hz, 1H), 8.14 (s, 1H), 7.99–7.97 (m, 1H), 7.93–7.83 (m, 3H), 7.72 (s, 1H), 7.63–7.61 (m, 3H), 7.44–7.39 (m, 6H), 7.12–7.10 (m, 1H), 7.02 (m, 1H), 4.06 (d, 2J = 19.5 Hz, 2H, CH2), 3.96–3.87 (m, 5H, CH2, CH3) ppm. 13C NMR (CDCl3): δ = 162.6 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.43 (s, 1H), 8.25 (d, J = 8.5 Hz, 1H), 8.14 (s, 1H), 7.99–7.97 (m, 1H), 7.93–7.83 (m, 3H), 7.72 (s, 1H), 7.63–7.61 (m, 3H), 7.44–7.39 (m, 6H), 7.12–7.10 (m, 1H), 7.02 (m, 1H), 4.06 (d, 2J = 19.5 Hz, 2H, CH2), 3.96–3.87 (m, 5H, CH2, CH3) ppm. 13C NMR (CDCl3): δ = 162.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.1 (C), 159.6 (C), 159.1 (C), 147.4 (C), 144.9 (C), 144.2 (C), 144.1 (C), 144.0 (C), 143.7 (C), 141.0 (C), 140.9 (C), 140.3 (C), 130.5 (C), 130.3 (C), 128.9 (CH), 128.5 (CH), 127.9 (CH), 127.5 (CH), 127.4 (CH), 127.1 (CH), 126.4 (CH), 126.2 (CH), 125.2 (CH), 125.1 (CH), 124.4 (CH), 120.6 (CH), 120.5 (CH), 120.2 (CH), 119.8 (CH), 114.7 (CH), 114.5 (CH), 103.2 (CN), 55.5 (CH3), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C39H26N2O: C, 86.96; H, 4.87; N, 5.20; found: C, 86.70; H, 4.59; N, 4.95.
N), 161.1 (C), 159.6 (C), 159.1 (C), 147.4 (C), 144.9 (C), 144.2 (C), 144.1 (C), 144.0 (C), 143.7 (C), 141.0 (C), 140.9 (C), 140.3 (C), 130.5 (C), 130.3 (C), 128.9 (CH), 128.5 (CH), 127.9 (CH), 127.5 (CH), 127.4 (CH), 127.1 (CH), 126.4 (CH), 126.2 (CH), 125.2 (CH), 125.1 (CH), 124.4 (CH), 120.6 (CH), 120.5 (CH), 120.2 (CH), 119.8 (CH), 114.7 (CH), 114.5 (CH), 103.2 (CN), 55.5 (CH3), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C39H26N2O: C, 86.96; H, 4.87; N, 5.20; found: C, 86.70; H, 4.59; N, 4.95.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.25 (d, J = 14.0 Hz, 2H), 8.12 (s, 1H), 8.00 (s, 1H), 7.94–7.82 (m, 4H), 7.70–7.68 (m, 1H), 7.60 (d, J = 14.0 Hz, 4H), 7.47–7.40 (m, 5H), 4.06 (d, 2J = 20.0 Hz, 2H, CH2), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.7 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.25 (d, J = 14.0 Hz, 2H), 8.12 (s, 1H), 8.00 (s, 1H), 7.94–7.82 (m, 4H), 7.70–7.68 (m, 1H), 7.60 (d, J = 14.0 Hz, 4H), 7.47–7.40 (m, 5H), 4.06 (d, 2J = 20.0 Hz, 2H, CH2), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 162.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.5 (C), 154.2 (C), 147.9 (C), 145.1 (C), 144.3 (C), 144.0 (C), 143.5 (C), 140.9 (C), 140.8 (C), 140.2 (C), 136.3 (C), 136.1 (C), 135.7 (C), 135.5 (C), 130.1 (CH), 129.5 (CH), 129.3 (CH), 129.1 (CH), 128.4 (CH), 128.1 (CH), 127.6 (CH), 127.4 (CH), 127.2 (CH), 127.0 (CH), 126.9 (CH), 126.3 (CH), 126.1 (CH), 125.2 (CH), 125.1 (CH), 124.3 (CH), 120.6 (CH), 120.5 (CH), 119.9 (CH), 117.8 (C), 103.7 (CN), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H23ClN2: C, 84.04; H, 4.27; N, 5.16; Cl, 6.53; found: C, 83.86; H, 4.05; N, 5.02; Cl, 6.30.
N), 159.5 (C), 154.2 (C), 147.9 (C), 145.1 (C), 144.3 (C), 144.0 (C), 143.5 (C), 140.9 (C), 140.8 (C), 140.2 (C), 136.3 (C), 136.1 (C), 135.7 (C), 135.5 (C), 130.1 (CH), 129.5 (CH), 129.3 (CH), 129.1 (CH), 128.4 (CH), 128.1 (CH), 127.6 (CH), 127.4 (CH), 127.2 (CH), 127.0 (CH), 126.9 (CH), 126.3 (CH), 126.1 (CH), 125.2 (CH), 125.1 (CH), 124.3 (CH), 120.6 (CH), 120.5 (CH), 119.9 (CH), 117.8 (C), 103.7 (CN), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H23ClN2: C, 84.04; H, 4.27; N, 5.16; Cl, 6.53; found: C, 83.86; H, 4.05; N, 5.02; Cl, 6.30.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.25 (m, 1H), 8.14 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.93–7.88 (m, 4H), 7.87–7.85 (m, 2H), 7.82 (d, J = 8.0 Hz, 1H), 7.67–7.61 (m, 3H), 7.47–7.44 (m, 2H), 7.43–7.39 (m, 2H), 4.08 (s, 2H, CH2), 4.00 (d, 2J = 24.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.1 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.25 (m, 1H), 8.14 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.93–7.88 (m, 4H), 7.87–7.85 (m, 2H), 7.82 (d, J = 8.0 Hz, 1H), 7.67–7.61 (m, 3H), 7.47–7.44 (m, 2H), 7.43–7.39 (m, 2H), 4.08 (s, 2H, CH2), 4.00 (d, 2J = 24.0 Hz, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 160.1 (C), 153.7 (C), 148.8 (C), 145.7 (C), 145.0 (C), 144.4 (C), 144.3 (C), 143.9 (C), 141.6 (C), 141.2 (C), 140.4 (C), 136.0 (C), 135.6 (C), 133.6 (CH), 133.1 (CH), 129.9 (CH), 128.8 (CH), 128.1 (CH), 127.9 (CH), 127.4 (CH), 127.3 (CH), 126.4 (CH), 125.6 (CH), 125.5 (CH), 124.5 (CH), 121.0 (CH), 120.9 (CH), 120.3 (CH), 120.1 (CH), 118.4 (C), 114.1 (C), 103.8 (CN), 37.4 (CH2), 37.3 (CH2) ppm. Elemental analysis: calcd for C39H23N3: C, 87.78; H, 4.34; N, 7.87; found: C, 87.70; H, 4.21; N, 7.69.
N), 160.1 (C), 153.7 (C), 148.8 (C), 145.7 (C), 145.0 (C), 144.4 (C), 144.3 (C), 143.9 (C), 141.6 (C), 141.2 (C), 140.4 (C), 136.0 (C), 135.6 (C), 133.6 (CH), 133.1 (CH), 129.9 (CH), 128.8 (CH), 128.1 (CH), 127.9 (CH), 127.4 (CH), 127.3 (CH), 126.4 (CH), 125.6 (CH), 125.5 (CH), 124.5 (CH), 121.0 (CH), 120.9 (CH), 120.3 (CH), 120.1 (CH), 118.4 (C), 114.1 (C), 103.8 (CN), 37.4 (CH2), 37.3 (CH2) ppm. Elemental analysis: calcd for C39H23N3: C, 87.78; H, 4.34; N, 7.87; found: C, 87.70; H, 4.21; N, 7.69.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.46 (d, J = 7.5 Hz, 2H), 8.38 (s, 1H), 8.29–8.25 (m, 1H), 8.14 (s, 1H), 8.01 (s, 1H), 7.95–7.83 (m, 5H), 7.73–7.63 (m, 4H), 7.49–7.41 (m, 4H), 4.06 (s, 2J = 21.0 Hz, 2H, CH2), 4.01 (s, 2H, CH2). 13C NMR (CDCl3): δ = 162.8 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.46 (d, J = 7.5 Hz, 2H), 8.38 (s, 1H), 8.29–8.25 (m, 1H), 8.14 (s, 1H), 8.01 (s, 1H), 7.95–7.83 (m, 5H), 7.73–7.63 (m, 4H), 7.49–7.41 (m, 4H), 4.06 (s, 2J = 21.0 Hz, 2H, CH2), 4.01 (s, 2H, CH2). 13C NMR (CDCl3): δ = 162.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.8 (C), 152.7 (C), 148.6 (C), 145.4 (C), 144.6 (C), 144.3 (C), 144.1 (C), 144.0 (C), 143.7 (C), 143.6 (C), 143.1 (C), 140.8 (C), 140.1 (C), 135.8 (C), 129.9 (CH), 128.7 (CH), 128.4 (CH), 128.2 (CH), 127.7 (CH), 127.6 (CH), 127.2 (CH), 127.1 (CH), 127.0 (CH), 126.3 (CH), 126.0 (CH), 125.3 (CH), 125.2 (CH), 124.7 (CH), 124.3 (CH), 120.7 (C), 120.6 (CH), 120.3 (CH), 119.9 (CH), 117.4 (C), 103.4 (CN), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H23N3O2: C, 82.44; H, 4.19; N, 7.59; found: C, 82.31; H, 4.22; N, 7.61.
N), 159.8 (C), 152.7 (C), 148.6 (C), 145.4 (C), 144.6 (C), 144.3 (C), 144.1 (C), 144.0 (C), 143.7 (C), 143.6 (C), 143.1 (C), 140.8 (C), 140.1 (C), 135.8 (C), 129.9 (CH), 128.7 (CH), 128.4 (CH), 128.2 (CH), 127.7 (CH), 127.6 (CH), 127.2 (CH), 127.1 (CH), 127.0 (CH), 126.3 (CH), 126.0 (CH), 125.3 (CH), 125.2 (CH), 124.7 (CH), 124.3 (CH), 120.7 (C), 120.6 (CH), 120.3 (CH), 119.9 (CH), 117.4 (C), 103.4 (CN), 37.1 (CH2), 36.9 (CH2) ppm. Elemental analysis: calcd for C38H23N3O2: C, 82.44; H, 4.19; N, 7.59; found: C, 82.31; H, 4.22; N, 7.61.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.46 (s, 1H), 8.36 (d, J = 14.5 Hz, 2H), 8.28–8.25 (m, 3H), 8.21–8.19 (m, 4H), 8.10–8.08 (m, 2H), 7.92 (s, 1H), 7.88–7.85 (m, 3H), 7.63–7.60 (m, 4H), 7.43–7.38 (m, 2H), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.7 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.46 (s, 1H), 8.36 (d, J = 14.5 Hz, 2H), 8.28–8.25 (m, 3H), 8.21–8.19 (m, 4H), 8.10–8.08 (m, 2H), 7.92 (s, 1H), 7.88–7.85 (m, 3H), 7.63–7.60 (m, 4H), 7.43–7.38 (m, 2H), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.3 (C), 154.7 (C), 144.2 (C), 144.1 (C), 144.0 (C), 140.8 (C), 136.7 (C), 132.0 (C), 130.8 (C), 130.0 (CH), 129.1 (CH), 128.7 (CH), 128.5 (CH), 127.5 (CH), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.8 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.5 (CH), 124.4 (CH), 120.5 (CH), 120.3 (CH), 118.8 (CH), 117.2 (C), 107.2 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C41H24N2: C, 90.42; H, 4.44; N, 5.14; found: C, 90.33; H, 4.31; N, 4.97.
N), 159.3 (C), 154.7 (C), 144.2 (C), 144.1 (C), 144.0 (C), 140.8 (C), 136.7 (C), 132.0 (C), 130.8 (C), 130.0 (CH), 129.1 (CH), 128.7 (CH), 128.5 (CH), 127.5 (CH), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.8 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.5 (CH), 124.4 (CH), 120.5 (CH), 120.3 (CH), 118.8 (CH), 117.2 (C), 107.2 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C41H24N2: C, 90.42; H, 4.44; N, 5.14; found: C, 90.33; H, 4.31; N, 4.97.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.34 (d, J = 6.0 Hz, 1H), 8.30–8.24 (m, 3H), 8.22–8.13 (m, 4H), 8.11–8.06 (m, 3H), 7.99–7.90 (m, 5H), 7.83 (s, 1H), 7.60–7.42 (m, 2H), 6.63 (d, J = 7.0 Hz, 2H), 3.94 (s, 2H, CH2), 3.07 (s, 6H, 2CH3) ppm. 13C NMR (CDCl3): δ = 156.1 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.34 (d, J = 6.0 Hz, 1H), 8.30–8.24 (m, 3H), 8.22–8.13 (m, 4H), 8.11–8.06 (m, 3H), 7.99–7.90 (m, 5H), 7.83 (s, 1H), 7.60–7.42 (m, 2H), 6.63 (d, J = 7.0 Hz, 2H), 3.94 (s, 2H, CH2), 3.07 (s, 6H, 2CH3) ppm. 13C NMR (CDCl3): δ = 156.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.9 (C), 146.4 (C), 144.5 (C), 143.3 (C), 140.4 (C), 134.7 (CH), 133.0 (C), 132.3 (C), 131.1 (C), 130.6 (C), 129.2 (C), 129.1 (CH), 129.0 (CH), 128.0 (CH), 127.7 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.1 (CH), 125.9 (CH), 125.7 (CH), 125.2 (CH), 124.9 (CH), 124.3 (CH), 124.0 (CH), 120.9 (CH), 119.6 (C), 118.6 (C), 111.6 (CH), 104.3 (CN), 40.0 (CH3), 36.9 (CH2) ppm. Elemental analysis: calcd for C43H29N3: C, 87.88; H, 4.97; N, 7.15; found: C, 87.76; H, 4.78; N, 6.99.
N), 153.9 (C), 146.4 (C), 144.5 (C), 143.3 (C), 140.4 (C), 134.7 (CH), 133.0 (C), 132.3 (C), 131.1 (C), 130.6 (C), 129.2 (C), 129.1 (CH), 129.0 (CH), 128.0 (CH), 127.7 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.1 (CH), 125.9 (CH), 125.7 (CH), 125.2 (CH), 124.9 (CH), 124.3 (CH), 124.0 (CH), 120.9 (CH), 119.6 (C), 118.6 (C), 111.6 (CH), 104.3 (CN), 40.0 (CH3), 36.9 (CH2) ppm. Elemental analysis: calcd for C43H29N3: C, 87.88; H, 4.97; N, 7.15; found: C, 87.76; H, 4.78; N, 6.99.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.55 (d, J = 5.0 Hz, 1H), 8.33–8.31 (m, 2H), 8.27–8.25 (m, 2H), 8.18–8.15 (m, 4H), 8.08 (s, 1H), 7.99–7.97 (m, 1H), 7.88–7.86 (m, 2H), 7.79 (d, J = 7.5 Hz, 2H), 7.62–7.59 (m, 2H), 7.45–7.43 (m, 1H), 7.39–7.37 (m, 1H), 7.12 (d, J = 7.5 Hz, 2H), 4.05 (s, 2H, CH2), 3.92 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 162.9 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.55 (d, J = 5.0 Hz, 1H), 8.33–8.31 (m, 2H), 8.27–8.25 (m, 2H), 8.18–8.15 (m, 4H), 8.08 (s, 1H), 7.99–7.97 (m, 1H), 7.88–7.86 (m, 2H), 7.79 (d, J = 7.5 Hz, 2H), 7.62–7.59 (m, 2H), 7.45–7.43 (m, 1H), 7.39–7.37 (m, 1H), 7.12 (d, J = 7.5 Hz, 2H), 4.05 (s, 2H, CH2), 3.92 (s, 3H, CH3) ppm. 13C NMR (CDCl3): δ = 162.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.2 (C), 154.8 (C), 144.0 (C), 143.5 (C), 140.9 (C), 132.2 (C), 131.3 (C), 130.8 (C), 130.4 (CH), 128.7 (CH), 128.6 (CH), 127.8 (CH), 127.4 (CH), 127.3 (CH), 126.9 (CH), 126.3 (CH), 125.9 (CH), 125.4 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 123.8 (CH), 120.5 (CH), 119.9 (CH), 118.4 (C), 114.5 (CH), 104.1 (CN), 55.5 (CH3), 37.1 (CH2) ppm. Elemental analysis: calcd for C42H26N2O: C, 87.78; H, 4.56; N, 4.87; found: C, 87.60; H, 4.44; N, 4.69.
N), 161.2 (C), 154.8 (C), 144.0 (C), 143.5 (C), 140.9 (C), 132.2 (C), 131.3 (C), 130.8 (C), 130.4 (CH), 128.7 (CH), 128.6 (CH), 127.8 (CH), 127.4 (CH), 127.3 (CH), 126.9 (CH), 126.3 (CH), 125.9 (CH), 125.4 (CH), 125.1 (CH), 124.9 (CH), 124.2 (CH), 123.8 (CH), 120.5 (CH), 119.9 (CH), 118.4 (C), 114.5 (CH), 104.1 (CN), 55.5 (CH3), 37.1 (CH2) ppm. Elemental analysis: calcd for C42H26N2O: C, 87.78; H, 4.56; N, 4.87; found: C, 87.60; H, 4.44; N, 4.69.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.37 (s, 1H), 8.33–8.26 (m, 4H), 8.20–8.17 (m, 4H), 8.09–8.05 (m, 3H), 7.92–7.87 (m, 2H), 7.79–7.77 (m, 1H), 7.62–7.59 (m, 2H), 7.44–7.38 (m, 2H), 7.30–7.28 (m, 1H), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.5 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.45 (s, 1H), 8.37 (s, 1H), 8.33–8.26 (m, 4H), 8.20–8.17 (m, 4H), 8.09–8.05 (m, 3H), 7.92–7.87 (m, 2H), 7.79–7.77 (m, 1H), 7.62–7.59 (m, 2H), 7.44–7.38 (m, 2H), 7.30–7.28 (m, 1H), 3.98 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 163.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.7 (C), 144.2 (C), 144.1 (C), 141.0 (C), 135.7 (C), 135.1 (C), 130.1 (CH), 129.4 (CH), 128.6 (CH), 128.0 (CH), 127.6 (CH), 127.5 (C), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.8 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.4 (CH), 124.3 (CH), 120.9 (CH), 120.6 (CH), 120.3 (CH), 119.6 (CH), 118.6 (C), 118.5 (CH), 116.9 (C), 107.9 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C41H23ClN2: C, 85.04; H, 4.00; N, 4.84; Cl, 6.12; found: C, 84.96; H, 4.09; N, 4.78; Cl, 6.04.
N), 159.7 (C), 144.2 (C), 144.1 (C), 141.0 (C), 135.7 (C), 135.1 (C), 130.1 (CH), 129.4 (CH), 128.6 (CH), 128.0 (CH), 127.6 (CH), 127.5 (C), 127.4 (CH), 127.0 (CH), 126.7 (CH), 126.2 (CH), 125.8 (CH), 125.5 (CH), 125.2 (CH), 124.6 (CH), 124.4 (CH), 124.3 (CH), 120.9 (CH), 120.6 (CH), 120.3 (CH), 119.6 (CH), 118.6 (C), 118.5 (CH), 116.9 (C), 107.9 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C41H23ClN2: C, 85.04; H, 4.00; N, 4.84; Cl, 6.12; found: C, 84.96; H, 4.09; N, 4.78; Cl, 6.04.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.46 (s, 1H), 8.38 (s, 1H), 8.30–8.27 (m, 4H), 8.21–8.18 (m, 4H), 8.10–8.05 (m, 2H), 7.94–7.87 (m, 6H), 7.60–7.58 (m, 1H), 7.44–7.39 (m, 2H), 3.99 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 160.5 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.46 (s, 1H), 8.38 (s, 1H), 8.30–8.27 (m, 4H), 8.21–8.18 (m, 4H), 8.10–8.05 (m, 2H), 7.94–7.87 (m, 6H), 7.60–7.58 (m, 1H), 7.44–7.39 (m, 2H), 3.99 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 160.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 160.1 (C), 153.0 (C), 145.0 (C), 144.4 (C), 141.3 (C), 141.0 (C), 135.6 (C), 133.2 (C), 133.0 (CH), 129.8 (CH), 128.8 (CH), 127.9 (CH), 127.7 (CH), 127.5 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.1 (CH), 125.8 (CH), 125.3 (CH), 124.8 (CH), 124.7 (CH), 124.5 (CH), 124.4 (CH), 120.7 (CH), 120.5 (CH), 118.8 (C), 118.4 (C), 116.9 (C), 114.3 (C), 110.8 (CN), 37.5 (CH2) ppm. Elemental analysis: calcd for C41H23N3O2: C, 83.52; H, 3.93; N, 7.13; found: C, 83.44; H, 3.79; N, 7.01.
N), 160.1 (C), 153.0 (C), 145.0 (C), 144.4 (C), 141.3 (C), 141.0 (C), 135.6 (C), 133.2 (C), 133.0 (CH), 129.8 (CH), 128.8 (CH), 127.9 (CH), 127.7 (CH), 127.5 (CH), 127.2 (CH), 127.0 (CH), 126.4 (CH), 126.1 (CH), 125.8 (CH), 125.3 (CH), 124.8 (CH), 124.7 (CH), 124.5 (CH), 124.4 (CH), 120.7 (CH), 120.5 (CH), 118.8 (C), 118.4 (C), 116.9 (C), 114.3 (C), 110.8 (CN), 37.5 (CH2) ppm. Elemental analysis: calcd for C41H23N3O2: C, 83.52; H, 3.93; N, 7.13; found: C, 83.44; H, 3.79; N, 7.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (CDCl3): δ = 8.50 (d, J = 7.5 Hz, 1H), 8.46 (s, 1H), 8.38 (s, 1H), 8.31–8.27 (m, 4H), 8.21–8.19 (m, 4H), 8.11–8.08 (m, 3H), 8.03–8.01 (m, 2H), 7.94–7.92 (m, 1H), 7.89–7.87 (m, 1H), 7.61–7.59 (m, 1H), 7.45–7.39 (m, 2H), 3.99 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 161.1 (C
N) cm−1. 1H NMR (CDCl3): δ = 8.50 (d, J = 7.5 Hz, 1H), 8.46 (s, 1H), 8.38 (s, 1H), 8.31–8.27 (m, 4H), 8.21–8.19 (m, 4H), 8.11–8.08 (m, 3H), 8.03–8.01 (m, 2H), 7.94–7.92 (m, 1H), 7.89–7.87 (m, 1H), 7.61–7.59 (m, 1H), 7.45–7.39 (m, 2H), 3.99 (s, 2H, CH2) ppm. 13C NMR (CDCl3): δ = 161.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 161.0 (C), 159.8 (C), 148.7 (C), 147.9 (C), 144.7 (C), 144.1 (C), 140.6 (C), 135.3 (C), 132.6 (C), 130.8 (C), 130.2 (CH), 129.2 (CH), 129.0 (CH), 128.1 (CH), 127.7 (CH), 127.4 (CH), 127.1 (CH), 126.6 (CH), 126.3 (CH), 125.9 (CH), 125.5 (CH), 124.9 (CH), 124.8 (CH), 124.6 (CH), 124.5 (CH), 120.9 (CH), 120.7 (CH), 118.6 (CH), 118.2 (C), 106.9 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H23N3: C, 88.55; H, 4.07; N, 7.38; found: C, 88.60; H, 4.15; N, 7.30.
N), 161.0 (C), 159.8 (C), 148.7 (C), 147.9 (C), 144.7 (C), 144.1 (C), 140.6 (C), 135.3 (C), 132.6 (C), 130.8 (C), 130.2 (CH), 129.2 (CH), 129.0 (CH), 128.1 (CH), 127.7 (CH), 127.4 (CH), 127.1 (CH), 126.6 (CH), 126.3 (CH), 125.9 (CH), 125.5 (CH), 124.9 (CH), 124.8 (CH), 124.6 (CH), 124.5 (CH), 120.9 (CH), 120.7 (CH), 118.6 (CH), 118.2 (C), 106.9 (CN), 37.0 (CH2) ppm. Elemental analysis: calcd for C42H23N3: C, 88.55; H, 4.07; N, 7.38; found: C, 88.60; H, 4.15; N, 7.30.Conflicts of interest
The authors confirm that there are no conflicts of interest to disclose.Acknowledgements
The authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University for the full financial support of this work through the project number 18-SCI-1-01-0009. S. A. Ahmed is highly indebted to the Alexander von Humboldt Foundation (AvH) and Prof. Jochen Mattay, Bielefeld University, Germany for help with some of the analytical and spectroscopic measurements in addition to some labware donations.Notes and references
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- I. Ugi, A. Dömling and W. Hörl, Endeavour, 1994, 18, 115–122 CrossRef CAS.
- A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed.
- S. Tu, B. Jiang, Y. Zhang, R. Jia, J. Zhang, C. Yao and F. Shi, Org. Biomol. Chem., 2007, 5, 355–359 RSC.
- C. B. Reddy, K. S. Kumar, M. A. Kumar, M. V. N. Reddy, B. S. Krishna, M. Naveen, M. K. Arunasree, C. S. Reddy, C. N. Raju and C. D. Reddy, Eur. J. Med. Chem., 2012, 47, 553–559 CrossRef CAS PubMed.
- J. A. Wells and C. L. McClendon, Nature, 2007, 450, 1001–1009 CrossRef CAS PubMed.
- Y. Feng, T. J. Mitchison, A. Bender, D. W. Young and J. A. Tallarico, Nat. Rev. Drug Discovery, 2009, 8, 567–578 CrossRef CAS PubMed.
- V. Azzarito, K. Long, N. S. Murphy and A. J. Wilson, Nat. Chem., 2013, 5, 161–173 CrossRef CAS PubMed.
- J. M. Grasshoff, J. L. Marshall, R. A. Minns, S. M. Ramos, S. G. Stroud, S. J. Telfer, H. Yang, R. A. Boggs and E. S. Kolb, Process and composition for generating acid for imaging compositions, Int. Pat., 9824000, June 4, 1998.
- N. Hisamatsu and S. Hiraishi, Thermal recording material providing yellow image, Japanese Pat., 10250237, September 22, 1998.
- C. S. Angadiyavar and R. Srinivasan, 2,4,6-Trisubstituted pyridine dye lasers, US. Pat., 506916, February 3, 1976.
- B. Garcia-Acosta, R. Martinez-Manez, F. Sancenon, J. Soto, K. Rurack, M. Spieles, E. Garcia-Breijo and L. Gil, Inorg. Chem., 2007, 46, 3123–3135 CrossRef CAS PubMed.
- B. Tang, F. Yu, P. Li, L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016–3023 CrossRef CAS PubMed.
- C. Z. Wang, X. Feng, Z. Kowsera, C. Wu, T. Akther, M. R. J. Elsegood, C. Redshaw and T. Yamato, Dyes Pigm., 2018, 153, 125–131 CrossRef CAS.
- J. Hu, M. Era, M. R. J. Elsegood and T. Yamato, Eur. J. Org. Chem., 2010, 72–79 CrossRef.
- M. Ottonelli, M. Piccardo, D. Duce, S. Thea and G. Dellepiane, J. Phys. Chem. A, 2012, 116, 611–630 CrossRef CAS PubMed.
- D. G. Vanga, M. Santra, A. Keerthi and S. Valiyaveettil, Org. Biomol. Chem., 2014, 12, 7914–7918 RSC.
- P. S. Reeta, A. Khetubol, T. Jella, V. Chukharev, F. Abou-Chahine, N. V. Tkachenko, L. Giribabu and H. Lemmetyinen, J. Porphyrins Phthalocyanines, 2015, 19, 288–300 CrossRef CAS.
- M. Nandakumar, E. Sankar and A. K. Mohanakrishnan, Synth. Commun., 2016, 46, 1810–1819 CrossRef CAS.
- C. Z. Wang, R. Kihara, X. Feng, P. Thuéry, C. Redshaw and T. Yamato, ChemistrySelect, 2017, 2, 1436–1441 CrossRef CAS.
- W. Yang, J. H. S. K. Monteiro, A. de Bettencourt-Dias and W. A. Chalifoux, Can. J. Chem., 2017, 95, 341–345 CrossRef CAS.
- J. Weng, Q. Mei, Q. Q. Ling, Q. Fan and W. Huang, Tetrahedron, 2012, 68, 3129–3134 CrossRef CAS.
- J. S. Yang, C. S. Lin and C. Y. Huang, Org. Lett., 2001, 3, 889–892 CrossRef CAS PubMed.
- S. K. Kim, J. H. Bok, R. A. Bartsch, J. Y. Lee and J. S. Kim, Org. Lett., 2005, 7, 4839–4842 CrossRef CAS PubMed.
- T. C. Chou, C. L. Hwa, J. J. Lin, K. C. Liao and J. C. Tseng, J. Org. Chem., 2005, 70, 9717–9726 CrossRef CAS PubMed.
- F. Gualtieri, E. Teodori, C. Bellucci, E. Pesce and G. Piacenza, J. Med. Chem., 1985, 28, 1621–1628 CrossRef CAS PubMed.
- C. A. Parker, Photoluminescence of Solutions, Elsevier, Amsterdam, the Netherlands, 1968 Search PubMed.
- S. R. Marder, B. Kippelen, A. K. Y. Jen and N. Peyghammbarian, Nature, 1997, 388, 845–851 CrossRef CAS.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. Dos Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS.
- J. Jo, C. Chi, S. Hoeger, G. Wegner and D. Y. Yoon, Chem. - Eur. J., 2004, 10, 2681–2688 CrossRef CAS PubMed.
- P. Thirumurugan and P. T. Perumal, Tetrahedron, 2009, 65, 7620–7629 CrossRef CAS.
- K. Zhao, X.-P. Xu, S.-L. Zhu, D.-Q. Shi, Y. Zhang and S.-J. Ji, Synthesis, 2009, 16, 2697–2708 Search PubMed.
- F. Zhang, Y. Zhao, L. Sun, L. Ding, Y. Gu and P. Gong, Eur. J. Med. Chem., 2011, 46, 3149–3157 CrossRef CAS PubMed.
- N. S. El-Sayed, A. N. Shirazi, M. G. El-Meligy, A. K. El-Ziaty, D. Rowley, J. Sun, Z. A. Nagib and K. Parang, Tetrahedron Lett., 2014, 55, 1154–1158 CrossRef CAS PubMed.
- E. E. Sizova, E. V. Arshinov, Y. A. Kotsareva, L. V. Glizdinskaya and G. P. Sagitullina, Chem. Heterocycl. Compd., 2017, 53, 1026–1032 CrossRef CAS.
- K. A. M. Abouzid, G. H. Al-Ansary and A. M. El-Naggar, Eur. J. Med. Chem., 2017, 134, 357–365 CrossRef CAS PubMed.
- S. K. Krishnammagari, S. G. Balwe, J. S. Kim, K. T. Lim and Y. T. Jeong, Monatsh. Chem., 2019, 150, 691–702 CrossRef CAS.
- E. M. Hussein, Z. Moussa, N. El Guesmi and S. A. Ahmed, RSC Adv., 2018, 8, 24116–24127 RSC.
- N. El Guesmi, E. M. Hussein and S. A. Ahmed, J. Photochem. Photobiol., A, 2019, 371, 306–314 CrossRef CAS.
- E. M. Hussein, Z. Naturforsch. B Chem. Sci, 2012, 67, 231–237 CAS.
- E. M. Hussein and A. M. El-Khawaga, J. Heterocycl. Chem., 2012, 49, 1296–1301 CrossRef CAS.
- E. M. Hussein, Monatsh. Chem., 2013, 144, 1691–1697 CrossRef CAS.
- E. M Hussein, Russ. J. Org. Chem., 2015, 51, 54–64 CrossRef.
- E. M. Hussein and S. A. Ahmed, Chem. Heterocycl. Compd., 2017, 53, 1148–1155 CrossRef CAS.
- (a) M. Barfield and D. M. Grant, J. Am. Chem. Soc., 1961, 83, 4726–4729 CrossRef CAS; (b) J. A. Pople and A. A. Bothner, J. Chem. Phys., 1965, 42, 1339–1349 CrossRef CAS; (c) R. C. Cookson, T. A. Crabb, J. J. Frankel and J. Hudec, Tetrahedron, 1966, 22, 355–390 CrossRef; (d) R. Cahill, R. C. Cookson and T. A. Crabb, Tetrahedron, 1969, 25, 4681–4709 CrossRef CAS.
- H. M. Manohara, C. T. Devaiah, B. Hemavathi and T. N. Ahipa, J. Lumin., 2019, 206, 284–291 CrossRef CAS.
- H. A. El-Sayed, A. M. Abdel Hamid, S. M. Mohammed and A. H. Moustafa, Synth. Commun., 2019, 49, 2096–2105 CrossRef CAS.
- O. V. Ershov, S. V. Fedoseev, M. Y. Ievlev and M. Y. Belikov, Dyes Pigm., 2016, 134, 459–464 CrossRef CAS.
- C.-S. Yao, K. Lu, B. Song, B. Liu, T.-J. Li and C.-X. Yub, J. Heterocycl. Chem., 2014, 51, 1807–1810 CrossRef CAS.
- T. N. Ahipa, V. Kumar and A. V. Adhikari, Struct. Chem., 2014, 25, 1165–1174 CrossRef CAS.
- E. P. Gutiérrez, M. J. Percino, V. M. Chapela, M. Cerón, J. L. Maldonado and G. R. Ortiz, Materials, 2011, 4, 562–574 CrossRef PubMed.
- M. D. Bowman, M. M. Jacobson and H. E. Blackwell, Org. Lett., 2006, 8, 1645–1648 CrossRef CAS PubMed.
- S. J. J. Titinchi, F. S. Kamounah, H. S. Abbo and O. Hammerich, ARKIVOC, 2008, 13, 91–105 Search PubMed.
- E. M. Hussein, S. A. Ahmed and I. I. Althagafi, Heterocycl. Commun., 2017, 23, 379–384 CAS.
- D. W. Robertson, J. H. Krushinski, E. E. Beedle, J. D. Leander, D. T. Wong and R. C. Rathbun, J. Med. Chem., 1986, 29, 1577–1586 CrossRef CAS PubMed.
- P. G. Baraldi, R. Romagnoli, M. G. Pavani, M. Nuñez, M. A. Tabrizi, J. C. Shryock, E. Leung, A. R. Moorman, C. Uluoglu, V. Iannotta, S. Merighi and P. A. Borea, J. Med. Chem., 2003, 46, 794–809 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09379f |
| This journal is © The Royal Society of Chemistry 2019 |