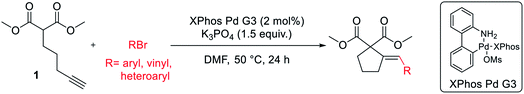Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-catalyzed intramolecular addition of active methylene compounds to alkynes with subsequent cross-coupling with (hetero)aryl halides†
Aleksandra Błockaa,
Paweł Woźnicki b,
Marek Stankevič
b,
Marek Stankevič b and
Wojciech Chaładaj
b and
Wojciech Chaładaj *a
*a
aInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: wojciech.chaladaj@icho.edu.pl
bDepartment of Organic Chemistry, Faculty of Chemistry, Marie Curie-Skłodowska University in Lublin, Gliniana 33, 20-614 Lublin, Poland
First published on 3rd December 2019
Abstract
We report an efficient protocol for tandem Pd-catalyzed intramolecular addition of active methylene compounds to alkynes, followed by subsequent cross-coupling with (hetero)aryl bromides and chlorides. The reaction proceeds under mild conditions, providing excellent functional group tolerance, including unprotected OH, NH2 groups, enolizable ketones, or a variety of heterocycles. Mechanistic studies point towards a catalytic cycle involving oxidative addition, intramolecular nucleophilic addition to the Pd(II)-activated alkyne, and reductive elimination, with 5-exo-dig cyclization being the rate limiting step.
Introduction
Palladium complexes emerge as some of the most versatile homogenous catalysts with a myriad of applications in both academic and industrial research. The most prominent area of palladium catalysis, awarded with the 2010 Nobel Price to R. Heck, A. Suzuki, and E. Negishi,1 covers cross-couplings of (hetero)aryl or vinyl(pseudo)halides with nucleophilic or organometallic partners. High efficiency of these and many other processes (e.g. Wacker oxidation) arises from the facile interconversion of palladium oxidation states through two-electron redox chemistry. Besides the most widespread Pd(0)/Pd(II) cycle, palladium is also able to enter radical processes or to serve as a carbophilic Lewis acid in redox–neutral transformations. The ability to mediate mechanistically distinct transformations makes palladium the catalyst of choice for the design of tandem reactions in which a single metal complex catalyzes a sequence of transformations.2 In our research, we are focused on the development of tandem processes combining the nucleophilic addition to alkynes and subsequent cross-coupling, which give the access to a wide set of carbo- and heterocyclic systems.3 In contrast to cross-coupling reactions, these transformations are highly underdeveloped and suffer from harsh reaction conditions (e.g. the use of strong bases), narrow substrate scope (usually limited to active aryl iodides), and poor functional group tolerance, as well as insufficient mechanistic understanding.In the late 1980s, Gore disclosed seminal works on a novel Pd-catalyzed dicarbofunctionalization of unsaturated C–C systems through arylation with iodobenzene and intramolecular nucleophilic additions of malonates to alkylidenecyclopropanes or alkenes.4 In subsequent accounts, the authors reported a sequential 5-exo-dig cyclization of malonates and β-ketoesters tethered to the alkyne moiety, followed by coupling with aryl iodides.5 The scope of the methodology was further extended to the use of haloalkynes,6 allyl halides and acetates7 as coupling partners. Recently, we have developed a protocol enabling the effective reaction of much less active aryl bromides with acetylenic β-ketoesters.8 A similar strategy, utilizing a 5-endo-dig cyclization has also been applied to the synthesis of cyclopentenes9 and indenes.10 Propargylmalonates led to substituted cyclopropanes via analogous cyclization/coupling protocol.11 On the other hand, propargyl-β-ketoesters underwent 5-exo-dig oxocyclization/coupling, leading to the formation of substituted furan systems due to ambident nature of enolates of β-ketoesters.12 Interestingly, the analogous transformation involving homopropargyl-β-ketoesters possessing an internal or terminal alkyne motif clearly led to either cyclopentenes9 or dihydropyranes,13 respectively.
The vast majority of the known methodologies utilizing sequential Pd-catalyzed nucleophilic cyclization and cross coupling are limited to aryl iodides. Moreover, the functional group compatibility appeared very narrow, which could possibly arise from the use of a strong base. Recently, we have addressed these challenges in a transformation involving acetylenic β-ketoesters which readily undergo cyclization. Extension of the scope with respect to activated methylene compounds still awaits investigation. Although there are examples of such transformations involving derivatives of ketoesters and malonates (with active aryl iodides), to the best of our knowledge, cyclization/coupling of haloarenes with acetylenic derivatives of malononitrile, cyanoacetates, diketones, as well as substrates bearing organophosphorus electron-withdrawing functions have not been reported.
Here, we report an efficient protocol for tandem Pd-catalyzed intramolecular addition of active methylene compounds to alkynes and subsequent cross-coupling with (hetero)aryl bromides and chlorides. The methodology features excellent tolerance for functionalities present in either reaction partner.
Results and discussion
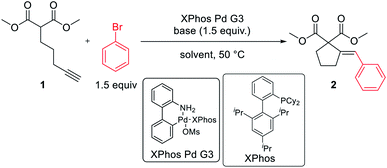
The reaction of dimethyl pent-4-yn-1-ylmalonate 1 with bromobenzene was chosen as a model transformation for the development of the reaction conditions. First, a range of Pd-complexes of mono- and diphosphine ligands were examined using 3rd-generation Buchwald-type palladacyclic system as a platform in order to identify an active catalyst system. Optimization revealed XPhos Pd G3 as the pre-catalyst of choice. Then, the benchmark reaction was evaluated against various reaction conditions, including base, solvent, catalyst loading, temperature, and time, among others (Table 1).14 A polar aprotic solvent appeared to be crucial for the efficiency of the cyclization. Reactions carried out in moderately polar, or nonpolar solvents (e.g. dioxane, THF, toluene) failed to proceed at all, or competitive Sonogashira coupling was observed. The best results were achieved for the reaction run for 24 h at 50 °C in DMF with potassium phosphate as the base. 2 mol% of palladium complex was necessary to achieve a high yield of desired product 2.| Entry | Solvent | Base | Time | Cat. loading | Yielda |
|---|---|---|---|---|---|
| a Determined by GC with mesitilene as an internal standard. | |||||
| 1 | Toluene | K3PO4 | 4 h | 1 mol% | 1% |
| 2 | Dioxane | K3PO4 | 4 h | 1 mol% | 3% |
| 3 | THF | K3PO4 | 4 h | 1 mol% | 2% |
| 4 | MeCN | K3PO4 | 4 h | 1 mol% | 8% |
| 5 | DMSO | K3PO4 | 4 h | 1 mol% | 47% |
| 6 | DMF | t-BuOK | 4 h | 1 mol% | 0% |
| 7 | DMF | KHMDS | 4 h | 1 mol% | 0% |
| 8 | DMF | K2CO3 | 4 h | 1 mol% | 22% |
| 9 | DMF | K3PO4 | 4h | 1 mol% | 22% |
| 10 | DMF | K3PO4 | 4 h | 1 mol% | 61% |
| 11 | DMF | K3PO4 | 24 h | 2 mol% | 90% |
With satisfactory conditions developed for the model substrate, we proceeded to investigate the scope of the reaction. First, we examined the performance of various aryl and heteroaryl bromides in the reaction with malonate 1 (Table 2).
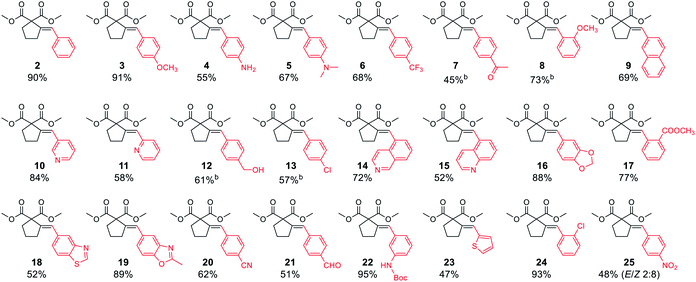
Both electron-rich and electron-poor bromoarenes smoothly underwent the reaction, affording the expected products with good to excellent yields and complete stereoselectivity on the olefinic bond. A range of functional groups including, inter alia, unprotected amines (4), alcohols (12), aldehydes (21), nitriles (20), nitro (25), carbamates (22), or enolizable ketones (7) were well tolerated. Furthermore, sterically hindered o-substituted bromo(hetero)arenes also proved to be complementary reaction partners (8, 17, 24). The use of various heteroaryl bromides enabled the introduction of the heterocyclic moiety to the product (10–11, 14–16, 18–19, 23), including pharmaceutically relevant N-heterocyclic motifs (10–11, 14–16, 18–19).
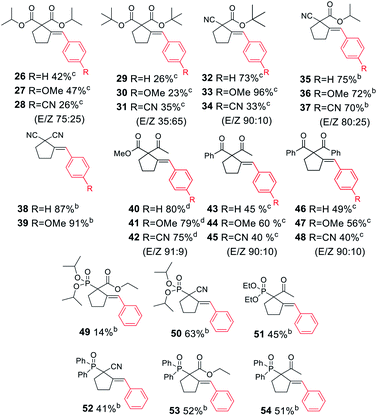
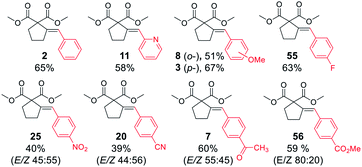
Next, we proceeded to examine the scope and limitations with respect to various acetylenic active methylene compounds (Table 3). Selected derivatives of malonates, cyanoacetates, cyanomalonates, β-ketoesters, and 1,3-diketones were subjected to the reaction with both electron-poor and electron-rich bromoarenes – bromobenzene, p-bromoanisole, and p-bromobenzonitrile. Transformations with more sterically hindered i-propyl and t-butyl malonates delivered the expected products (26–31), although with diminished yields, compared to the less sterically demanding methyl malonate 1.
The considerably more C–H acidic cyanoacetates, cyanomalonates, and β-ketoesters appeared to be the more reactive substrates, usually providing the appropriate products (32–43) with very good yields (70–96%). The only exception was a reaction of electron-deficient bromoarenes with cyanomalonate and t-butyl cyanoacetate, which afforded products (34 and 40) with moderate yields (33–40%). Notably, reactions involving electron-deficient bromoarenes and all of the above-mentioned acetylenic substrates proceeded with high, but not complete diastereoselectivity (E/Z selectivity). All reactions involving electronically neutral, or electron-rich bromoarenes provided complete selectivity.
Next, we investigated various phosphorus-substituted acetylenes as potential reaction partners. We were pleased to find that esters, ketones, and nitriles bearing phosphoryl or phosphinoyl functions entered the reaction with bromobenzene, affording the target cyclopentanes (49–54) with moderate to good yields and complete diastereoselectivity. Compound 49 was isolated with a low yield due to difficulties in the isolation and purification.
Finally, we were pleased to find that the developed protocol is also applicable to the remarkably less active aryl chlorides (Table 4). Both electron-rich and electron-deficient chloroarenes, as well as heteroaryl chlorides (2-chloropyridine) entered the reaction, yielding the expected products in moderate to good yields (39–69%). Interestingly, electron-deficient chloroarenes gave products with low diastereoselectivity, in contrast to their corresponding aryl bromides which provided the products as single isomers (except 4-nitrobromobenzene).
| a Reaction conditions: dimethyl pent-4-yn-1-ylmalonate 1 (0.400 mmol), aryl chloride (0.500 mmol), K3PO4 (0.600 mmol), XPhos Pd G3 (8.0 μmol, 2 mol%), DMF (1 ml), 80 °C, 24 h. |
|---|
 |
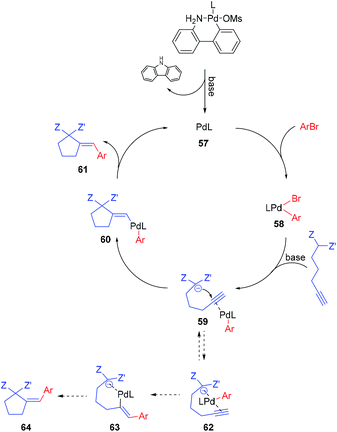
The postulated mechanism, based on the observations of the reaction outcome, several control experiments, and literature data, is depicted in Scheme 1. First, the bromoarene undergoes fast oxidative addition to Pd(0) complex 57 (formed upon the activation of the precatalyst with a base)15 leading to the formation of aryl–Pd(II) species 58 which coordinates to the alkyne moiety. Then, intramolecular nucleophilic addition to the activated unsaturated system occurs, providing vinyl–Pd(II) species 60 which undergoes facile reductive elimination affording the expected product 61 and reconstituting the Pd(0) complex 57. Although the above mechanism seems viable for the majority of the investigated reactions, for some specific combinations of substrates, alternative scenarios should also be considered. For instance, the formation of chelate 62 (possibly being in equilibrium with 59), in which palladium is bound by both alkyne and active methylene moieties, could facilitate the insertion of the Pd–arene to the alkyne (syn-carbometallation), and thus rationalize the formation of some amount of another diastereoisomer of the product with altered configuration at the exocyclic double bond (64).
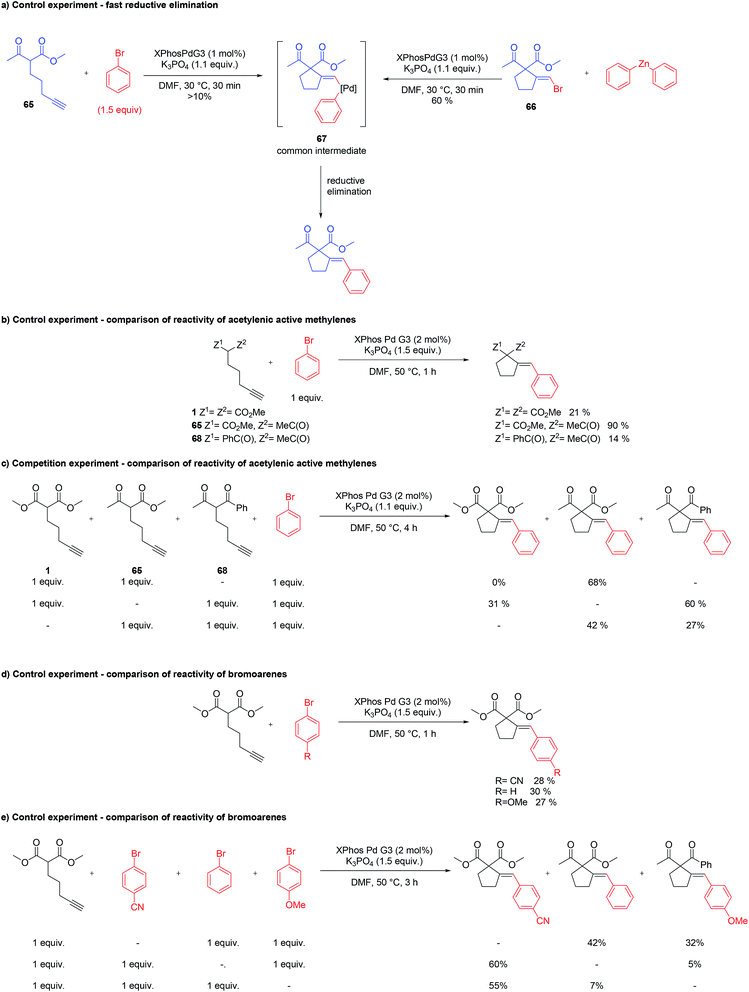
Oxidative addition to Pd(0) ligated to a single electron-rich monophosphine is fast. In fact, oxidative addition of bromoarene to XPhos–Pd(0) complex proceeds within minutes at room temperature, as observed by 31P NMR spectroscopy. Reductive elimination from Pd complexes of sterically demanding ligands is also facile. In particular, we have recently shown that the reductive elimination is not a rate-limiting step in the XPhos–Pd-catalyzed tandem cyclization/coupling of ε-acetylenic β-ketoesters with aryl bromides (Scheme 2a).8 The tandem reaction of ketoester 65 with bromobenzene is much slower than Negishi coupling of compound 66 with diphenylzinc, both proceeding through reductive elimination from a common intermediate 67. This points towards the conclusion that the cyclization step is a bottleneck of the transformation. In order to shed more light on the influence of the structure of reagents on the reaction outcome, we compared the rate of reactions of bromobenzene with three acetylenic substrates – derivatives of malonate 1, β-ketoester 65, and β-diketone 68 (Scheme 2b). As expected, malonate 1 reacted significantly slower than ketoester 65, providing the corresponding product in only 21% yield after 1 h, compared to 90% for 65. This is due to considerably lower C–H acidity of the malonate. Surprisingly, under identical conditions, the more C–H acidic β-diketone 68 delivered the product with only 14% yield. Competition experiments, involving pairs of acetylenic substrates (1 equiv. of each) and bromobenzene (1 equiv.) were also conducted (Scheme 2c). A reaction involving ketoester 65 and malonate 1 delivered only the product of the cyclization/coupling of 65, demonstrating the huge difference in their reactivity. Despite diketone 68 reacting slower than malonate in a parallel experiment (see: Scheme 2b), in the competition experiment it provided higher yield of the corresponding product (60% and 31%, respectively). Similarly, the cyclization of ketoester and diketone occurred at comparable rates under the competition conditions (42% and 27%, respectively), in contrast to the parallel experiment (90% vs. 14%). The remarkably slow reaction of diketone 68 could be attributed either to the lower nucleophilicity of its enolate due to extended resonance stabilization, or the capability for the formation of stable complexes with palladium.16 The relatively stable palladium complex with diketone (or its anion) could possibly be in tautomeric equilibrium with Pd–alkyne complex suitable for intramolecular nucleophilic addition leading to 61. Thus, the involvement of arylpalladium 58 in complexation with diketone 68 could make it less available for the catalytic transformation of the more reactive ketoester 65 in the competition experiment.
Competition experiments of malonate 1 with pairs of electronically divergent bromoarenes revealed the preference for the reaction with the more electron-deficient substrate (Scheme 2e). This stays in contrast with the outcome of the parallel experiments of 1 with each of the above bromoarenes showing comparable rates (Scheme 2d). Apparently, oxidative addition is not a rate limiting step, although in control experiments it determines the ratio of aryl–Pd(II) intermediates, which in turn dictates the final product distribution.
Another factor used for better understanding the reaction mechanism is the stereochemical outcome of the transformation. All of the reactions with malonates proceeded with complete diastereoselectivity, arising from anti-carbopalladation of the alkyne moiety. Similarly, other acetylenic active methylene compounds delivered the corresponding products as single isomers, unless electron-deficient bromoarenes (e.g. p-bromobenzonitrile) were used as coupling partners. In this case, the isomer with the alternate configuration on the double bond was formed to some extent, suggesting an alternative pathway for these sets of substrates (Scheme 1, dashed lines).
Experimental
All manipulations were performed in a nitrogen-filled glovebox or under an argon atmosphere using Schlenk techniques, unless mentioned otherwise. Flash chromatography was performed using Merck silica gel 60 (230–400 mesh). TLC analysis of reaction mixtures was performed on Merck silica gel 60 F254 TLC plates and visualized with cerium molybdate stain (Hanessian's stain). 1H, 13C{1H}, and 19F NMR spectra were recorded with a Bruker AV 400 spectrometer. 1H and 13C chemical shifts are given in ppm relative to TMS. Solvent signals were used as references (CDCl3δH = 7.26 ppm, δC = 77.0 ppm) and the chemical shift converted to the TMS scale. Coupling constants (J) are reported in Hz, and the following abbreviations were used to denote multiplets: s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet (denotes a complex pattern), dd = doublet of doublets, dt = doublet of triplets and br = broad signal. Infrared spectra were recorded with a Jasco FTIR-6200 spectrometer. Electron ionization high-resolution mass spectra (EI-HR) were recorded with an Autospec Premier (Waters Inc) mass spectrometer using the narrow-range high-voltage scan technique with low-boiling perfluorokerosene (PFK) as internal standard. Samples were introduced by using a heated direct insertion probe. Electrospray ionization high-resolution mass spectra (ESI-HR) were recorded with MALDISynapt G2-S HDMS (Waters Inc) mass spectrometer equipped with an electrospray ion source and q-TOF type mass analyzer. ESI-MS spectra were recorded in the positive ion mode (source parameters: capillary voltage 3.15 kV, sampling cone 25 V, source temperature 120 °C, desolvation temperature 150 °C).Unless otherwise noted, all commercially available compounds (ABCR, Acros, Fluorochem, TCI, Sigma-Aldrich, Strem) were used as received. Phosphine ligands were purchased from Aldrich or Fluorochem, Pd(OAc)2 was purchased from Strem. Buchwald-type 3rd-generation palladacyclic precatalysts (Ligand Pd G3) were prepared following literature procedures,15 and showed similar reactivity to the commercial samples (XPhos Pd G3 was compared with commercial samples). Dimethyl pent-4-yn-1-ylmalonate 1 and other acetylenic active methylene compounds were synthesized by alkylation of dimethyl malonate or other C–H acids with 1-iodo-pentyne, according to typical literature procedures.
General procedure A for Pd-catalyzed carbocyclization-coupling of aryl bromides with acetylenic active methylene compounds
In a drybox, a 4 ml screw-cap vial was charged with XPhos Pd G3 (6.8 mg, 8 μmol), aryl halide (0.5 mmol), K3PO4 (127.2 mg, 0.6 mmol), DMF (1 ml), and a magnetic stirring bar. Then, acetylenic active methylene compound (e.g. dimethyl pent-4-yn-1-ylmalonate 1) was added (0.4 mmol), the vial was tightly sealed and removed from drybox. The reaction mixture was stirred for 24 h at 50 °C in a heating block, then cooled to room temperature, quenched with 20 ml of an NH4Cl solution, added to 10 ml of water, and extracted with MTBE (3 × 10 ml). The combined organic phases were dried with Na2SO4, filtered, and concentrated. The crude product was purified by column chromatography on silica gel.Dimethyl (2E)-2-benzylidenecyclopentane-1,1-dicarboxylate (2)
Prepared in reaction of dimethyl 4-pentenylmalonate and bromobenzene following general procedure (105 mg, 90%) or in reaction with chlorobenzene following modified general procedure (run at 80 °C) (71 mg, yield 65%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.37–7.30 (m, 4H), 7.24–7.19 (m, 1H), 6.71 (t, J = 2.4 Hz, 1H), 3.78 (s, 6H), 2.72 (td, J = 7.2, 2.5 Hz, 2H), 2.40 (t, J = 6.9 Hz, 2H), 1.84 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.0, 137.6, 128.7, 128.2, 127.4, 126.8, 65.4, 52.8, 35.7, 32.0, 24.8; IR (CH2Cl2): 3053, 3024, 2953, 2878, 2842, 1733, 1431, 1263, 1152, 773, 696 cm−1; HRMS (ESI) m/z calcd for C16H18O4Na 297.1103; found 297.1097.
5). 1H NMR (400 MHz, CDCl3) δ 7.37–7.30 (m, 4H), 7.24–7.19 (m, 1H), 6.71 (t, J = 2.4 Hz, 1H), 3.78 (s, 6H), 2.72 (td, J = 7.2, 2.5 Hz, 2H), 2.40 (t, J = 6.9 Hz, 2H), 1.84 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.0, 137.6, 128.7, 128.2, 127.4, 126.8, 65.4, 52.8, 35.7, 32.0, 24.8; IR (CH2Cl2): 3053, 3024, 2953, 2878, 2842, 1733, 1431, 1263, 1152, 773, 696 cm−1; HRMS (ESI) m/z calcd for C16H18O4Na 297.1103; found 297.1097.
Dimethyl (2E)-2-(4-methoxybenzylidene)cyclopentane-1,1-dicarboxylate (3)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromoanisole following general procedure (110 mg, yield 91%) or in reaction with 4-chloroanisole following modified general procedure (run at 80 °C) (81 mg, yield 67%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 6.89–6.84 (m, 2H), 6.63 (t, J = 2.6 Hz, 1H), 3.80 (s, 3H), 3.76 (s, 6H), 2.69 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.5, 158.4, 138.7, 130.4, 129.9, 126.8, 113.6, 65.3, 55.2, 52.7, 35.7, 31.9, 24.8; IR (CH2Cl2): 2954, 2838, 1732, 1606, 1512, 1435, 1251, 1177, 1033, 826 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1196.
20). 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 2H), 6.89–6.84 (m, 2H), 6.63 (t, J = 2.6 Hz, 1H), 3.80 (s, 3H), 3.76 (s, 6H), 2.69 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.5, 158.4, 138.7, 130.4, 129.9, 126.8, 113.6, 65.3, 55.2, 52.7, 35.7, 31.9, 24.8; IR (CH2Cl2): 2954, 2838, 1732, 1606, 1512, 1435, 1251, 1177, 1033, 826 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1196.
Dimethyl (2E)-2-(4-aminobenzylidene)cyclopentane-1,1-dicarboxylate (4)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromoaniline following general procedure (63 mg, yield 55%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 → 70
20 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 1H NMR (400 MHz, CDCl3) δ 7.16 (d, J = 8.4 Hz, 2H), 6.64 (d, J = 8.4 Hz, 2H), 6.57 (t, J = 2.3 Hz, 1H), 3.75 (s, 6H), 2.68 (td, J = 7.2, 2.4 Hz, 2H), 2.36 (t, J = 7.0 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.7, 145.3, 137.2, 129.9, 128.2, 127.2, 114.7, 65.3, 52.7, 35.8, 31.9, 24.9; IR (CH2Cl2): 3467, 3378, 2953, 1728, 1623, 1516, 1264, 1180, 1153, 825, 526 cm−1; MS (EI): m/z (%) = 290(21), 289(67)[M+], 231(29), 230(100), 229(21), 202(19), 171(30), 170(94), 143(21), 106(22), 73(38), 57(28), 55(17), 43(23); HRMS (EI): m/z calcd for C16H19NO4 289.1314; found 289.1316.
30) 1H NMR (400 MHz, CDCl3) δ 7.16 (d, J = 8.4 Hz, 2H), 6.64 (d, J = 8.4 Hz, 2H), 6.57 (t, J = 2.3 Hz, 1H), 3.75 (s, 6H), 2.68 (td, J = 7.2, 2.4 Hz, 2H), 2.36 (t, J = 7.0 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.7, 145.3, 137.2, 129.9, 128.2, 127.2, 114.7, 65.3, 52.7, 35.8, 31.9, 24.9; IR (CH2Cl2): 3467, 3378, 2953, 1728, 1623, 1516, 1264, 1180, 1153, 825, 526 cm−1; MS (EI): m/z (%) = 290(21), 289(67)[M+], 231(29), 230(100), 229(21), 202(19), 171(30), 170(94), 143(21), 106(22), 73(38), 57(28), 55(17), 43(23); HRMS (EI): m/z calcd for C16H19NO4 289.1314; found 289.1316.
Dimethyl (2E)-2-(4-(dimethylamino)benzylidene)cyclopentane-1,1-dicarboxylate (5)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromo-N,N-dimethylaniline following general procedure (85 mg, yield 67%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 70
10 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 2H), 6.70 (d, J = 8.8 Hz, 2H), 6.60 (t, J = 2.3 Hz, 1H), 3.76 (s, 6H), 2.96 (s, 6H), 2.72 (td, J = 7.2, 2.4 Hz, 2H), 2.38 (t, J = 7.0 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.8, 149.3, 136.5, 129.7, 127.2, 126.2, 112.1, 65.3, 52.6, 40.4, 35.8, 31.9, 24.9; IR (CH2Cl2): 2952, 2881, 2804, 1730, 1608, 1522, 1434, 1355, 1247, 1162, 1064, 813, 530 cm−1; MS (EI): m/z (%) = 318(21), 317(69)[M+], 259(29), 258(100), 199(20), 198(49), 171(12), 153(9), 134(13), 77(5), 59(7); HRMS (EI): m/z calcd for C18H23NO4 317.1627; found 317.1636.
30). 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 2H), 6.70 (d, J = 8.8 Hz, 2H), 6.60 (t, J = 2.3 Hz, 1H), 3.76 (s, 6H), 2.96 (s, 6H), 2.72 (td, J = 7.2, 2.4 Hz, 2H), 2.38 (t, J = 7.0 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.8, 149.3, 136.5, 129.7, 127.2, 126.2, 112.1, 65.3, 52.6, 40.4, 35.8, 31.9, 24.9; IR (CH2Cl2): 2952, 2881, 2804, 1730, 1608, 1522, 1434, 1355, 1247, 1162, 1064, 813, 530 cm−1; MS (EI): m/z (%) = 318(21), 317(69)[M+], 259(29), 258(100), 199(20), 198(49), 171(12), 153(9), 134(13), 77(5), 59(7); HRMS (EI): m/z calcd for C18H23NO4 317.1627; found 317.1636.
Dimethyl (2E)-2-(4-(trifluoromethyl)benzylidene)cyclopentane-1,1-dicarboxylate (6)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromobenzotrifluoride following general procedure (91 mg, yield 67%) or in reaction with 4-chlorobenzotrifluoride following modified general procedure (run at 80 °C) (76 mg, yield 56%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt/DCM 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5
9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5). 1H NMR (400 MHz, CDCl3 δ 7.58 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H), 6.74 (s, 1H), 3.78 (s, 6H), 2.71 (td, J = 7.2, 2.5 Hz, 2H), 2.41 (t, J = 7.0 Hz, 2H), 1.85 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 143.8, 141.1, 128.8, 128.6 (q, J = 32.5 Hz), 126.3, 125.0 (q, J = 3.6 Hz), 124.2 (q, J = 271.8 Hz) 65.5, 52.9, 35.6, 32.1, 24.7; 19F NMR (376 MHz, CDCl3) δ −62.52; IR (CH2Cl2): 2956, 1735, 1615, 1435, 1327, 1265, 1125, 1068, 830, 598 cm−1; MS (EI): m/z (%) = 343(12), 342(39)[M+], 323(18), 310(24), 283(40), 282(61), 252(25), 251(83), 250(41), 224(27), 223(100), 159(37), 77(14), 59(38); HRMS (EI): m/z calcd for C17H17F3O4 342.1079; found 342.1084.
0.5). 1H NMR (400 MHz, CDCl3 δ 7.58 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H), 6.74 (s, 1H), 3.78 (s, 6H), 2.71 (td, J = 7.2, 2.5 Hz, 2H), 2.41 (t, J = 7.0 Hz, 2H), 1.85 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 143.8, 141.1, 128.8, 128.6 (q, J = 32.5 Hz), 126.3, 125.0 (q, J = 3.6 Hz), 124.2 (q, J = 271.8 Hz) 65.5, 52.9, 35.6, 32.1, 24.7; 19F NMR (376 MHz, CDCl3) δ −62.52; IR (CH2Cl2): 2956, 1735, 1615, 1435, 1327, 1265, 1125, 1068, 830, 598 cm−1; MS (EI): m/z (%) = 343(12), 342(39)[M+], 323(18), 310(24), 283(40), 282(61), 252(25), 251(83), 250(41), 224(27), 223(100), 159(37), 77(14), 59(38); HRMS (EI): m/z calcd for C17H17F3O4 342.1079; found 342.1084.
Dimethyl (2E)-2-(4-acetylbenzylidene)cyclopentane-1,1-dicarboxylate (7)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromoacetophenone following general procedure (57 mg, yield 45%) or in reaction with 4-chloroacetophenone following modified general procedure (run at 80 °C) (75 mg, yield 60%, isomer E/Z 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90
45). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 80
10 → 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3): δ 7.92–7.88 (m, 2H), 7.43–7.38 (m, 2H), 6.73 (t, J = 2.6 Hz, 1H), 3.76 (s, 6H), 2.71 (td, J = 7.2, 2.6 Hz, 2H), 2.57 (s, 3H), 2.39 (t, J = 6.9 Hz, 2H), 1.84 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 197.5, 171.0, 144.0, 142.2, 135.2, 128.7, 128.2, 126.6, 65.6, 52.8, 35.6, 32.2, 26.5, 24.7; IR (CH2Cl2): 2954, 1732, 1682, 1602, 1435, 1360, 1268, 590 cm−1; HRMS (ESI): m/z calcd for C18H20O5Na 339.1208; found: 339.1201.
20). 1H NMR (400 MHz, CDCl3): δ 7.92–7.88 (m, 2H), 7.43–7.38 (m, 2H), 6.73 (t, J = 2.6 Hz, 1H), 3.76 (s, 6H), 2.71 (td, J = 7.2, 2.6 Hz, 2H), 2.57 (s, 3H), 2.39 (t, J = 6.9 Hz, 2H), 1.84 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3): δ 197.5, 171.0, 144.0, 142.2, 135.2, 128.7, 128.2, 126.6, 65.6, 52.8, 35.6, 32.2, 26.5, 24.7; IR (CH2Cl2): 2954, 1732, 1682, 1602, 1435, 1360, 1268, 590 cm−1; HRMS (ESI): m/z calcd for C18H20O5Na 339.1208; found: 339.1201.
Dimethyl (2E)-2-(2-methoxybenzylidene)cyclopentane-1,1-dicarboxylate (8)
Prepared in reaction of dimethyl 4-pentenylmalonate and 2-bromoanisole following general procedure (88 mg, yield 73%) or in reaction with 2-chloroanisole following modified general procedure (run at 80 °C) (63 mg, yield 51%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 7.6, 1.7 Hz, 1H), 7.23–7.17 (m, 1H), 6.97 (t, J = 2.6 Hz, 1H), 6.92 (td, J = 7.5, 1.2 Hz, 1H), 6.85 (dd, J = 8.3, 1.1 Hz, 1H), 3.81 (s, 3H), 3.78 (s, 6H), 2.64 (td, J = 7.2, 2.6 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.80 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.5, 157.0, 140.5, 128.9, 128.1, 126.7, 122.3, 120.0, 110.5, 64.9, 55.5, 52.6, 35.7, 31.8, 24.7; IR (CH2Cl2): 2953, 2839, 1732, 1597, 1487, 1461, 1436, 1248, 1136, 755 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1203.
15). 1H NMR (400 MHz, CDCl3) δ 7.34 (dd, J = 7.6, 1.7 Hz, 1H), 7.23–7.17 (m, 1H), 6.97 (t, J = 2.6 Hz, 1H), 6.92 (td, J = 7.5, 1.2 Hz, 1H), 6.85 (dd, J = 8.3, 1.1 Hz, 1H), 3.81 (s, 3H), 3.78 (s, 6H), 2.64 (td, J = 7.2, 2.6 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.80 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.5, 157.0, 140.5, 128.9, 128.1, 126.7, 122.3, 120.0, 110.5, 64.9, 55.5, 52.6, 35.7, 31.8, 24.7; IR (CH2Cl2): 2953, 2839, 1732, 1597, 1487, 1461, 1436, 1248, 1136, 755 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1203.
Dimethyl (2E)-2-(naphthalen-2-ylmethylidene)cyclopentane-1,1-dicarboxylate (9)
Prepared in reaction of dimethyl 4-pentenylmalonate and 2-bromonaphthalene following general procedure (90 mg, yield 69%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.84–7.79 (m, 4H), 7.52 (dd, J = 8.5, 1.4 Hz, 1H), 7.48–7.44 (m, 2H), 6.90 (t, J = 2.3 Hz, 1H), 3.82 (s, 6H), 2.84 (td, J = 7.2, 2.5 Hz, 2H), 2.46 (t, J = 7.0 Hz, 2H), 1.89 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.5, 135.1, 133.3, 132.23, 128.0, 127.6, 127.6, 127.5, 126.8, 126.0, 125.8, 65.5, 52.8, 35.7, 32.1, 24.8; IR (CH2Cl2): 3053, 2953, 2879, 1732, 1434, 1262, 1065, 1016, 817, 748, 477 cm−1; HRMS (ESI): m/z calcd for C20H20O4Na 347.1248; found 347.1259.
10). 1H NMR (400 MHz, CDCl3) δ 7.84–7.79 (m, 4H), 7.52 (dd, J = 8.5, 1.4 Hz, 1H), 7.48–7.44 (m, 2H), 6.90 (t, J = 2.3 Hz, 1H), 3.82 (s, 6H), 2.84 (td, J = 7.2, 2.5 Hz, 2H), 2.46 (t, J = 7.0 Hz, 2H), 1.89 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.5, 135.1, 133.3, 132.23, 128.0, 127.6, 127.6, 127.5, 126.8, 126.0, 125.8, 65.5, 52.8, 35.7, 32.1, 24.8; IR (CH2Cl2): 3053, 2953, 2879, 1732, 1434, 1262, 1065, 1016, 817, 748, 477 cm−1; HRMS (ESI): m/z calcd for C20H20O4Na 347.1248; found 347.1259.
Dimethyl (2E)-2-(pyridin-3-ylmethylidene)cyclopentane-1,1-dicarboxylate (10)
Prepared in reaction of dimethyl 4-pentenylmalonate and 3-bromopyridine following general procedure (92 mg, yield 84%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 → 70
20 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 2.0 Hz, 1H), 8.41 (dd, J = 4.8, 1.5 Hz, 1H), 7.61 (dt, J = 7.9, 1.7 Hz, 1H), 7.24–7.19 (m, 1H), 6.64 (t, J = 2.4 Hz, 1H), 3.75 (s, 6H), 2.67 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 7.0 Hz, 2H), 1.82 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.0, 150.0, 147.7, 143.6, 135.1, 133.2, 124.0, 123.0, 65.4, 52.8, 35.6, 32.0, 24.7; IR (CH2Cl2): 3027, 2953, 2879, 1732, 1567, 1434, 1266, 1065, 1021, 804, 710 cm−1; HRMS (ESI): m/z calcd for C15H17NO4 276.1212; found 276.1232.
30). 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 2.0 Hz, 1H), 8.41 (dd, J = 4.8, 1.5 Hz, 1H), 7.61 (dt, J = 7.9, 1.7 Hz, 1H), 7.24–7.19 (m, 1H), 6.64 (t, J = 2.4 Hz, 1H), 3.75 (s, 6H), 2.67 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 7.0 Hz, 2H), 1.82 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.0, 150.0, 147.7, 143.6, 135.1, 133.2, 124.0, 123.0, 65.4, 52.8, 35.6, 32.0, 24.7; IR (CH2Cl2): 3027, 2953, 2879, 1732, 1567, 1434, 1266, 1065, 1021, 804, 710 cm−1; HRMS (ESI): m/z calcd for C15H17NO4 276.1212; found 276.1232.
Dimethyl (2E)-2-(pyridin-2-ylmethylidene)cyclopentane-1,1-dicarboxylate (11)
Prepared in reaction of dimethyl 4-pentenylmalonate and 2-bromopyridine following general procedure (64 mg, yield 58%) or in reaction with 2-chloropyridine following modified general procedure (run at 80 °C) (60 mg, yield 55%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 → 70
20 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (400 MHz, CDCl3) δ 8.57 (dd, J = 4.8, 1.9, 0.9 Hz, 1H), 7.59 (td, J = 7.7, 1.9 Hz, 1H), 7.28–7.20 (m, 1H), 7.07–7.02 (m, 1H), 6.74 (t, J = 2.7 Hz, 1H), 3.74 (s, 6H), 2.94 (td, J = 7.3, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 156.4, 149.1, 146.1, 135.8, 126.6, 124.07, 121.0, 65.8, 52.8, 35.6, 32.7, 24.6; IR (CH2Cl2): 3050, 2954, 2280, 1732, 1584, 1438, 1433, 1263, 1151, 738, 747 cm−1; HRMS (ESI): m/z calcd for C15H17NO4 276.1236; found 276.1223.
30). 1H NMR (400 MHz, CDCl3) δ 8.57 (dd, J = 4.8, 1.9, 0.9 Hz, 1H), 7.59 (td, J = 7.7, 1.9 Hz, 1H), 7.28–7.20 (m, 1H), 7.07–7.02 (m, 1H), 6.74 (t, J = 2.7 Hz, 1H), 3.74 (s, 6H), 2.94 (td, J = 7.3, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 156.4, 149.1, 146.1, 135.8, 126.6, 124.07, 121.0, 65.8, 52.8, 35.6, 32.7, 24.6; IR (CH2Cl2): 3050, 2954, 2280, 1732, 1584, 1438, 1433, 1263, 1151, 738, 747 cm−1; HRMS (ESI): m/z calcd for C15H17NO4 276.1236; found 276.1223.
Dimethyl (2E)-2-(4-(hydroxymethyl)benzylidene)cyclopentane-1,1-dicarboxylate (12)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromobenzyl alcohol following general procedure (72 mg, yield 60%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). 1H NMR (400 MHz, CDCl3) δ 7.34–7.27 (m, 4H), 6.67 (t, J = 2.5 Hz, 1H), 4.64 (s, 2H), 3.75 (s, 6H), 2.68 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 2.11 (s, 1H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.0, 139.5, 136.9, 128.8, 127.1, 126.7, 65.4, 64.8, 52.8, 35.7, 32.0, 24.7; IR (CH2Cl2): 3426, 2953, 2877, 1730, 1435, 1265, 1163, 1013 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1205.
40). 1H NMR (400 MHz, CDCl3) δ 7.34–7.27 (m, 4H), 6.67 (t, J = 2.5 Hz, 1H), 4.64 (s, 2H), 3.75 (s, 6H), 2.68 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 2.11 (s, 1H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 141.0, 139.5, 136.9, 128.8, 127.1, 126.7, 65.4, 64.8, 52.8, 35.7, 32.0, 24.7; IR (CH2Cl2): 3426, 2953, 2877, 1730, 1435, 1265, 1163, 1013 cm−1; HRMS (ESI): m/z calcd for C17H20O5Na 327.1208; found 327.1205.
Dimethyl (2E)-2-(4-chlorobenzylidene)cyclopentane-1,1-dicarboxylate (13)
Prepared in reaction of dimethyl 4-pentenylmalonate and 1-bromo-4-chlorobenzene following general procedure (70 mg, 57%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3): δ 7.31–7.23 (m, 4H), 6.65 (t, J = 2.5 Hz, 1H), 3.76 (s, 6H), 2.66 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.88–1.80 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 171.2, 141.7, 136.0, 132.5, 129.9, 128.3, 126.3, 65.4, 52.8, 35.7, 32.0, 24.7; IR (CH2Cl2): 2953, 1733, 1491, 1434, 1265, 821, 519 cm−1; HRMS (ESI): m/z calcd for C16H17O4ClNa 331.0713; found 331.0706.
10). 1H NMR (400 MHz, CDCl3): δ 7.31–7.23 (m, 4H), 6.65 (t, J = 2.5 Hz, 1H), 3.76 (s, 6H), 2.66 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.88–1.80 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 171.2, 141.7, 136.0, 132.5, 129.9, 128.3, 126.3, 65.4, 52.8, 35.7, 32.0, 24.7; IR (CH2Cl2): 2953, 1733, 1491, 1434, 1265, 821, 519 cm−1; HRMS (ESI): m/z calcd for C16H17O4ClNa 331.0713; found 331.0706.
Dimethyl (2E)-2-(isoquinolin-5-ylmethylidene)cyclopentane-1,1-dicarboxylate (14)
Prepared in reaction of dimethyl 4-pentenylmalonate and 5-bromoisoquinoline following general procedure (93 mg, yield 72%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 → 60
30 → 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H), 8.53 (d, J = 6.0 Hz, 1H), 7.82 (dd, J = 15.3, 6.9 Hz, 2H), 7.61–7.52 (m, 2H), 7.19 (s, 1H), 3.82 (s, 6H), 2.49–2.42 (m, 4H), 1.75 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.2, 152.7, 144.2, 143.2, 134.3, 134.1, 129.5, 128.6, 126.7, 126.5, 123.7, 117.5, 64.6, 52.9, 35.9, 31.6, 24.4; IR (CH2Cl2): 2953, 1732, 1617, 1584, 1434, 1261, 1152, 832, 762, 475 cm−1; MS (EI): m/z (%) = 326(12), 325(57)[M+], 275(9), 267(4), 234(75), 207(30), 206(100), 204(26), 156(13), 142(14), 98(2), 77(6), 43(12); HRMS (EI): m/z calcd for C19H19NO4 325.1314, found: 325.1317.
40). 1H NMR (400 MHz, CDCl3) δ 9.21 (s, 1H), 8.53 (d, J = 6.0 Hz, 1H), 7.82 (dd, J = 15.3, 6.9 Hz, 2H), 7.61–7.52 (m, 2H), 7.19 (s, 1H), 3.82 (s, 6H), 2.49–2.42 (m, 4H), 1.75 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.2, 152.7, 144.2, 143.2, 134.3, 134.1, 129.5, 128.6, 126.7, 126.5, 123.7, 117.5, 64.6, 52.9, 35.9, 31.6, 24.4; IR (CH2Cl2): 2953, 1732, 1617, 1584, 1434, 1261, 1152, 832, 762, 475 cm−1; MS (EI): m/z (%) = 326(12), 325(57)[M+], 275(9), 267(4), 234(75), 207(30), 206(100), 204(26), 156(13), 142(14), 98(2), 77(6), 43(12); HRMS (EI): m/z calcd for C19H19NO4 325.1314, found: 325.1317.
Dimethyl (2E)-2-(quinolin-5-ylmethylidene)cyclopentane-1,1-dicarboxylate (15)
Prepared in reaction of dimethyl 4-pentenylmalonate and 5-bromoquinoline following general procedure (68 mg, yield 52%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 → 60
30 → 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). 1H NMR (400 MHz, CDCl3) δ 8.88 (dd, J = 4.2, 1.6 Hz, 1H), 8.41–8.36 (m, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.67–7.62 (m, 1H), 7.44–7.37 (m, 2H), 7.18 (s, 1H), 3.81 (s, 6H), 2.44 (td, J = 7.0, 2.1 Hz, 4H), 1.73 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 150.1, 148.3, 144.1, 135.4, 133.2, 128.7, 128.6, 126.8, 126.0, 124.1, 120.9, 64.5, 52.9, 35.9, 31.5, 24.4; IR (CH2Cl2): 2952, 1731, 1593, 1572, 1434, 1254, 1148, 806 cm−1; MS (EI): m/z (%) = 326(10), 325(41)[M+], 265(23), 248(20), 235(27), 234(73), 207(35), 206(100), 204(36), 152(9), 142(24), 59(11); HRMS (EI): m/z calcd for C19H19NO4 325.1314; found 325.1312.
40). 1H NMR (400 MHz, CDCl3) δ 8.88 (dd, J = 4.2, 1.6 Hz, 1H), 8.41–8.36 (m, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.67–7.62 (m, 1H), 7.44–7.37 (m, 2H), 7.18 (s, 1H), 3.81 (s, 6H), 2.44 (td, J = 7.0, 2.1 Hz, 4H), 1.73 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 150.1, 148.3, 144.1, 135.4, 133.2, 128.7, 128.6, 126.8, 126.0, 124.1, 120.9, 64.5, 52.9, 35.9, 31.5, 24.4; IR (CH2Cl2): 2952, 1731, 1593, 1572, 1434, 1254, 1148, 806 cm−1; MS (EI): m/z (%) = 326(10), 325(41)[M+], 265(23), 248(20), 235(27), 234(73), 207(35), 206(100), 204(36), 152(9), 142(24), 59(11); HRMS (EI): m/z calcd for C19H19NO4 325.1314; found 325.1312.
Dimethyl (2E)-2-(1,3-benzodioxol-5-ylmethylidene)cyclopentane-1,1-dicarboxylate (16)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromo-1,2-methylenedioxybenzene following general procedure (112 mg, yield 88%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 → 50
30 → 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 1H NMR (400 MHz, CDCl3) δ 6.87 (s, 1H), 6.83–6.74 (m, 2H), 6.59 (t, J = 2.3 Hz, 1H), 5.93 (s, 2H), 3.76 (s, 6H), 2.67 (td, J = 7.1, 2.4 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 147.5, 146.4, 139.3, 131.9, 127.1, 123.0, 108.6, 108.1, 100.9, 65.3, 52.7, 35.7, 31.9, 24.8; IR (CH2Cl2): 2963, 2890, 1730, 1491, 1442, 1254, 1038, 930, 809 cm−1; MS (EI): m/z (%) = 319(25), 318(81)[M+], 260(18), 259(63), 258(38), 231(29), 227(23), 200(31), 199(100), 169(36), 141(34), 135(30), 115(27), 77(13), 59(21); HRMS (EI): m/z calcd for C17H18O6 318.1103; found 318.1095.
50). 1H NMR (400 MHz, CDCl3) δ 6.87 (s, 1H), 6.83–6.74 (m, 2H), 6.59 (t, J = 2.3 Hz, 1H), 5.93 (s, 2H), 3.76 (s, 6H), 2.67 (td, J = 7.1, 2.4 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.4, 147.5, 146.4, 139.3, 131.9, 127.1, 123.0, 108.6, 108.1, 100.9, 65.3, 52.7, 35.7, 31.9, 24.8; IR (CH2Cl2): 2963, 2890, 1730, 1491, 1442, 1254, 1038, 930, 809 cm−1; MS (EI): m/z (%) = 319(25), 318(81)[M+], 260(18), 259(63), 258(38), 231(29), 227(23), 200(31), 199(100), 169(36), 141(34), 135(30), 115(27), 77(13), 59(21); HRMS (EI): m/z calcd for C17H18O6 318.1103; found 318.1095.
Dimethyl (2E)-2-(2-(methoxycarbonyl)benzylidene)cyclopentane-1,1-dicarboxylate (17)
Prepared in reaction of dimethyl 4-pentenylmalonate and methyl 2-bromobenzoate following general procedure (102 mg, yield 77%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 70
10 → 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30).1H NMR (400 MHz, CDCl3) δ 7.89 (dd, J = 7.8, 1.4 Hz, 1H), 7.48–7.39 (m, 2H), 7.30–7.25 (m, 1H), 7.19 (t, J = 2.6 Hz, 1H), 3.85 (s, 3H), 3.80 (s, 6H), 2.49 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.77 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 167.5, 141.1, 138.9, 131.4, 130.3, 129.8, 129.3, 126.9, 126.7, 64.4, 52.7, 51.9, 35.8, 31.3, 24.6; IR (CH2Cl2): 2953, 1729, 1598, 1569, 1434, 1257, 1127, 1078, 777, 740 cm−1; MS (EI): m/z (%) = 332(8)[M+], 301(12), 300(24), 273(23), 268(12), 241(42), 240(100), 213(38), 182(27), 181(72), 153(32), 128(17), 115(18), 91(12), 77(14), 59(23); HRMS (EI): m/z calcd for C18H20O6 332.1260; found 332.1255.
30).1H NMR (400 MHz, CDCl3) δ 7.89 (dd, J = 7.8, 1.4 Hz, 1H), 7.48–7.39 (m, 2H), 7.30–7.25 (m, 1H), 7.19 (t, J = 2.6 Hz, 1H), 3.85 (s, 3H), 3.80 (s, 6H), 2.49 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.77 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 167.5, 141.1, 138.9, 131.4, 130.3, 129.8, 129.3, 126.9, 126.7, 64.4, 52.7, 51.9, 35.8, 31.3, 24.6; IR (CH2Cl2): 2953, 1729, 1598, 1569, 1434, 1257, 1127, 1078, 777, 740 cm−1; MS (EI): m/z (%) = 332(8)[M+], 301(12), 300(24), 273(23), 268(12), 241(42), 240(100), 213(38), 182(27), 181(72), 153(32), 128(17), 115(18), 91(12), 77(14), 59(23); HRMS (EI): m/z calcd for C18H20O6 332.1260; found 332.1255.
Dimethyl (2E)-2-(1,3-benzothiazol-5-ylmethylidene)cyclopentane-1,1-dicarboxylate (18)
Prepared in reaction of dimethyl 4-pentenylmalonate and 5-bromobenzothiazole following general procedure (69 mg, yield 52%). Product was isolated as orange solid after column chromatography on silica gel (15 g, hex/AcOEt 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H), 8.11 (d, J = 1.7 Hz, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.41 (dd, J = 8.4, 1.7 Hz, 1H), 6.85 (t, J = 2.6 Hz, 1H), 3.78 (s, 6H), 2.78 (td, J = 7.2, 2.6 Hz, 2H), 2.41 (t, J = 6.9 Hz, 2H), 1.86 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 154.3, 153.6, 141.9, 136.1, 132.0, 126.8, 126.8, 123.1, 121.3, 65.4, 52.8, 35.7, 32.1, 24.8; IR (CH2Cl2): 2952, 1731, 1540, 1438, 1264, 1153, 1065, 849 cm−1; MS (EI): m/z (%) = 332(21), 331(62)[M+], 272(10), 241(20), 240(62), 213(29), 212(100), 186(18), 152(14), 148(28), 59(14); HRMS (EI): m/z calcd for C17H17NO4S: 331.0878; found 331.0885.
25). 1H NMR (400 MHz, CDCl3) δ 8.97 (s, 1H), 8.11 (d, J = 1.7 Hz, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.41 (dd, J = 8.4, 1.7 Hz, 1H), 6.85 (t, J = 2.6 Hz, 1H), 3.78 (s, 6H), 2.78 (td, J = 7.2, 2.6 Hz, 2H), 2.41 (t, J = 6.9 Hz, 2H), 1.86 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 154.3, 153.6, 141.9, 136.1, 132.0, 126.8, 126.8, 123.1, 121.3, 65.4, 52.8, 35.7, 32.1, 24.8; IR (CH2Cl2): 2952, 1731, 1540, 1438, 1264, 1153, 1065, 849 cm−1; MS (EI): m/z (%) = 332(21), 331(62)[M+], 272(10), 241(20), 240(62), 213(29), 212(100), 186(18), 152(14), 148(28), 59(14); HRMS (EI): m/z calcd for C17H17NO4S: 331.0878; found 331.0885.
Dimethyl (2E)-2-((2-methyl-1,3-benzoxazol-5-yl)methylidene)cyclopentane-1,1-dicarboxylate(19)
Prepared in reaction of dimethyl 4-pentenylmalonate and 5-bromo-2-methyl-1,3-benzoxazole following general procedure (118 mg, yield 89%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 2.0 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 7.23 (dd, J = 8.5, 1.8 Hz, 1H), 6.77 (t, J = 2.6 Hz, 1H), 3.75 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.59 (s, 3H), 2.37 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 164.2, 149.8, 141.6, 140.6, 134.1, 127.1, 125.9, 119.0, 109.6, 65.3, 52.7, 35.7, 31.9, 24.8, 14.4; IR (CH2Cl2): 3456, 2954, 1732, 1578, 1434, 1265, 919, 812 cm−1; MS (EI): m/z (%) = 330(15), 329(45)[M+], 269(37), 252(23), 238(59), 211(30), 210(100), 169(33), 146(28), 141(31), 115(23), 91(5), 77(9), 59(19); HRMS (EI): m/z calcd for C18H19NO5 329.1263; found 329.1274.
30). 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 2.0 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 7.23 (dd, J = 8.5, 1.8 Hz, 1H), 6.77 (t, J = 2.6 Hz, 1H), 3.75 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.59 (s, 3H), 2.37 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 164.2, 149.8, 141.6, 140.6, 134.1, 127.1, 125.9, 119.0, 109.6, 65.3, 52.7, 35.7, 31.9, 24.8, 14.4; IR (CH2Cl2): 3456, 2954, 1732, 1578, 1434, 1265, 919, 812 cm−1; MS (EI): m/z (%) = 330(15), 329(45)[M+], 269(37), 252(23), 238(59), 211(30), 210(100), 169(33), 146(28), 141(31), 115(23), 91(5), 77(9), 59(19); HRMS (EI): m/z calcd for C18H19NO5 329.1263; found 329.1274.
Dimethyl (2E)-2-(4-cyanobenzylidene)cyclopentane-1,1-dicarboxylate (20)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromobenzonitrile following general procedure (73 mg, yield 62%) or in reaction with 4-chlorobenzonitrile following modified general procedure (run at 80 °C) (47 mg, yield 39%, isomer E/Z 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 98
44). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 → 90
2 → 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.3 Hz, 2H), 7.41 (d, J = 8.3 Hz, 2H), 6.71 (t, J = 2.4 Hz, 1H), 3.76 (s, 6H), 2.68 (td, J = 7.2, 2.5 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.89–1.80 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 170.8, 145.1, 142.0, 131.9, 129.1, 126.1, 118.9, 110.1, 65.6, 52.9, 35.5, 32.2, 24.7; IR (CH2Cl2): 2955, 2225, 1732, 1604, 1435, 1264, 1115, 826, 555 cm−1; HRMS (ESI): m/z calcd for C17H17NO4Na 322.1055; found: 322.1045.
10). 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.3 Hz, 2H), 7.41 (d, J = 8.3 Hz, 2H), 6.71 (t, J = 2.4 Hz, 1H), 3.76 (s, 6H), 2.68 (td, J = 7.2, 2.5 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.89–1.80 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 170.8, 145.1, 142.0, 131.9, 129.1, 126.1, 118.9, 110.1, 65.6, 52.9, 35.5, 32.2, 24.7; IR (CH2Cl2): 2955, 2225, 1732, 1604, 1435, 1264, 1115, 826, 555 cm−1; HRMS (ESI): m/z calcd for C17H17NO4Na 322.1055; found: 322.1045.
Dimethyl (2E)-2-(4-formylbenzylidene)cyclopentane-1,1-dicarboxylate (21)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromobenzaldehyde following general procedure (62 mg, yield 51%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 80
10 → 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3) δ 9.97 (s, 1H), 7.83 (d, J = 7.9 Hz, 2H), 7.48 (d, J = 7.9 Hz, 2H), 6.75 (t, J = 2.8 Hz, 1H), 3.77 (d, J = 1.1 Hz, 6H), 2.72 (td, J = 7.3, 2.6 Hz, 2H), 2.42–2.37 (m, 2H), 1.85 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 191.7, 171.0, 144.8, 143.7, 134.6, 129.6, 129.1, 126.6, 65.6, 52.9, 35.6, 32.3, 24.8; IR (CH2Cl2): 2954, 2840, 1731, 1696, 1602, 1565, 1434, 1264, 1168, 822, 792, 523 cm−1; MS (EI): m/z (%) = 303(17), 302(63)[M+], 270(41), 243(42), 242(50), 213(21), 211(99), 210(54), 183(73), 156(27), 155(100), 153(49), 128(33), 115(31), 91(37), 77(27), 59(33); HRMS (EI): m/z calcd for C17H18O5 302.1154; found 302.1159.
20). 1H NMR (400 MHz, CDCl3) δ 9.97 (s, 1H), 7.83 (d, J = 7.9 Hz, 2H), 7.48 (d, J = 7.9 Hz, 2H), 6.75 (t, J = 2.8 Hz, 1H), 3.77 (d, J = 1.1 Hz, 6H), 2.72 (td, J = 7.3, 2.6 Hz, 2H), 2.42–2.37 (m, 2H), 1.85 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 191.7, 171.0, 144.8, 143.7, 134.6, 129.6, 129.1, 126.6, 65.6, 52.9, 35.6, 32.3, 24.8; IR (CH2Cl2): 2954, 2840, 1731, 1696, 1602, 1565, 1434, 1264, 1168, 822, 792, 523 cm−1; MS (EI): m/z (%) = 303(17), 302(63)[M+], 270(41), 243(42), 242(50), 213(21), 211(99), 210(54), 183(73), 156(27), 155(100), 153(49), 128(33), 115(31), 91(37), 77(27), 59(33); HRMS (EI): m/z calcd for C17H18O5 302.1154; found 302.1159.
Dimethyl (2E)-2-(3-((tert-butoxycarbonyl)amino)benzylidene)cyclopentane-1,1-dicarboxylate (22)
Prepared in reaction of dimethyl 4-pentenylmalonate and N-(tert-butoxycarbonyl)-3-bromoaniline following general procedure (148 mg, yield 95%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 → 80
15 → 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3) δ 7.39 (s, 1H), 7.24–7.16 (m, 2H), 7.00 (dt, J = 7.2, 1.7 Hz, 1H), 6.65 (t, J = 2.7 Hz, 1H), 6.60 (s, 1H), 3.75 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.1 Hz, 2H), 1.50 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 171.3, 152.7, 141.4, 138.4, 138.3, 128.6, 127.2, 123.4, 118.7, 117.1, 80.3, 65.4, 52.7, 35.7, 32.0, 28.3, 24.8; IR (CH2Cl2): 3361, 2976, 2955, 1729, 1538, 1435, 1237, 1160, 1065, 888, 737, 693, 463 cm−1; HRMS (EI): m/z calcd for C21H27NO6 389.1736; found 389.1736.
20). 1H NMR (400 MHz, CDCl3) δ 7.39 (s, 1H), 7.24–7.16 (m, 2H), 7.00 (dt, J = 7.2, 1.7 Hz, 1H), 6.65 (t, J = 2.7 Hz, 1H), 6.60 (s, 1H), 3.75 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.37 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.1 Hz, 2H), 1.50 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 171.3, 152.7, 141.4, 138.4, 138.3, 128.6, 127.2, 123.4, 118.7, 117.1, 80.3, 65.4, 52.7, 35.7, 32.0, 28.3, 24.8; IR (CH2Cl2): 3361, 2976, 2955, 1729, 1538, 1435, 1237, 1160, 1065, 888, 737, 693, 463 cm−1; HRMS (EI): m/z calcd for C21H27NO6 389.1736; found 389.1736.
Dimethyl (2E)-2-(thiophen-2-ylmethylidene)cyclopentane-1,1-dicarboxylate (23)
Prepared in reaction of dimethyl 4-pentenylmalonate and 2-bromothiophene following general procedure (53 mg, yield 47%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 → 90
2 → 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.31–7.27 (m, 1H), 7.04–7.01 (m, 2H), 6.93 (t, J = 2.7 Hz, 1H), 3.76 (s, 6H), 2.69 (td, J = 7.3, 2.6 Hz, 2H), 2.40 (t, J = 6.9 Hz, 2H), 1.90 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 141.3, 138.7, 127.4, 126.9, 125.6, 120.8, 65.3, 52.8, 36.1, 32.2, 24.5; IR (CH2Cl2): 2952, 1731, 1433, 1261, 1148, 701 cm−1; MS (EI): m/z (%) = 280(53)[M+], 222(36), 221(100), 220(84), 190(21), 189(62), 167(30), 161(87), 128(32), 115(20), 97(52), 77(23), 59(32); HRMS (EI): m/z calcd for C14H16O4S 280.0769; found 280.0761.
10). 1H NMR (400 MHz, CDCl3) δ 7.31–7.27 (m, 1H), 7.04–7.01 (m, 2H), 6.93 (t, J = 2.7 Hz, 1H), 3.76 (s, 6H), 2.69 (td, J = 7.3, 2.6 Hz, 2H), 2.40 (t, J = 6.9 Hz, 2H), 1.90 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 141.3, 138.7, 127.4, 126.9, 125.6, 120.8, 65.3, 52.8, 36.1, 32.2, 24.5; IR (CH2Cl2): 2952, 1731, 1433, 1261, 1148, 701 cm−1; MS (EI): m/z (%) = 280(53)[M+], 222(36), 221(100), 220(84), 190(21), 189(62), 167(30), 161(87), 128(32), 115(20), 97(52), 77(23), 59(32); HRMS (EI): m/z calcd for C14H16O4S 280.0769; found 280.0761.
Dimethyl (2E)-2-(2-chlorobenzylidene)cyclopentane-1,1-dicarboxylate (24)
Prepared in reaction of dimethyl 4-pentenylmalonate and 1-chloro-2-bromobenzene following general procedure (114 mg, yield 93%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.40–7.34 (m, 2H), 7.24–7.19 (m, 1H), 7.18–7.13 (m, 1H), 6.95 (t, J = 2.6 Hz, 1H), 3.79 (s, 6H), 2.58 (td, J = 7.2, 2.6 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 142.9, 135.7, 133.7, 129.6, 129.3, 128.1, 126.2, 124.5, 64.7, 52.8, 35.7, 31.6, 24.7; IR (CH2Cl2): 2953, 1733, 1590, 1436, 1258, 1138, 751, 606 cm−1; MS (EI): m/z (%) = 310(22), 308(48)[M+], 276(27), 249(42), 248(48), 219(42), 217(83), 213(94), 191(48), 189(100), 153(65), 125(62), 115(26), 77(27); HRMS (EI) m/z calcd for C16H17O4Cl 308.0815; found 308.0819.
10). 1H NMR (400 MHz, CDCl3) δ 7.40–7.34 (m, 2H), 7.24–7.19 (m, 1H), 7.18–7.13 (m, 1H), 6.95 (t, J = 2.6 Hz, 1H), 3.79 (s, 6H), 2.58 (td, J = 7.2, 2.6 Hz, 2H), 2.39 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.0 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.1, 142.9, 135.7, 133.7, 129.6, 129.3, 128.1, 126.2, 124.5, 64.7, 52.8, 35.7, 31.6, 24.7; IR (CH2Cl2): 2953, 1733, 1590, 1436, 1258, 1138, 751, 606 cm−1; MS (EI): m/z (%) = 310(22), 308(48)[M+], 276(27), 249(42), 248(48), 219(42), 217(83), 213(94), 191(48), 189(100), 153(65), 125(62), 115(26), 77(27); HRMS (EI) m/z calcd for C16H17O4Cl 308.0815; found 308.0819.
Dimethyl (2E)-2-(4-nitrobenzylidene)cyclopentane-1,1-dicarboxylate (25)
Prepared in reaction of dimethyl 4-pentenylmalonate and 4-bromonitrobenzene following modified general procedure (run at 80 °C) (61 mg, yield 48%, isomer E/Z 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) or with 4-chloronitrobenzene following modified general procedure (run at 80 °C) (50 mg, yield 40%, isomer E/Z 45
80) or with 4-chloronitrobenzene following modified general procedure (run at 80 °C) (50 mg, yield 40%, isomer E/Z 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 75
55). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). 1H NMR (400 MHz, CDCl3) δ 8.14–8.08 (m, 4H), 5.41–5.38 (m, 1H), 3.67 (s, 6H), 3.64 (q, J = 2.3 Hz, 2H), 2.57–2.51 (m, 2H), 2.40–2.33 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 171.2, 170.5, 147.5, 146.5, 146.4, 145.3, 143.5, 139.9, 134.1, 123.0, 129.2, 124.9, 123.4, 122.9, 67.6, 64.0, 52.6, 52.5, 39.4, 35.0, 35.0, 33.9, 30.4, 22.6. Indicative signals of minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.49–7.45 (m, 2H), 7.37–7.32 (m, 2H), 6.67 (t, J = 2.4 Hz, 1H), 3.47 (s, 6H), 2.68 (td, J = 7.6, 2.2 Hz, 2H), 2.44 (t, J = 7.0 Hz, 2H), 1.76 (p, J = 7.3 Hz, 2H); MS (EI): m/z (%) = 319(36)[M+], 287(33), 260(48), 259(100), 229(28), 228(99), 227(48), 200(73), 154(66), 128(31), 115(30), 106(14), 90(19), 77(25), 59(49), 39(16); IR (CH2Cl2): 2953, 2854, 1732, 1597, 1519, 1434, 1346, 1266, 1156, 1066, 857 cm−1; HRMS (EI): m/z calcd for C16H17NO6 319.1056; found 319.1057.
25). 1H NMR (400 MHz, CDCl3) δ 8.14–8.08 (m, 4H), 5.41–5.38 (m, 1H), 3.67 (s, 6H), 3.64 (q, J = 2.3 Hz, 2H), 2.57–2.51 (m, 2H), 2.40–2.33 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 171.2, 170.5, 147.5, 146.5, 146.4, 145.3, 143.5, 139.9, 134.1, 123.0, 129.2, 124.9, 123.4, 122.9, 67.6, 64.0, 52.6, 52.5, 39.4, 35.0, 35.0, 33.9, 30.4, 22.6. Indicative signals of minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.49–7.45 (m, 2H), 7.37–7.32 (m, 2H), 6.67 (t, J = 2.4 Hz, 1H), 3.47 (s, 6H), 2.68 (td, J = 7.6, 2.2 Hz, 2H), 2.44 (t, J = 7.0 Hz, 2H), 1.76 (p, J = 7.3 Hz, 2H); MS (EI): m/z (%) = 319(36)[M+], 287(33), 260(48), 259(100), 229(28), 228(99), 227(48), 200(73), 154(66), 128(31), 115(30), 106(14), 90(19), 77(25), 59(49), 39(16); IR (CH2Cl2): 2953, 2854, 1732, 1597, 1519, 1434, 1346, 1266, 1156, 1066, 857 cm−1; HRMS (EI): m/z calcd for C16H17NO6 319.1056; found 319.1057.
Di(propan-2-yl) (2E)-2-benzylidenecyclopentane-1,1-dicarboxylate (26)
Prepared in reaction of dipropan-2-yl 2-pent-4-ynylpropanedioate and bromobenzene following modified general procedure (run at 80 °C) (56 mg, yield 42%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.34–7.32 (m, 4H), 7.23–7.18 (m, 1H), 6.76 (t, J = 2.6 Hz, 1H), 5.10 (hept, J = 6.3 Hz, 2H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.36 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H), 1.28 (dd, J = 6.3, 4.1 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.3, 141.2, 137.9, 128.6, 128.1, 127.2, 126.6, 68.8, 65.2, 35.6, 32.1, 24.7, 21.6, 21.5; IR (CH2Cl2): 3450, 2980, 2875, 1722, 1449, 1374, 1251, 1104, 909, 777, 699, 517 cm−1. MS (EI): m/z (%) = 330(27)[M+], 244(35), 202(59), 201(72), 184(54), 183(57), 173(44), 155(100), 129(43), 115(29), 91(56), 77(25), 43(95); HRMS (EI): m/z calcd for C20H26O4 330.1831; found 330.1822.
5). 1H NMR (400 MHz, CDCl3) δ 7.34–7.32 (m, 4H), 7.23–7.18 (m, 1H), 6.76 (t, J = 2.6 Hz, 1H), 5.10 (hept, J = 6.3 Hz, 2H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.36 (t, J = 6.9 Hz, 2H), 1.82 (p, J = 7.1 Hz, 2H), 1.28 (dd, J = 6.3, 4.1 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.3, 141.2, 137.9, 128.6, 128.1, 127.2, 126.6, 68.8, 65.2, 35.6, 32.1, 24.7, 21.6, 21.5; IR (CH2Cl2): 3450, 2980, 2875, 1722, 1449, 1374, 1251, 1104, 909, 777, 699, 517 cm−1. MS (EI): m/z (%) = 330(27)[M+], 244(35), 202(59), 201(72), 184(54), 183(57), 173(44), 155(100), 129(43), 115(29), 91(56), 77(25), 43(95); HRMS (EI): m/z calcd for C20H26O4 330.1831; found 330.1822.
Di(propan-2-yl) (2E)-2-(4-methoxybenzylidene)cyclopentane-1,1-dicarboxylate (27)
Prepared in reaction of dipropan-2-yl 2-pent-4-ynylpropanedioate and 4-bromoanisole following modified general procedure (run at 80 °C) (68 mg, yield 47%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 2H), 6.88–6.84 (m, 2H), 6.68 (t, J = 2.6 Hz, 1H), 5.08 (hept, J = 6.2 Hz, 2H), 3.80 (s, 3H), 2.67 (td, J = 7.2, 2.6 Hz, 2H), 2.33 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.1 Hz, 2H), 1.26 (dd, J = 6.3, 4.0 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.4, 158.3, 139.0, 130.7, 129.9, 126.7, 113.6, 68.7, 65.1, 55.2, 35.6, 32.0, 24.7, 21.6, 21.5; IR (CH2Cl2): 2979, 2936, 1724, 1607, 1511, 1466, 1250, 1103, 827, 530 cm−1; MS (EI): m/z (%) = 361(14), 360(43) [M+], 317(11), 273(36), 232(32), 231(100), 214(33), 213(30), 185(53), 171(12), 159(13), 135(17), 121(28), 115(21), 43(50), 41(24); HRMS (EI): m/z calcd for C21H28O5 360.1937; found 360.1936.
10). 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 2H), 6.88–6.84 (m, 2H), 6.68 (t, J = 2.6 Hz, 1H), 5.08 (hept, J = 6.2 Hz, 2H), 3.80 (s, 3H), 2.67 (td, J = 7.2, 2.6 Hz, 2H), 2.33 (t, J = 6.9 Hz, 2H), 1.81 (p, J = 7.1 Hz, 2H), 1.26 (dd, J = 6.3, 4.0 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.4, 158.3, 139.0, 130.7, 129.9, 126.7, 113.6, 68.7, 65.1, 55.2, 35.6, 32.0, 24.7, 21.6, 21.5; IR (CH2Cl2): 2979, 2936, 1724, 1607, 1511, 1466, 1250, 1103, 827, 530 cm−1; MS (EI): m/z (%) = 361(14), 360(43) [M+], 317(11), 273(36), 232(32), 231(100), 214(33), 213(30), 185(53), 171(12), 159(13), 135(17), 121(28), 115(21), 43(50), 41(24); HRMS (EI): m/z calcd for C21H28O5 360.1937; found 360.1936.
Di(propan-2-yl) (2E)-2-(4-cyanobenzylidene)cyclopentane-1,1-dicarboxylate (28)
Prepared in reaction of dipropan-2-yl 2-pent-4-ynylpropanedioate and 4-bromobenzonitrile following modified general procedure (run at 80 °C) (37 mg, yield 26%, isomer E/Z 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 95
25). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.55–7.49 (m, 4H), 6.62 (t, J = 2.2 Hz, 1H), 4.81 (hept, J = 6.3 Hz, 2H), 2.68 (td, J = 7.6, 2.2 Hz, 2H), 2.43 (t, J = 7.0 Hz, 2H), 1.76 (p, J = 7.2 Hz, 2H), 1.13 (d, J = 6.2 Hz, 6H), 1.04 (d, J = 6.3 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 169.6, 144.7, 132.1, 131.5, 130.2, 129.3, 125.2, 110.1, 69.5, 64.3, 39.8, 35.8, 22.7, 21.4, 21.3; IR (CH2Cl2): 2981, 2937, 2226, 1724, 1604, 1375, 1265, 1102, 1128, 845, 696, 515 cm−1; Indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.59–7.55 (m, 2H), 7.35–7.30 (m, 2H), 5.23 (p, J = 2.1 Hz, 1H), 5.05 (hept, J = 6.3 Hz, 2H), 3.61–3.55 (m, 2H), 2.55–2.48 (m, 2H), 2.37–2.30 (m, 2H), 1.25 (dd, J = 6.3, 5.1 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.5, 145.8, 141.7, 140.6, 133.3, 119.1, 110.1, 69.0, 68.0, 35.3, 33.8, 30.3, 21.6, 21.6; MS (EI): m/z (%) = 355(11)[M+], 313(7), 269(20), 227(54), 209(30), 180(40), 154(19), 116(29), 77(10), 57(11); HRMS (EI): m/z calcd for C21H25NO4 355.1784; found 355.1781.
5). 1H NMR (400 MHz, CDCl3) δ 7.55–7.49 (m, 4H), 6.62 (t, J = 2.2 Hz, 1H), 4.81 (hept, J = 6.3 Hz, 2H), 2.68 (td, J = 7.6, 2.2 Hz, 2H), 2.43 (t, J = 7.0 Hz, 2H), 1.76 (p, J = 7.2 Hz, 2H), 1.13 (d, J = 6.2 Hz, 6H), 1.04 (d, J = 6.3 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 169.6, 144.7, 132.1, 131.5, 130.2, 129.3, 125.2, 110.1, 69.5, 64.3, 39.8, 35.8, 22.7, 21.4, 21.3; IR (CH2Cl2): 2981, 2937, 2226, 1724, 1604, 1375, 1265, 1102, 1128, 845, 696, 515 cm−1; Indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.59–7.55 (m, 2H), 7.35–7.30 (m, 2H), 5.23 (p, J = 2.1 Hz, 1H), 5.05 (hept, J = 6.3 Hz, 2H), 3.61–3.55 (m, 2H), 2.55–2.48 (m, 2H), 2.37–2.30 (m, 2H), 1.25 (dd, J = 6.3, 5.1 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ 170.5, 145.8, 141.7, 140.6, 133.3, 119.1, 110.1, 69.0, 68.0, 35.3, 33.8, 30.3, 21.6, 21.6; MS (EI): m/z (%) = 355(11)[M+], 313(7), 269(20), 227(54), 209(30), 180(40), 154(19), 116(29), 77(10), 57(11); HRMS (EI): m/z calcd for C21H25NO4 355.1784; found 355.1781.
Di-tert-butyl (2E)-2-benzylidenecyclopentane-1,1-dicarboxyl-ate (29)
Prepared in reaction of di-tert-butyl pent-4-yn-1-ylpropanedioate and bromobenzene following modified general procedure (run at 80 °C) (37 mg, yield 26%). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (m, 4H), 7.23–7.17 (m, 1H), 6.77 (t, J = 2.6 Hz, 1H), 2.68 (td, J = 7.2, 2.6 Hz, 2H), 2.30 (t, J = 6.9 Hz, 2H), 1.79 (p, J = 7.0 Hz, 2H), 1.50 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 169.9, 141.5, 138.1, 128.6, 128.1, 127.0, 126.5, 81.2, 66.51, 35.7, 32.1, 27.9, 24.6; MS (EI): m/z (%) = 247(3), 246(12), 202(34), 185(14), 184(21), 183(11), 155(21), 142(7), 129(14), 115(13), 106(9), 91(16), 79(16), 57(100), 41(34); IR (CH2Cl2): 3054, 2977, 2930, 1725, 1599, 1368, 1270, 1166, 1128, 845, 696, 515 cm−1; HRMS (EI): m/z calcd for C22H30O4 358.2144; found 358.2115.
2). 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (m, 4H), 7.23–7.17 (m, 1H), 6.77 (t, J = 2.6 Hz, 1H), 2.68 (td, J = 7.2, 2.6 Hz, 2H), 2.30 (t, J = 6.9 Hz, 2H), 1.79 (p, J = 7.0 Hz, 2H), 1.50 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 169.9, 141.5, 138.1, 128.6, 128.1, 127.0, 126.5, 81.2, 66.51, 35.7, 32.1, 27.9, 24.6; MS (EI): m/z (%) = 247(3), 246(12), 202(34), 185(14), 184(21), 183(11), 155(21), 142(7), 129(14), 115(13), 106(9), 91(16), 79(16), 57(100), 41(34); IR (CH2Cl2): 3054, 2977, 2930, 1725, 1599, 1368, 1270, 1166, 1128, 845, 696, 515 cm−1; HRMS (EI): m/z calcd for C22H30O4 358.2144; found 358.2115.
Di-tert-butyl (2E)-2-(4-methoxybenzylidene)cyclopentane-1,1-dicarboxylate (30)
Prepared in reaction of di-tert-butyl pent-4-yn-1-ylpropanedioate and 4-bromoanisole following modified general procedure (run at 80 °C) (35 mg, yield 23%). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.29–7.25 (m, 2H), 6.88–6.84 (m, 2H), 6.69 (t, J = 2.5 Hz, 1H), 3.80 (s, 3H), 2.65 (td, J = 7.2, 2.6 Hz, 2H), 2.27 (t, J = 6.9 Hz, 2H), 1.78 (p, J = 7.1 Hz, 2H), 1.48 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 170.1, 158.3, 139.3, 130.9, 129.9, 126.4, 113.6, 81.2, 66.5, 55.2, 35.7, 32.1, 27.9, 27.7, 24.6; IR (CH2Cl2) 3449, 2977, 2934, 1725, 1607, 1511, 1456, 1368, 1251, 1167, 1129, 1036, 848, 828 cm−1; MS (EI): m/z (%) = 388(7)[M+], 276(22), 232(40), 231(100), 214(25), 203(11), 185(36), 171(12), 121(21), 115(15), 91(4), 77(8), 57(96), 43(12), 41(33); HRMS (EI): m/z calcd for C23H32O5 388.2250; found 388.2242.
10). 1H NMR (400 MHz, CDCl3) δ 7.29–7.25 (m, 2H), 6.88–6.84 (m, 2H), 6.69 (t, J = 2.5 Hz, 1H), 3.80 (s, 3H), 2.65 (td, J = 7.2, 2.6 Hz, 2H), 2.27 (t, J = 6.9 Hz, 2H), 1.78 (p, J = 7.1 Hz, 2H), 1.48 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 170.1, 158.3, 139.3, 130.9, 129.9, 126.4, 113.6, 81.2, 66.5, 55.2, 35.7, 32.1, 27.9, 27.7, 24.6; IR (CH2Cl2) 3449, 2977, 2934, 1725, 1607, 1511, 1456, 1368, 1251, 1167, 1129, 1036, 848, 828 cm−1; MS (EI): m/z (%) = 388(7)[M+], 276(22), 232(40), 231(100), 214(25), 203(11), 185(36), 171(12), 121(21), 115(15), 91(4), 77(8), 57(96), 43(12), 41(33); HRMS (EI): m/z calcd for C23H32O5 388.2250; found 388.2242.
Di-tert-butyl (2E)-2-(4-cyanobenzylidene)cyclopentane-1,1-dicarboxylate (31)
Prepared in reaction of di-tert-butyl pent-4-yn-1-ylpropanedioate and 4-bromobenzonitrile following modified general procedure (run at 80 °C) (54 mg, yield 35%, isomer E/Z 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 95
65). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.55–7.50 (m, 4H), 6.57 (t, 1H), 2.65 (td, J = 7.5, 2.3 Hz, 3H), 2.37 (t, J = 6.9 Hz, 2H), 1.76–1.69 (m, 2H), 1.28 (s, 18H). 13C NMR (101 MHz, CDCl3) δ 169.2, 145.0, 141.8, 131.5, 129.5, 124.7, 109.9, 109.8, 81.9, 65.3, 40.5, 36.5, 27.6, 22.7; indicative signals of minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.61–7.56 (m, 2H), 7.42–7.37 (m, 2H), 6.76 (t, J = 2.6 Hz, 1H), 2.29 (t, J = 6.9 Hz, 2H), 1.84–1.76 (m, 2H), 1.47 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 169.36, 145.66, 142.49, 131.90, 129.00, 125.56, 119.06, 81.62, 66.83, 35.46, 32.37, 27.84, 24.48; IR (CH2Cl2): 3434, 2978, 2933, 2226, 1724, 1604, 1456, 1368, 1128, 1065, 844, 555 cm−1; MS (EI): m/z (%) = 384(1)[M+], 327(4), 283(10), 271(13), 254(9), 227(42), 210(15), 180(13), 153(11), 116(12), 77(3), 57(100), 43(6), 41(23).
5). 1H NMR (400 MHz, CDCl3) δ 7.55–7.50 (m, 4H), 6.57 (t, 1H), 2.65 (td, J = 7.5, 2.3 Hz, 3H), 2.37 (t, J = 6.9 Hz, 2H), 1.76–1.69 (m, 2H), 1.28 (s, 18H). 13C NMR (101 MHz, CDCl3) δ 169.2, 145.0, 141.8, 131.5, 129.5, 124.7, 109.9, 109.8, 81.9, 65.3, 40.5, 36.5, 27.6, 22.7; indicative signals of minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.61–7.56 (m, 2H), 7.42–7.37 (m, 2H), 6.76 (t, J = 2.6 Hz, 1H), 2.29 (t, J = 6.9 Hz, 2H), 1.84–1.76 (m, 2H), 1.47 (s, 18H); 13C NMR (101 MHz, CDCl3) δ 169.36, 145.66, 142.49, 131.90, 129.00, 125.56, 119.06, 81.62, 66.83, 35.46, 32.37, 27.84, 24.48; IR (CH2Cl2): 3434, 2978, 2933, 2226, 1724, 1604, 1456, 1368, 1128, 1065, 844, 555 cm−1; MS (EI): m/z (%) = 384(1)[M+], 327(4), 283(10), 271(13), 254(9), 227(42), 210(15), 180(13), 153(11), 116(12), 77(3), 57(100), 43(6), 41(23).
tert-Butyl (2E)-2-benzylidene-1-cyanocyclopentanecarboxylate (32)
Prepared in reaction of tert-butyl 2-cyanohept-6-ynoate and bromobenzene following general procedure (82 mg, yield 73%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.39–7.31 (m, 4H), 7.29–7.24 (m, 1H), 6.85 (t, J = 2.6 Hz, 1H), 2.78–2.71 (m, 2H), 2.59–2.50 (m, 1H), 2.32–2.24 (m, 1H), 2.14–2.03 (m, 1H), 2.01–1.90 (m, 1H), 1.52 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.7, 141.3, 136.4, 128.6, 128.5, 128.3, 127.5, 127.1, 120.1, 83.8, 53.6, 36.4, 30.8, 27.7, 25.1; MS (EI): m/z (%) = 283(1)[M+], 182(37), 153(10), 128(12), 115(15), 102(5), 91(17), 77(12), 57(100), 43(12), 41(26); IR (CH2Cl2): 3447, 2978, 2877, 2240, 2214, 1737, 1449, 1370, 1256, 1150, 840, 695, 513 cm−1; HRMS (EI): m/z calcd for C18H21NO2 283.1572; found 283.1564.
10). 1H NMR (400 MHz, CDCl3) δ 7.39–7.31 (m, 4H), 7.29–7.24 (m, 1H), 6.85 (t, J = 2.6 Hz, 1H), 2.78–2.71 (m, 2H), 2.59–2.50 (m, 1H), 2.32–2.24 (m, 1H), 2.14–2.03 (m, 1H), 2.01–1.90 (m, 1H), 1.52 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.7, 141.3, 136.4, 128.6, 128.5, 128.3, 127.5, 127.1, 120.1, 83.8, 53.6, 36.4, 30.8, 27.7, 25.1; MS (EI): m/z (%) = 283(1)[M+], 182(37), 153(10), 128(12), 115(15), 102(5), 91(17), 77(12), 57(100), 43(12), 41(26); IR (CH2Cl2): 3447, 2978, 2877, 2240, 2214, 1737, 1449, 1370, 1256, 1150, 840, 695, 513 cm−1; HRMS (EI): m/z calcd for C18H21NO2 283.1572; found 283.1564.
tert-Butyl (2E)-1-cyano-2-(4-methoxybenzylidene)cyclopentanecarboxylate (33)
Prepared in reaction of tert-butyl 2-cyanohept-6-ynoate and 4-bromoanisole following modified general procedure (run at 80 °C) (121 mg, yield 96%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20).1H NMR (400 MHz, CDCl3) δ 7.32–7.23 (m, 2H), 6.93–6.85 (m, 2H), 6.77 (t, J = 2.6 Hz, 1H), 3.80 (s, 3H), 2.78–2.64 (m, 2H), 2.58–2.46 (m, 1H), 2.31–2.20 (m, 1H), 2.13–2.01 (m, 1H), 2.00–1.88 (m, 1H), 1.51 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.9, 158.9, 138.9, 129.9, 129.2, 126.6, 120.3, 113.8, 83.7, 55.17, 53.60, 36.5, 30.7, 27.7, 25.1; IR (CH2Cl2): 2978, 2935, 2838, 2240, 1735, 1607, 1512, 1462, 1370, 1253, 1152, 1034, 836, 513 cm−1; MS (EI): m/z (%) = 313(10)[M+], 213(65), 212(75), 198(28), 167(32), 121(16), 115(14), 91(8), 77(13), 57(100), 43(11), 41(27); HRMS (EI): m/z calcd for C19H23NO3 313.1678; found 313.1680.
20).1H NMR (400 MHz, CDCl3) δ 7.32–7.23 (m, 2H), 6.93–6.85 (m, 2H), 6.77 (t, J = 2.6 Hz, 1H), 3.80 (s, 3H), 2.78–2.64 (m, 2H), 2.58–2.46 (m, 1H), 2.31–2.20 (m, 1H), 2.13–2.01 (m, 1H), 2.00–1.88 (m, 1H), 1.51 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.9, 158.9, 138.9, 129.9, 129.2, 126.6, 120.3, 113.8, 83.7, 55.17, 53.60, 36.5, 30.7, 27.7, 25.1; IR (CH2Cl2): 2978, 2935, 2838, 2240, 1735, 1607, 1512, 1462, 1370, 1253, 1152, 1034, 836, 513 cm−1; MS (EI): m/z (%) = 313(10)[M+], 213(65), 212(75), 198(28), 167(32), 121(16), 115(14), 91(8), 77(13), 57(100), 43(11), 41(27); HRMS (EI): m/z calcd for C19H23NO3 313.1678; found 313.1680.
tert-Butyl (2E)-1-cyano-2-(4-cyanobenzylidene)cyclopentane-carboxylate (34)
Prepared in reaction of tert-butyl 2-cyanohept-6-ynoate and 4-bromobenzonitrile following modified general procedure (run at 80 °C) (40 mg, yield 33%, isomer E/Z 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90
77). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.61–7.56 (m, 2H), 7.35–7.29 (m, 2H), 5.49–5.45 (m, 1H), 3.58 (dq, J = 16.6, 2.1 Hz, 1H), 3.46 (dq, J = 16.5, 2.2 Hz, 1H), 2.66–2.57 (m, 1H), 2.54–2.44 (m, 3H), 1.45 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.7, 143.3, 138.5, 134.0, 132.2, 132.1, 130.1, 129.0, 110.6, 84.1, 56.7, 36.1, 34.61, 31.0, 27.6; Indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.66–7.60 (m, 2H), 7.41 (d, J = 8.3 Hz, 2H), 6.82 (t, J = 2.7 Hz, 1H), 2.73 (td, J = 7.3, 2.6 Hz, 2H), 2.32–2.24 (m, 1H), 2.14–2.03 (m, 1H), 2.02–1.91 (m, 1H), 1.50 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.2, 145.3, 140.7, 119.6, 118.5, 110.9, 84.3, 53.9, 36.4, 25.0; IR (CH2Cl2): 3059, 2879, 2935, 2228, 1736, 1606, 1370, 1254, 1152 cm−1; MS (EI): m/z (%) = 308(1)[M+], 252(10), 208(26), 207(30), 153(6), 116(28), 77(8), 57(100), 43(16), 41(28); HRMS (ESI): m/z calcd for C19H20N2O2Na 331.1525; found 331.1397.
10). 1H NMR (400 MHz, CDCl3) δ 7.61–7.56 (m, 2H), 7.35–7.29 (m, 2H), 5.49–5.45 (m, 1H), 3.58 (dq, J = 16.6, 2.1 Hz, 1H), 3.46 (dq, J = 16.5, 2.2 Hz, 1H), 2.66–2.57 (m, 1H), 2.54–2.44 (m, 3H), 1.45 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.7, 143.3, 138.5, 134.0, 132.2, 132.1, 130.1, 129.0, 110.6, 84.1, 56.7, 36.1, 34.61, 31.0, 27.6; Indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.66–7.60 (m, 2H), 7.41 (d, J = 8.3 Hz, 2H), 6.82 (t, J = 2.7 Hz, 1H), 2.73 (td, J = 7.3, 2.6 Hz, 2H), 2.32–2.24 (m, 1H), 2.14–2.03 (m, 1H), 2.02–1.91 (m, 1H), 1.50 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 166.2, 145.3, 140.7, 119.6, 118.5, 110.9, 84.3, 53.9, 36.4, 25.0; IR (CH2Cl2): 3059, 2879, 2935, 2228, 1736, 1606, 1370, 1254, 1152 cm−1; MS (EI): m/z (%) = 308(1)[M+], 252(10), 208(26), 207(30), 153(6), 116(28), 77(8), 57(100), 43(16), 41(28); HRMS (ESI): m/z calcd for C19H20N2O2Na 331.1525; found 331.1397.
Propan-2-yl (2E)-2-benzylidene-1-cyanocyclopentane-carboxylate (35)
Prepared in reaction of propan-2-yl 2-cyanohept-6-ynoate and bromobenzene following modified general procedure (run for 4 h) (80 mg, yield 75%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.39–7.30 (m, 4H), 7.29–7.24 (m, 1H), 6.84 (t, J = 2.6 Hz, 1H), 5.10 (hept, J = 6.3 Hz, 1H), 2.82–2.68 (m, 2H), 2.61–2.53 (m, 1H), 2.35–2.26 (m, 1H), 2.16–2.05 (m, 1H), 2.03–1.91 (m, 1H), 1.32 (t, J = 6.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 167.4, 141.0, 136.3, 128.6, 128.4, 127.6, 127.4, 119.9, 70.9, 52.9, 36.6, 30.8, 25.1, 21.4, 21.4; IR (CH2Cl2): 2981, 2241, 1737, 1450, 1376, 1237, 1103, 762, 695, 513 cm−1; MS (EI): m/z (%) = 270(3), 269(14), 184(5), 183(62), 182(82), 155(5), 129(22), 115(20), 102(8), 91(24), 77(18), 52(13), 43(100); HRMS (EI) m/z calcd for C17H19NO2 269.1416; found 269.1422.
5). 1H NMR (400 MHz, CDCl3) δ 7.39–7.30 (m, 4H), 7.29–7.24 (m, 1H), 6.84 (t, J = 2.6 Hz, 1H), 5.10 (hept, J = 6.3 Hz, 1H), 2.82–2.68 (m, 2H), 2.61–2.53 (m, 1H), 2.35–2.26 (m, 1H), 2.16–2.05 (m, 1H), 2.03–1.91 (m, 1H), 1.32 (t, J = 6.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 167.4, 141.0, 136.3, 128.6, 128.4, 127.6, 127.4, 119.9, 70.9, 52.9, 36.6, 30.8, 25.1, 21.4, 21.4; IR (CH2Cl2): 2981, 2241, 1737, 1450, 1376, 1237, 1103, 762, 695, 513 cm−1; MS (EI): m/z (%) = 270(3), 269(14), 184(5), 183(62), 182(82), 155(5), 129(22), 115(20), 102(8), 91(24), 77(18), 52(13), 43(100); HRMS (EI) m/z calcd for C17H19NO2 269.1416; found 269.1422.
Propan-2-yl (2E)-1-cyano-2-(4-methoxybenzylidene)cyclopentanecarboxylate (36)
Prepared in reaction of propan-2-yl 2-cyanohept-6-ynoate and 4-bromoanisole following modified general procedure (run for 4 h) (86 mg, yield 72%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.31–7.23 (m, 2H), 6.92–6.84 (m, 2H), 6.76 (t, J = 2.6 Hz, 1H), 5.08 (hept, J = 6.3 Hz, 1H), 3.80 (s, 3H), 2.80–2.64 (m, 2H), 2.59–2.49 (m, 1H), 2.33–2.22 (m, 1H), 2.15–2.04 (m, 1H), 2.01–1.89 (m, 1H), 1.31 (t, J = 6.3 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 167.5, 159.0, 138.6, 129.9, 129.0, 126.8, 120.0, 113.8, 70.7, 55.2, 52.9, 36.5, 30.7, 25.1, 21.4, 21.3. IR (CH2Cl2): 2981, 2937, 2240, 1736, 1606, 1512, 1465, 1253, 1178, 1103, 1034, 831, 531 cm−1; MS (EI): m/z (%) = 299(19)[M+], 256(4), 212(100), 198(10), 170(12), 121(13), 115(12), 91(6), 77(10), 43(52); HRMS (EI): m/z calcd for C18H21NO3 299.1521; found 299.1527.
10). 1H NMR (400 MHz, CDCl3) δ 7.31–7.23 (m, 2H), 6.92–6.84 (m, 2H), 6.76 (t, J = 2.6 Hz, 1H), 5.08 (hept, J = 6.3 Hz, 1H), 3.80 (s, 3H), 2.80–2.64 (m, 2H), 2.59–2.49 (m, 1H), 2.33–2.22 (m, 1H), 2.15–2.04 (m, 1H), 2.01–1.89 (m, 1H), 1.31 (t, J = 6.3 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 167.5, 159.0, 138.6, 129.9, 129.0, 126.8, 120.0, 113.8, 70.7, 55.2, 52.9, 36.5, 30.7, 25.1, 21.4, 21.3. IR (CH2Cl2): 2981, 2937, 2240, 1736, 1606, 1512, 1465, 1253, 1178, 1103, 1034, 831, 531 cm−1; MS (EI): m/z (%) = 299(19)[M+], 256(4), 212(100), 198(10), 170(12), 121(13), 115(12), 91(6), 77(10), 43(52); HRMS (EI): m/z calcd for C18H21NO3 299.1521; found 299.1527.
Propan-2-yl (2E)-1-cyano-2-(4-cyanobenzylidene)cyclopentanecarboxylate (37)
Prepared in reaction of propan-2-yl 2-cyanohept-6-ynoate and 4-bromobenzonitrile following modified general procedure (run for 4 h) (83 mg, yield 70%, isomer E/Z 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90
80). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.60–7.55 (m, 2H), 7.32–7.27 (m, 2H), 5.50 (p, J = 1.9 Hz, 1H), 4.95 (hept, J = 6.3 Hz, 1H), 3.61–3.52 (m, 1H), 3.48–3.40 (m, 1H), 2.67–2.58 (m, 1H), 2.56–2.46 (m, 2H), 1.26–1.21 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 167.3, 143.1, 138.2, 134.3, 132.7, 132.4, 132.2, 132.1, 130.0, 129.4, 129.0, 127.8, 118.6, 118.5, 110.6, 71.0, 56.0, 36.1, 34.5, 31.0, 21.4, 21.3, 21.3, 21.3; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.78–7.72 (m, 1H), 7.71–7.65 (m, 1H), 7.65–7.57 (m, 1H), 7.40 (d, J = 8.4 Hz, 1H), 6.81 (t, J = 2.7 Hz, 1H), 5.08 (p, J = 6.2 Hz, 1H), 2.77–2.70 (m, 1H), 2.36–2.26 (m, 2H), 2.16–2.05 (m, 1H), 2.03–1.95 (m, 1H), 1.95–1.88 (m, 1H), 1.32–1.27 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 166.8, 160.8, 145.0, 143.3, 142.5, 140.6, 125.8, 119.3, 118.2, 112.2, 110.9, 110.81, 110.2, 53.2, 37.1, 36.4, 35.5, 34.1, 25.0, 22.3; IR (CH2Cl2): 3452, 3060, 2983, 2938, 2228, 1738, 1606, 1326, 1248, 1178, 1104, 834, 553 cm−1; MS (EI): m/z (%) = 294(7)[M+], 252(10), 208(63), 204(34), 180(18), 153(15), 140(19), 116(45), 104(16), 89(20), 77(14), 43(100); HRMS (EI): m/z calcd for C18H18N2O2 294.1368; found 294.1363.
10). 1H NMR (400 MHz, CDCl3) δ 7.60–7.55 (m, 2H), 7.32–7.27 (m, 2H), 5.50 (p, J = 1.9 Hz, 1H), 4.95 (hept, J = 6.3 Hz, 1H), 3.61–3.52 (m, 1H), 3.48–3.40 (m, 1H), 2.67–2.58 (m, 1H), 2.56–2.46 (m, 2H), 1.26–1.21 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 167.3, 143.1, 138.2, 134.3, 132.7, 132.4, 132.2, 132.1, 130.0, 129.4, 129.0, 127.8, 118.6, 118.5, 110.6, 71.0, 56.0, 36.1, 34.5, 31.0, 21.4, 21.3, 21.3, 21.3; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.78–7.72 (m, 1H), 7.71–7.65 (m, 1H), 7.65–7.57 (m, 1H), 7.40 (d, J = 8.4 Hz, 1H), 6.81 (t, J = 2.7 Hz, 1H), 5.08 (p, J = 6.2 Hz, 1H), 2.77–2.70 (m, 1H), 2.36–2.26 (m, 2H), 2.16–2.05 (m, 1H), 2.03–1.95 (m, 1H), 1.95–1.88 (m, 1H), 1.32–1.27 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 166.8, 160.8, 145.0, 143.3, 142.5, 140.6, 125.8, 119.3, 118.2, 112.2, 110.9, 110.81, 110.2, 53.2, 37.1, 36.4, 35.5, 34.1, 25.0, 22.3; IR (CH2Cl2): 3452, 3060, 2983, 2938, 2228, 1738, 1606, 1326, 1248, 1178, 1104, 834, 553 cm−1; MS (EI): m/z (%) = 294(7)[M+], 252(10), 208(63), 204(34), 180(18), 153(15), 140(19), 116(45), 104(16), 89(20), 77(14), 43(100); HRMS (EI): m/z calcd for C18H18N2O2 294.1368; found 294.1363.
(2E)-2-Benzylidenecyclopentane-1,1-dicarbonitrile (38)
Prepared in reaction of pent-4-yn-1-ylpropanedinitrile and bromobenzene following modified general procedure (run for 4 h) (72 mg, yield 87%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.44–7.31 (m, 5H), 6.99 (t, J = 2.7 Hz, 1H), 2.83 (td, J = 7.3, 2.7 Hz, 2H), 2.50 (t, J = 6.9 Hz, 2H), 2.15 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 136.5, 135.0, 129.9, 128.8, 128.7, 128.6, 128.4, 115.4, 40.3, 38.8, 29.3, 24.3; IR (CH2Cl2): 3058, 3029, 2953, 2246, 1492, 1449, 1194, 921, 760, 694, 512 cm−1; MS (EI): m/z (%) = 208(100)[M+], 207(55), 180(47), 153(30), 115(69), 102(21), 91(33), 77(26), 51(28), 39(26); HRMS (EI): m/z calcd for C14H12N2 208.1000; found 208.1006.
10). 1H NMR (400 MHz, CDCl3) δ 7.44–7.31 (m, 5H), 6.99 (t, J = 2.7 Hz, 1H), 2.83 (td, J = 7.3, 2.7 Hz, 2H), 2.50 (t, J = 6.9 Hz, 2H), 2.15 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 136.5, 135.0, 129.9, 128.8, 128.7, 128.6, 128.4, 115.4, 40.3, 38.8, 29.3, 24.3; IR (CH2Cl2): 3058, 3029, 2953, 2246, 1492, 1449, 1194, 921, 760, 694, 512 cm−1; MS (EI): m/z (%) = 208(100)[M+], 207(55), 180(47), 153(30), 115(69), 102(21), 91(33), 77(26), 51(28), 39(26); HRMS (EI): m/z calcd for C14H12N2 208.1000; found 208.1006.
(2E)-2-(4-Methoxybenzylidene)cyclopentane-1,1-dicarbo-nitrile (39)
Prepared in reaction of pent-4-yn-1-ylpropanedinitrile and 4-bromoanisole following modified general procedure (run for 4 h) (86 mg, yield 91%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3) δ 7.34–7.28 (m, 2H), 6.95–6.89 (m, 3H), 3.83 (s, 3H), 2.79 (td, J = 7.3, 2.7 Hz, 2H), 2.47 (t, J = 6.9 Hz, 2H), 2.13 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 159.6, 133.8, 130.2, 129.3, 127.7, 115.6, 114.0, 55.2, 40.3, 38.8, 29.2, 24.4; IR (CH2Cl2): 2956, 2839, 2246, 1606, 1513, 1463, 1254, 1179, 1032, 890, 829, 531 cm−1; MS (EI): m/z (%) = 239(29), 238(100)[M+], 237(27), 223(16), 210(29), 195(21), 170(19), 160(40), 145(40), 129(25), 115(27), 91(17), 77(20), 51(17), 43(13), 39(18); HRMS (EI): m/z calcd for C15H14N2O 238.1106; found 238.1111.
20). 1H NMR (400 MHz, CDCl3) δ 7.34–7.28 (m, 2H), 6.95–6.89 (m, 3H), 3.83 (s, 3H), 2.79 (td, J = 7.3, 2.7 Hz, 2H), 2.47 (t, J = 6.9 Hz, 2H), 2.13 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 159.6, 133.8, 130.2, 129.3, 127.7, 115.6, 114.0, 55.2, 40.3, 38.8, 29.2, 24.4; IR (CH2Cl2): 2956, 2839, 2246, 1606, 1513, 1463, 1254, 1179, 1032, 890, 829, 531 cm−1; MS (EI): m/z (%) = 239(29), 238(100)[M+], 237(27), 223(16), 210(29), 195(21), 170(19), 160(40), 145(40), 129(25), 115(27), 91(17), 77(20), 51(17), 43(13), 39(18); HRMS (EI): m/z calcd for C15H14N2O 238.1106; found 238.1111.
(E)-Methyl 1-acetyl-2-benzylidenecyclopentanecarboxylate (40)
Prepared in reaction of methyl 2-acetylhept-6-ynoate and bromobenzene following modified general procedure (run for 2 h) (yield: 80%) product was isolated as oil after column chromatography on silica gel (15 g, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 → 90
5 → 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.37–7.32 (m, 4H), 7.27–7.20 (m, 1H), 6.60 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.80–2.63 (m, 2H), 2.50–2.41 (m, 1H), 2.26 (s, 3H), 2.25–2.16 (m, 1H), 1.89–1.75 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.0, 171.8, 141.5, 137.4, 128.6, 128.2, 127.6, 126.9, 72.2, 52.6, 34.4, 32.0, 26.8, 24.8; IR (CH2Cl2): 3410, 2953, 1737, 1714, 1493, 1447, 1433, 1356, 1238, 697 cm−1; MS (EI), m/z (%): 258 (7, M+), 216 (80), 184 (100), 167 (13), 155 (86), 141 (19), 128 (34), 115 (29), 105 (14), 91 (35), 77 (23), 43 (46); HRMS (EI): m/z calcd for C16H18O3: 258.1256. Found 258.1255.
10 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.37–7.32 (m, 4H), 7.27–7.20 (m, 1H), 6.60 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.80–2.63 (m, 2H), 2.50–2.41 (m, 1H), 2.26 (s, 3H), 2.25–2.16 (m, 1H), 1.89–1.75 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.0, 171.8, 141.5, 137.4, 128.6, 128.2, 127.6, 126.9, 72.2, 52.6, 34.4, 32.0, 26.8, 24.8; IR (CH2Cl2): 3410, 2953, 1737, 1714, 1493, 1447, 1433, 1356, 1238, 697 cm−1; MS (EI), m/z (%): 258 (7, M+), 216 (80), 184 (100), 167 (13), 155 (86), 141 (19), 128 (34), 115 (29), 105 (14), 91 (35), 77 (23), 43 (46); HRMS (EI): m/z calcd for C16H18O3: 258.1256. Found 258.1255.
(E)-Methyl 1-acetyl-2-(4-methoxybenzylidene)cyclopentane-carboxylate (41)
Prepared in reaction of methyl 2-acetylhept-6-ynoate and 4-bromoanisole following modified general procedure (run for 2 h) (yield: 79%). Product was isolated as oil after column chromatography on silica gel (15 g column, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 80
10 → 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.33–7.26 (m, J = 8.7 Hz, 2H), 6.91–6.85 (m, 2H), 6.53 (t, J = 2.4 Hz, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 2.79–2.59 (m, 2H), 2.44 (dt, J = 13.5, 6.9 Hz, 1H), 2.25 (s, 3H), 2.24–2.13 (m, 1H), 1.90–1.76 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.3, 172.0, 158.5, 139.2, 130.2, 129.9, 127.1, 113.7, 72.2, 55.2, 52.6, 34.4, 31.9, 26.7, 24.8; IR (CH2Cl2): 2954, 1737, 1712, 1606, 1512, 1461, 1435, 1355, 1251, 1177, 1034, 826, 531 cm−1; MS (EI), m/z (%): 288 (24, M+), 245 (79), 229 (16), 214 (50), 185 (100), 171 (16), 159 (14), 141 (13), 128 (14), 121 (24), 115 (23), 77 (10), 43 (32); HRMS (EI): m/z calcd for C17H20O4: 288.1362. Found 288.1364.
20 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.33–7.26 (m, J = 8.7 Hz, 2H), 6.91–6.85 (m, 2H), 6.53 (t, J = 2.4 Hz, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 2.79–2.59 (m, 2H), 2.44 (dt, J = 13.5, 6.9 Hz, 1H), 2.25 (s, 3H), 2.24–2.13 (m, 1H), 1.90–1.76 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.3, 172.0, 158.5, 139.2, 130.2, 129.9, 127.1, 113.7, 72.2, 55.2, 52.6, 34.4, 31.9, 26.7, 24.8; IR (CH2Cl2): 2954, 1737, 1712, 1606, 1512, 1461, 1435, 1355, 1251, 1177, 1034, 826, 531 cm−1; MS (EI), m/z (%): 288 (24, M+), 245 (79), 229 (16), 214 (50), 185 (100), 171 (16), 159 (14), 141 (13), 128 (14), 121 (24), 115 (23), 77 (10), 43 (32); HRMS (EI): m/z calcd for C17H20O4: 288.1362. Found 288.1364.
(E)-Methyl 1-acetyl-2-(4-cyanobenzylidene)cyclopentane-carboxylate (42)
Prepared in reaction of methyl 2-acetylhept-6-ynoate and 4-bromobenzonitrile following modified general procedure (run for 2 h) (yield: 75%, E/Z 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) Product was isolated as oil after column chromatography on silica gel (15 g column, 9
9) Product was isolated as oil after column chromatography on silica gel (15 g column, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 8
1 → 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.63–7.56 (m, 2H), 7.40 (d, J = 8.3 Hz, 2H), 6.57 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.76–2.59 (m, 2H), 2.53–2.43 (m, 1H), 2.24 (s, 3H), 2.22–2.14 (m, 1H), 1.91–1.75 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 202.9, 171.3, 145.4, 141.9, 132.0, 129.1, 126.2, 118.9, 110.2, 72.4, 52.9, 34.3, 32.3, 26.8, 24.7; indicative signals of Z isomer: 1H NMR (400 MHz, CDCl3) δ 7.52–7.48 (m, 2H), 7.28 (d, J = 8.1 Hz, 2H), 6.68 (s, 1H), 3.40 (s, 3H), 2.06 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 204.3, 145.2, 141.3, 131.6, 125.4, 110.5, 70.3, 52.3, 38.4, 35.8, 23.1; IR (CH2Cl2): 2954, 2880, 2842, 2226, 1738, 1713, 1604, 1503, 1433, 1357, 1239, 1177, 1153, 1129, 886, 827, 555; HRMS (ESI): m/z calcd for C17H17NO3Na ([M + Na]+): 306.1106. Found 306.1107.
2 hexanes/EtOAc). 1H NMR (400 MHz, CDCl3) δ 7.63–7.56 (m, 2H), 7.40 (d, J = 8.3 Hz, 2H), 6.57 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.76–2.59 (m, 2H), 2.53–2.43 (m, 1H), 2.24 (s, 3H), 2.22–2.14 (m, 1H), 1.91–1.75 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 202.9, 171.3, 145.4, 141.9, 132.0, 129.1, 126.2, 118.9, 110.2, 72.4, 52.9, 34.3, 32.3, 26.8, 24.7; indicative signals of Z isomer: 1H NMR (400 MHz, CDCl3) δ 7.52–7.48 (m, 2H), 7.28 (d, J = 8.1 Hz, 2H), 6.68 (s, 1H), 3.40 (s, 3H), 2.06 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 204.3, 145.2, 141.3, 131.6, 125.4, 110.5, 70.3, 52.3, 38.4, 35.8, 23.1; IR (CH2Cl2): 2954, 2880, 2842, 2226, 1738, 1713, 1604, 1503, 1433, 1357, 1239, 1177, 1153, 1129, 886, 827, 555; HRMS (ESI): m/z calcd for C17H17NO3Na ([M + Na]+): 306.1106. Found 306.1107.
1-((2E)-1-Benzoyl-2-benzylidenecyclopentyl)ethanone (43)
Prepared in reaction of 2-(pent-4-ynyl)-1-phenylbutane-1,3-dione and bromobenzene following modified general procedure (run at 80 °C) (55 mg, yield 45%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.83–7.78 (m, 2H), 7.54–7.49 (m, 1H), 7.44–7.39 (m, 2H), 7.36–7.31 (m, 4H), 7.26–7.21 (m, 1H), 2.90–2.80 (m, 2H), 2.79–2.69 (m, 1H), 2.34 (s, 3H), 2.32–2.24 (m, 1H), 1.92–1.84 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.8, 199.2, 142.2, 137.4, 135.5, 132.5, 129.3, 128.8, 128.6, 128.3, 128.2, 127.0, 77.2, 34.9, 31.8, 27.4, 24.8; IR (CH2Cl2): 3056, 3025, 2959, 2876, 1683, 1597, 1446, 1258, 1231, 735, 696, 516 cm−1; MS (EI): m/z (%) = 304(5)[M+], 262(13), 233(12), 199(11), 182(13), 155(10), 128(15), 105(100), 91(19), 77(48), 51(15), 43(32); HRMS (EI) m/z calcd for C21H20O2 304.1463; found 304.1462.
10). 1H NMR (400 MHz, CDCl3) δ 7.83–7.78 (m, 2H), 7.54–7.49 (m, 1H), 7.44–7.39 (m, 2H), 7.36–7.31 (m, 4H), 7.26–7.21 (m, 1H), 2.90–2.80 (m, 2H), 2.79–2.69 (m, 1H), 2.34 (s, 3H), 2.32–2.24 (m, 1H), 1.92–1.84 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.8, 199.2, 142.2, 137.4, 135.5, 132.5, 129.3, 128.8, 128.6, 128.3, 128.2, 127.0, 77.2, 34.9, 31.8, 27.4, 24.8; IR (CH2Cl2): 3056, 3025, 2959, 2876, 1683, 1597, 1446, 1258, 1231, 735, 696, 516 cm−1; MS (EI): m/z (%) = 304(5)[M+], 262(13), 233(12), 199(11), 182(13), 155(10), 128(15), 105(100), 91(19), 77(48), 51(15), 43(32); HRMS (EI) m/z calcd for C21H20O2 304.1463; found 304.1462.
1-((2E)-1-Benzoyl-2-(4-methoxybenzylidene)cyclopentyl)ethanone (44)
Prepared in reaction of 2-(pent-4-ynyl)-1-phenylbutane-1,3-dione and 4-bromoanisole following modified general procedure (run at 80 °C) (78 mg, yield 60%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 1H NMR (400 MHz, CDCl3) δ 7.81–7.76 (m, 2H), 7.52–7.47 (m, 1H), 7.42–7.36 (m, 2H), 7.29–7.24 (m, 2H), 6.90–6.85 (m, 2H), 6.40 (t, J = 2.5 Hz, 1H), 3.80 (s, 3H), 2.87–2.74 (m, 2H), 2.73–2.66 (m, 1H), 2.32 (s, 3H), 2.29–2.22 (m, 1H), 1.92–1.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.9, 199.4, 158.6, 139.9, 135.6, 132.4, 130.2, 129.9, 129.2, 128.3, 128.2, 113.7, 77.2, 55.2, 34.9, 31.7, 27.3, 24.9; IR (CH2Cl2): 3059, 2968, 2837, 1684, 1605, 1511, 1446, 1251, 1177, 1032, 880, 829, 701 cm−1; MS (EI): m/z (%) = 334(25)[M+], 292(50), 291(34), 229(100), 187(33), 135(26), 121(31), 105(89), 77(57), 43(51); HRMS (EI) m/z calcd for C22H22O3 334.1569; found 334.1574.
20). 1H NMR (400 MHz, CDCl3) δ 7.81–7.76 (m, 2H), 7.52–7.47 (m, 1H), 7.42–7.36 (m, 2H), 7.29–7.24 (m, 2H), 6.90–6.85 (m, 2H), 6.40 (t, J = 2.5 Hz, 1H), 3.80 (s, 3H), 2.87–2.74 (m, 2H), 2.73–2.66 (m, 1H), 2.32 (s, 3H), 2.29–2.22 (m, 1H), 1.92–1.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.9, 199.4, 158.6, 139.9, 135.6, 132.4, 130.2, 129.9, 129.2, 128.3, 128.2, 113.7, 77.2, 55.2, 34.9, 31.7, 27.3, 24.9; IR (CH2Cl2): 3059, 2968, 2837, 1684, 1605, 1511, 1446, 1251, 1177, 1032, 880, 829, 701 cm−1; MS (EI): m/z (%) = 334(25)[M+], 292(50), 291(34), 229(100), 187(33), 135(26), 121(31), 105(89), 77(57), 43(51); HRMS (EI) m/z calcd for C22H22O3 334.1569; found 334.1574.
4-(((1E)-2-Acetyl-2-benzoylcyclopentylidene)methyl)benzo-nitrile (45)
Prepared in reaction of 2-(pent-4-ynyl)-1-phenylbutane-1,3-dione and 4-brombenzonitrile following modified general procedure (run at 80 °C) (51 mg, yield 40%, isomer E/Z 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90
30). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.77–7.73 (m, 2H), 7.65–7.58 (m, 2H), 7.46–7.38 (m, 5H), 6.45 (t, J = 2.6 Hz, 1H), 2.88–2.69 (m, 4H), 2.31 (s, 3H), 1.95–1.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.3, 198.2, 145.9, 141.9, 135.3, 132.8, 132.1, 132.0, 130.3, 129.2, 129.1, 128.9, 128.6, 128.5, 127.1, 35.0, 32.1, 27.4, 24.8; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 1.9 Hz, 1H), 7.56–7.50 (m, 5H), 7.36–7.30 (m, 3H), 5.41 (p, J = 2.0 Hz, 1H), 2.58–2.35 (m, 6H), 2.24 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 206.5, 199.4, 145.3, 135.2, 133.6, 133.0, 118.8, 110.3, 79.3, 35.4, 33.3, 31.1, 27.3; IR (CH2Cl2): 3058, 2962, 2226, 1695, 1692, 1603, 1446, 1357, 1232, 700, 553 cm−1; MS (EI): m/z (%) = 329(1)[M+], 287(39), 286(16), 258(8), 153(9), 127(6), 116(12), 105(100), 77(48), 51(15), 43(30); HRMS (EI): m/z calcd for C22H19NO2 329.1416; found 329.1404.
10). 1H NMR (400 MHz, CDCl3) δ 7.77–7.73 (m, 2H), 7.65–7.58 (m, 2H), 7.46–7.38 (m, 5H), 6.45 (t, J = 2.6 Hz, 1H), 2.88–2.69 (m, 4H), 2.31 (s, 3H), 1.95–1.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 204.3, 198.2, 145.9, 141.9, 135.3, 132.8, 132.1, 132.0, 130.3, 129.2, 129.1, 128.9, 128.6, 128.5, 127.1, 35.0, 32.1, 27.4, 24.8; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 1.9 Hz, 1H), 7.56–7.50 (m, 5H), 7.36–7.30 (m, 3H), 5.41 (p, J = 2.0 Hz, 1H), 2.58–2.35 (m, 6H), 2.24 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 206.5, 199.4, 145.3, 135.2, 133.6, 133.0, 118.8, 110.3, 79.3, 35.4, 33.3, 31.1, 27.3; IR (CH2Cl2): 3058, 2962, 2226, 1695, 1692, 1603, 1446, 1357, 1232, 700, 553 cm−1; MS (EI): m/z (%) = 329(1)[M+], 287(39), 286(16), 258(8), 153(9), 127(6), 116(12), 105(100), 77(48), 51(15), 43(30); HRMS (EI): m/z calcd for C22H19NO2 329.1416; found 329.1404.
((2E)-1-Benzoyl-2-benzylidenecyclopentyl)(phenyl)methanone (46)
Prepared in reaction of 2-(pent-4-ynyl)-1,3-diphenylpropane-1,3-dione and bromobenzene following modified general procedure (run at 80 °C) (71 mg, yield 49%). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.85–7.81 (m, 4H), 7.50–7.45 (m, 2H), 7.41–7.36 (m, 4H), 7.34–7.28 (m, 4H), 7.24–7.20 (m, 1H), 6.41 (t, J = 2.5 Hz, 1H), 2.92 (td, J = 7.4, 2.5 Hz, 2H), 2.68 (t, J = 7.2 Hz, 2H), 1.89 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 199.0, 143.1, 137.6, 136.2, 132.4, 129.3, 128.9, 128.8, 128.4, 128.1, 126.8, 75.5, 36.7, 32.0, 24.2; IR (CH2Cl2): 3059, 3026, 2959, 1689, 1659, 1597, 1447, 1264, 1125, 697 cm−1; MS (EI): m/z (%) = 366(2)[M+], 262(7), 261(20), 245(12), 244(21), 183(4), 155(6), 128(7), 115(7), 105(100), 91(13), 77(44), 51(11); HRMS (EI): m/z calcd for C26H22O2 366.1620; found 366.1620.
5). 1H NMR (400 MHz, CDCl3) δ 7.85–7.81 (m, 4H), 7.50–7.45 (m, 2H), 7.41–7.36 (m, 4H), 7.34–7.28 (m, 4H), 7.24–7.20 (m, 1H), 6.41 (t, J = 2.5 Hz, 1H), 2.92 (td, J = 7.4, 2.5 Hz, 2H), 2.68 (t, J = 7.2 Hz, 2H), 1.89 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 199.0, 143.1, 137.6, 136.2, 132.4, 129.3, 128.9, 128.8, 128.4, 128.1, 126.8, 75.5, 36.7, 32.0, 24.2; IR (CH2Cl2): 3059, 3026, 2959, 1689, 1659, 1597, 1447, 1264, 1125, 697 cm−1; MS (EI): m/z (%) = 366(2)[M+], 262(7), 261(20), 245(12), 244(21), 183(4), 155(6), 128(7), 115(7), 105(100), 91(13), 77(44), 51(11); HRMS (EI): m/z calcd for C26H22O2 366.1620; found 366.1620.
((2E)-1-Benzoyl-2-(4-methoxybenzylidene)cyclopentyl)(phenyl)methanone (47)
Prepared in reaction of 2-(pent-4-ynyl)-1,3-diphenylpropane-1,3-dione and 4-bromoanisole following modified general procedure (run at 80 °C) (88 mg, yield 56%). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt/dioxane 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (400 MHz, CDCl3) δ 7.84–7.80 (m, 4H), 7.49–7.43 (m, 2H), 7.40–7.34 (m, 4H), 7.27–7.22 (m, 2H), 6.89–6.84 (m, 2H), 6.34 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.92–2.87 (m, 2H), 2.65 (t, J = 7.2 Hz, 2H), 1.88 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 199.2, 158.4, 140.8, 136.2, 132.3, 130.3, 130.0, 129.3, 128.3, 128.3, 113.5, 75.5, 55.1, 36.7, 31.9, 24.3; IR (CH2Cl2): 3058, 2956, 2836, 1687, 1659, 1606, 1510, 1251, 1177, 1033, 828, 701, 531 cm−1; MS (EI): m/z (%) = 397(5)[M+], 369(15), 292(33), 291(100), 274(25), 263(19), 155(11), 135(19), 105(75), 91(13), 77(54), 51(15); HRMS (EI): m/z calcd for C27H24O3 396.1725; found 396.1719.
5). 1H NMR (400 MHz, CDCl3) δ 7.84–7.80 (m, 4H), 7.49–7.43 (m, 2H), 7.40–7.34 (m, 4H), 7.27–7.22 (m, 2H), 6.89–6.84 (m, 2H), 6.34 (t, J = 2.5 Hz, 1H), 3.78 (s, 3H), 2.92–2.87 (m, 2H), 2.65 (t, J = 7.2 Hz, 2H), 1.88 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 199.2, 158.4, 140.8, 136.2, 132.3, 130.3, 130.0, 129.3, 128.3, 128.3, 113.5, 75.5, 55.1, 36.7, 31.9, 24.3; IR (CH2Cl2): 3058, 2956, 2836, 1687, 1659, 1606, 1510, 1251, 1177, 1033, 828, 701, 531 cm−1; MS (EI): m/z (%) = 397(5)[M+], 369(15), 292(33), 291(100), 274(25), 263(19), 155(11), 135(19), 105(75), 91(13), 77(54), 51(15); HRMS (EI): m/z calcd for C27H24O3 396.1725; found 396.1719.
4-(((1E)-2,2-Dibenzoylcyclopentylidene)methyl)benzonitrile (48)
Prepared in reaction of 2-(pent-4-ynyl)-1,3-diphenylpropane-1,3-dione and 4-bromobenzonitrile following modified general procedure (run at 80 °C) (63 mg, yield 40%). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.81–7.76 (m, 4H), 7.59–7.56 (m, 2H), 7.50–7.45 (m, 2H), 7.40–7.34 (m, 6H), 6.38 (t, J = 2.6 Hz, 1H), 2.88 (td, J = 7.4, 2.6 Hz, 2H), 2.69 (t, J = 7.2 Hz, 2H), 1.90 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 198.5, 147.3, 142.0, 135.8, 132.6, 131.9, 129.3, 129.2, 128.5, 128.5, 127.2, 118.9, 110.1, 75.7, 36.6, 32.2, 24.2; IR (CH2Cl2): 3361, 3060, 2961, 2226, 1659, 1601, 1446, 1265, 1225, 1178, 879, 832, 736, 701, 554 cm−1; MS (EI): m/z (%) = 391(1)[M+], 287(2), 285(7), 269(4), 201(6), 153(3), 130(4), 105(100), 77(40), 51(10); HRMS (EI): m/z calcd for C27H21NO2 391.1572; found 391.1586.
10). 1H NMR (400 MHz, CDCl3) δ 7.81–7.76 (m, 4H), 7.59–7.56 (m, 2H), 7.50–7.45 (m, 2H), 7.40–7.34 (m, 6H), 6.38 (t, J = 2.6 Hz, 1H), 2.88 (td, J = 7.4, 2.6 Hz, 2H), 2.69 (t, J = 7.2 Hz, 2H), 1.90 (p, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 198.5, 147.3, 142.0, 135.8, 132.6, 131.9, 129.3, 129.2, 128.5, 128.5, 127.2, 118.9, 110.1, 75.7, 36.6, 32.2, 24.2; IR (CH2Cl2): 3361, 3060, 2961, 2226, 1659, 1601, 1446, 1265, 1225, 1178, 879, 832, 736, 701, 554 cm−1; MS (EI): m/z (%) = 391(1)[M+], 287(2), 285(7), 269(4), 201(6), 153(3), 130(4), 105(100), 77(40), 51(10); HRMS (EI): m/z calcd for C27H21NO2 391.1572; found 391.1586.
Ethyl (2E)-2-benzylidene-1-(diethylphosphono)cyclopentanecarboxylate (49)
Prepared in reaction of ethyl 2-(diethylphosphono)hept-6-ynoate and bromobenzene following general procedure (20 mg, yield 14%). Product was isolated as oil after HPLC chromatography (DCM/MeOH 99,5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0,5 → 99
0,5 → 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (m, 4H), 7.27–7.17 (m, 1H), 6.98 (7.01–6.94 (m, 1H), 4.28–4.10 (m, 6H), 2.79–2.68 (m, 1H), 2.69–2.59 (m, 1H), 2.58–2.45 (m, 1H), 2.45–2.33 (m, 1H), 2.01–1.87 (m, 1H), 1.84–1.73 (m, 1H), 1.34–1.25 (m, 9H); 13C NMR (101 MHz, CDCl3) δ 170.5, 140.3 (d, J = 7.7 Hz), 137.9 (d, J = 3.9 Hz), 128.7 (d, J = 1.8 Hz), 128.2, 127.5 (d, J = 7.5 Hz), 126.7, 63.3 (d, J = 6.9 Hz), 62.9 (d, J = 7.3 Hz), 61.6, 58.9 (d, J = 143.8 Hz), 33.7 (d, J = 3.4 Hz), 32.7 (d, J = 5.3 Hz), 29.7, 25.4 (d, J = 6.3 Hz), 16.5 (d, J = 5.7 Hz), 14.0; 31P NMR (162 MHz, CDCl3) δ 23.4; IR (CH2Cl2): 3233, 2979, 2928, 1728, 1446, 1248, 1025, 966, 759, 698, 572 cm−1; MS (EI): m/z (%) = 367(16), 366(41)[M+], 293(33), 229(42), 184(33), 183(82), 156(30), 155(100), 129(26), 115(29), 105(23), 91(38), 77(25), 43(8); HRMS (EI): m/z calcd for C19H27O5P 366.1596; found 366.1604.
1). 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (m, 4H), 7.27–7.17 (m, 1H), 6.98 (7.01–6.94 (m, 1H), 4.28–4.10 (m, 6H), 2.79–2.68 (m, 1H), 2.69–2.59 (m, 1H), 2.58–2.45 (m, 1H), 2.45–2.33 (m, 1H), 2.01–1.87 (m, 1H), 1.84–1.73 (m, 1H), 1.34–1.25 (m, 9H); 13C NMR (101 MHz, CDCl3) δ 170.5, 140.3 (d, J = 7.7 Hz), 137.9 (d, J = 3.9 Hz), 128.7 (d, J = 1.8 Hz), 128.2, 127.5 (d, J = 7.5 Hz), 126.7, 63.3 (d, J = 6.9 Hz), 62.9 (d, J = 7.3 Hz), 61.6, 58.9 (d, J = 143.8 Hz), 33.7 (d, J = 3.4 Hz), 32.7 (d, J = 5.3 Hz), 29.7, 25.4 (d, J = 6.3 Hz), 16.5 (d, J = 5.7 Hz), 14.0; 31P NMR (162 MHz, CDCl3) δ 23.4; IR (CH2Cl2): 3233, 2979, 2928, 1728, 1446, 1248, 1025, 966, 759, 698, 572 cm−1; MS (EI): m/z (%) = 367(16), 366(41)[M+], 293(33), 229(42), 184(33), 183(82), 156(30), 155(100), 129(26), 115(29), 105(23), 91(38), 77(25), 43(8); HRMS (EI): m/z calcd for C19H27O5P 366.1596; found 366.1604.
Dipropan-2-yl ((2E)-2-benzylidene-1-cyanocyclopentyl) phosphonate (50)
Prepared in reaction of dipropan-2-yl (1-cyanohex-5-yn-1-yl)phosphonate and bromobenzene following general procedure (88 mg, yield 63%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). 1H NMR (400 MHz, CDCl3) δ 7.37–7.28 (m, 4H), 7.27–7.18 (m, 1H), 6.98–6.91 (m, 1H), 4.86–4.73 (m, 2H), 2.80–2.61 (m, 2H), 2.54–2.41 (m, 1H), 2.37–2.25 (m, 1H), 2.14–2.03 (m, 1H), 1.89–1.77 (m, 1H), 1.39–1.27 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 138.1 (d, J = 8.1 Hz), 136.5 (d, J = 3.7 Hz), 128.5 (d, J = 2.0 Hz), 128.3 (d, J = 7.7 Hz), 128.2, 127.2, 119.8 (d, J = 6.4 Hz), 73.0 (d, J = 7.2 Hz), 73.0 (d, J = 7.2 Hz), 46.1 (d, J = 148.1 Hz), 34.6 (d, J = 4.6 Hz), 31.3 (d, J = 3.6 Hz), 24.8 (d, J = 4.1 Hz), 24.0 (d, J = 3.3 Hz), 24.0 (d, J = 3.3 Hz), 23.6 (d, J = 3.9 Hz), 23.5 (d, J = 3.8 Hz); 31P NMR (162 MHz, CDCl3) δ 16.7; IR (CH2Cl2): 3458, 3253, 2981, 2936, 2235, 1450, 1387, 1255, 1103, 989, 762, 696, 585 cm−1; MS (EI): m/z (%) = 348(3), 347(10)[M+], 305(9), 264(20), 263(68), 210(10), 183(50), 182(100), 181(12), 166(32), 155(22), 129(19), 115(22), 91(25), 77(15), 51(7); HRMS (EI): m/z calcd for C19H26NO3P 347.1650; found 347.1647.
40). 1H NMR (400 MHz, CDCl3) δ 7.37–7.28 (m, 4H), 7.27–7.18 (m, 1H), 6.98–6.91 (m, 1H), 4.86–4.73 (m, 2H), 2.80–2.61 (m, 2H), 2.54–2.41 (m, 1H), 2.37–2.25 (m, 1H), 2.14–2.03 (m, 1H), 1.89–1.77 (m, 1H), 1.39–1.27 (m, 12H); 13C NMR (101 MHz, CDCl3) δ 138.1 (d, J = 8.1 Hz), 136.5 (d, J = 3.7 Hz), 128.5 (d, J = 2.0 Hz), 128.3 (d, J = 7.7 Hz), 128.2, 127.2, 119.8 (d, J = 6.4 Hz), 73.0 (d, J = 7.2 Hz), 73.0 (d, J = 7.2 Hz), 46.1 (d, J = 148.1 Hz), 34.6 (d, J = 4.6 Hz), 31.3 (d, J = 3.6 Hz), 24.8 (d, J = 4.1 Hz), 24.0 (d, J = 3.3 Hz), 24.0 (d, J = 3.3 Hz), 23.6 (d, J = 3.9 Hz), 23.5 (d, J = 3.8 Hz); 31P NMR (162 MHz, CDCl3) δ 16.7; IR (CH2Cl2): 3458, 3253, 2981, 2936, 2235, 1450, 1387, 1255, 1103, 989, 762, 696, 585 cm−1; MS (EI): m/z (%) = 348(3), 347(10)[M+], 305(9), 264(20), 263(68), 210(10), 183(50), 182(100), 181(12), 166(32), 155(22), 129(19), 115(22), 91(25), 77(15), 51(7); HRMS (EI): m/z calcd for C19H26NO3P 347.1650; found 347.1647.
Diethyl ((2E)-1-acetyl-2-benzylidenecyclopentyl)phosphonate (51)
Prepared in reaction of diethyl (2-oxooct-7-yn-3-yl)phosphonate and bromobenzene following general procedure (60 mg, yield 45%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt/dioxane 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.36–7.31 (m, 4H), 7.26–7.18 (m, 1H), 7.01–6.96 (m, 1H), 4.22–4.05 (m, 4H), 2.74–2.64 (m, 2H), 2.51–2.38 (m, 1H), 2.37 (s, 3H), 2.35–2.23 (m, 1H), 1.96–1.83 (m, 1H), 1.77–1.65 (m, 1H), 1.33–1.29 (m, 3H), 1.26 (td, J = 7.1, 0.6 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 203.8, 140.5 (d, J = 6.6 Hz), 137.6 (d, J = 3.7 Hz), 128.6 (d, J = 1.8 Hz), 128.2, 128.2 (d, J = 7.4 Hz), 126.8, 66.7 (d, J = 139.8 Hz), 63.3 (d, J = 7.0 Hz), 62.5 (d, J = 7.3 Hz), 32.7 (d, J = 0.9 Hz), 32.6 (d, J = 4.2 Hz), 27.5, 25.0, 24.9 (d, J = 6.9 Hz), 16.4 (d, J = 5.8 Hz), 16.3 (d, J = 5.8 Hz); 31P NMR (162 MHz, CDCl3) δ 23.8, 23.8; IR (CH2Cl2): 3455, 3230, 2980, 1706, 1445, 1227, 1049, 1024, 956, 760, 698, 599 cm−1; MS (EI): m/z (%) = 336(4)[M+], 295(29), 294(100), 266(15), 237(13), 220(5), 156(31), 155(84), 128(22), 115(19), 105(11), 91(29), 77(16), 43(29); HRMS (EI): m/z calcd for C18H25O4P 336.1490; found 336.1502.
10). 1H NMR (400 MHz, CDCl3) δ 7.36–7.31 (m, 4H), 7.26–7.18 (m, 1H), 7.01–6.96 (m, 1H), 4.22–4.05 (m, 4H), 2.74–2.64 (m, 2H), 2.51–2.38 (m, 1H), 2.37 (s, 3H), 2.35–2.23 (m, 1H), 1.96–1.83 (m, 1H), 1.77–1.65 (m, 1H), 1.33–1.29 (m, 3H), 1.26 (td, J = 7.1, 0.6 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 203.8, 140.5 (d, J = 6.6 Hz), 137.6 (d, J = 3.7 Hz), 128.6 (d, J = 1.8 Hz), 128.2, 128.2 (d, J = 7.4 Hz), 126.8, 66.7 (d, J = 139.8 Hz), 63.3 (d, J = 7.0 Hz), 62.5 (d, J = 7.3 Hz), 32.7 (d, J = 0.9 Hz), 32.6 (d, J = 4.2 Hz), 27.5, 25.0, 24.9 (d, J = 6.9 Hz), 16.4 (d, J = 5.8 Hz), 16.3 (d, J = 5.8 Hz); 31P NMR (162 MHz, CDCl3) δ 23.8, 23.8; IR (CH2Cl2): 3455, 3230, 2980, 1706, 1445, 1227, 1049, 1024, 956, 760, 698, 599 cm−1; MS (EI): m/z (%) = 336(4)[M+], 295(29), 294(100), 266(15), 237(13), 220(5), 156(31), 155(84), 128(22), 115(19), 105(11), 91(29), 77(16), 43(29); HRMS (EI): m/z calcd for C18H25O4P 336.1490; found 336.1502.
(2E)-2-Benzylidene-1-(diphenylphosphoryl)cyclopentane-carbonitrile (52)
Prepared in reaction of 2-(diphenylphosphoryl)hept-6-ynenitrile and bromobenzene following general procedure (62 mg, yield 41%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 1H NMR (400 MHz, CDCl3) δ 8.28–8.18 (m, 2H), 8.05–7.96 (m, 2H), 7.67–7.52 (m, 4H), 7.51–7.41 (m, 2H), 7.35–7.26 (m, 2H), 7.26–7.18 (m, 1H), 7.15 (d, J = 7.2 Hz, 2H), 5.97–5.91 (m, 1H), 2.89–2.70 (m, 2H), 2.65–2.51 (m, 1H), 2.44–2.29 (m, 1H), 2.23–2.11 (m, 1H), 1.90–1.75 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 138.6 (d, J = 6.6 Hz), 136.3 (d, J = 3.4 Hz), 132.8 (d, J = 8.4 Hz), 132.7 (d, J = 2.9 Hz), 132.6 (d, J = 2.6 Hz), 131.9 (d, J = 8.5 Hz), 129.9 (d, J = 97.2 Hz), 128.85 (d, J = 11.7 Hz), 128.78 (d, J = 6.6 Hz), 128.5 (d, J = 1.9 Hz), 128.4 (d, J = 101.2 Hz), 128.21 (d, J = 12.4 Hz), 128.15, 127.3, 121.6 (d, J = 2.8 Hz), 47.5 (d, J = 64.7 Hz), 34.4, 31.8, 25.3 (d, J = 2.2 Hz); 31P NMR (162 MHz, CDCl3) δ 30.4; IR (CH2Cl2): 3057, 2961, 2871, 2230, 1438, 1203, 1115, 725, 695, 607, 548, 526 cm−1; MS (EI): m/z (%) = 384(12), 383(28)[M+], 382(6), 258(4), 202(25), 201(100), 182(15), 154(9), 115(7), 91(8), 77(25), 51(13); HRMS (EI): m/z calcd for C25H22NOP 383.1439; found 383.1428.
50). 1H NMR (400 MHz, CDCl3) δ 8.28–8.18 (m, 2H), 8.05–7.96 (m, 2H), 7.67–7.52 (m, 4H), 7.51–7.41 (m, 2H), 7.35–7.26 (m, 2H), 7.26–7.18 (m, 1H), 7.15 (d, J = 7.2 Hz, 2H), 5.97–5.91 (m, 1H), 2.89–2.70 (m, 2H), 2.65–2.51 (m, 1H), 2.44–2.29 (m, 1H), 2.23–2.11 (m, 1H), 1.90–1.75 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 138.6 (d, J = 6.6 Hz), 136.3 (d, J = 3.4 Hz), 132.8 (d, J = 8.4 Hz), 132.7 (d, J = 2.9 Hz), 132.6 (d, J = 2.6 Hz), 131.9 (d, J = 8.5 Hz), 129.9 (d, J = 97.2 Hz), 128.85 (d, J = 11.7 Hz), 128.78 (d, J = 6.6 Hz), 128.5 (d, J = 1.9 Hz), 128.4 (d, J = 101.2 Hz), 128.21 (d, J = 12.4 Hz), 128.15, 127.3, 121.6 (d, J = 2.8 Hz), 47.5 (d, J = 64.7 Hz), 34.4, 31.8, 25.3 (d, J = 2.2 Hz); 31P NMR (162 MHz, CDCl3) δ 30.4; IR (CH2Cl2): 3057, 2961, 2871, 2230, 1438, 1203, 1115, 725, 695, 607, 548, 526 cm−1; MS (EI): m/z (%) = 384(12), 383(28)[M+], 382(6), 258(4), 202(25), 201(100), 182(15), 154(9), 115(7), 91(8), 77(25), 51(13); HRMS (EI): m/z calcd for C25H22NOP 383.1439; found 383.1428.
Ethyl (2E)-2-benzylidene-1-(diphenylphosphoryl)cyclopentanecarboxylate (53)
Prepared in reaction of ethyl 2-(diphenylphosphoryl)hept-6-ynoate and bromobenzene following general procedure (83 mg, yield 52%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 → 50
40 → 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). 1H NMR (400 MHz, CDCl3) δ 7.97–7.89 (m, 2H), 7.88–7.80 (m, 2H), 7.54–7.47 (m, 2H), 7.46–7.38 (m, 4H), 7.34–7.28 (m, 2H), 7.24–7.18 (m, 3H), 6.50 (p, J = 7.0 Hz, 1H), 4.22–4.06 (m, 2H), 2.69–2.51 (m, 2H), 2.47–2.34 (m, 1H), 1.85–1.68 (m, 2H), 1.28–1.22 (m, 1H), 1.11 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.0 (d, J = 2.0 Hz), 140.5 (d, J = 6.5 Hz), 137.6 (d, J = 3.2 Hz), 133.1 (d, J = 8.7 Hz), 132.2 (d, J = 8.7 Hz), 131.9 (d, J = 97.4 Hz), 131.68 (d, J = 2.8 Hz), 131.65 (d, J = 2.8 Hz), 131.1 (d, J = 100.3 Hz), 128.63, 128.61, 128.56 (d, J = 7.0 Hz), 128.2 (d, J = 11.7 Hz), 128.1, 127.7 (d, J = 11.7 Hz), 61.6, 61.2 (d, J = 65.9 Hz), 33.4, 33.1 (d, J = 2.9 Hz), 25.7 (d, J = 5.1 Hz), 13.7; 31P NMR (162 MHz, CDCl3) δ 34.1, 31.2; IR (CH2Cl2): 3431, 3057, 2959, 1721, 1438, 1228, 1113, 724, 697, 549 cm−1; MS (EI): m/z (%) = 431(18), 430(36)[M+], 357(10), 301(7), 288(6), 229(12), 219(32), 202(59), 201(100), 184(68), 183(46), 155(59), 129(24), 105(23), 91(32), 77(25), 43(8); HRMS (EI): m/z calcd for C27H27O3P 430.1698; found 430.1705.
50). 1H NMR (400 MHz, CDCl3) δ 7.97–7.89 (m, 2H), 7.88–7.80 (m, 2H), 7.54–7.47 (m, 2H), 7.46–7.38 (m, 4H), 7.34–7.28 (m, 2H), 7.24–7.18 (m, 3H), 6.50 (p, J = 7.0 Hz, 1H), 4.22–4.06 (m, 2H), 2.69–2.51 (m, 2H), 2.47–2.34 (m, 1H), 1.85–1.68 (m, 2H), 1.28–1.22 (m, 1H), 1.11 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.0 (d, J = 2.0 Hz), 140.5 (d, J = 6.5 Hz), 137.6 (d, J = 3.2 Hz), 133.1 (d, J = 8.7 Hz), 132.2 (d, J = 8.7 Hz), 131.9 (d, J = 97.4 Hz), 131.68 (d, J = 2.8 Hz), 131.65 (d, J = 2.8 Hz), 131.1 (d, J = 100.3 Hz), 128.63, 128.61, 128.56 (d, J = 7.0 Hz), 128.2 (d, J = 11.7 Hz), 128.1, 127.7 (d, J = 11.7 Hz), 61.6, 61.2 (d, J = 65.9 Hz), 33.4, 33.1 (d, J = 2.9 Hz), 25.7 (d, J = 5.1 Hz), 13.7; 31P NMR (162 MHz, CDCl3) δ 34.1, 31.2; IR (CH2Cl2): 3431, 3057, 2959, 1721, 1438, 1228, 1113, 724, 697, 549 cm−1; MS (EI): m/z (%) = 431(18), 430(36)[M+], 357(10), 301(7), 288(6), 229(12), 219(32), 202(59), 201(100), 184(68), 183(46), 155(59), 129(24), 105(23), 91(32), 77(25), 43(8); HRMS (EI): m/z calcd for C27H27O3P 430.1698; found 430.1705.
1-((2E)-2-Benzylidene-1-(diphenylphosphoryl)cyclopentyl) ethanone (54)
Prepared in reaction of 3-(diphenylphosphoryl)oct-7-yn-2-one and bromobenzene following general procedure (81 mg, yield 51%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt/dioxane 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.94–7.79 (m, 4H), 7.54–7.46 (m, 2H), 7.46–7.38 (m, 4H), 7.36–7.30 (m, 2H), 7.27–7.19 (m, 3H), 6.68 (s, 1H), 2.75–2.65 (m, 1H), 2.62–2.52 (m, 2H), 2.42 (s, 3H), 2.38–2.28 (m, 1H), 1.76–1.63 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 205.3, 140.8 (d, J = 5.7 Hz), 137.5 (d, J = 2.8 Hz), 133.0 (d, J = 9.2 Hz), 132.6 (d, J = 8.6 Hz), 131.8 (d, J = 2.7 Hz), 131.7 (d, J = 2.7 Hz), 131.6 (d, J = 97.8 Hz), 131.0 (d, J = 97.8 Hz), 129.3 (d, J = 6.5 Hz), 128.6, 128.21, 128.17 (d, J = 11.4 Hz), 128.0 (d, J = 11.9 Hz), 127.0, 68.8 (d, J = 64.4 Hz), 32.8 (d, J = 3.7 Hz), 32.7, 28.3, 25.4 (d, J = 5.5 Hz); 31P NMR (162 MHz, CDCl3) δ 35.80, 34.60; IR (CH2Cl2): 3378, 3058, 2960, 2925, 2854, 1699, 1437, 1179, 1112, 750, 722, 696, 543 cm−1; MS (EI): m/z (%) = 400(24)[M+], 359(42), 358(100), 281(16), 219(24), 202(47), 201(98), 182(45), 167(53), 155(40), 128(34), 115(25), 105(21), 91(36), 77(63), 51(33), 43(65); HRMS (EI): m/z calcd for C26H25O2P 400.1592; found 400.1587.
10). 1H NMR (400 MHz, CDCl3) δ 7.94–7.79 (m, 4H), 7.54–7.46 (m, 2H), 7.46–7.38 (m, 4H), 7.36–7.30 (m, 2H), 7.27–7.19 (m, 3H), 6.68 (s, 1H), 2.75–2.65 (m, 1H), 2.62–2.52 (m, 2H), 2.42 (s, 3H), 2.38–2.28 (m, 1H), 1.76–1.63 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 205.3, 140.8 (d, J = 5.7 Hz), 137.5 (d, J = 2.8 Hz), 133.0 (d, J = 9.2 Hz), 132.6 (d, J = 8.6 Hz), 131.8 (d, J = 2.7 Hz), 131.7 (d, J = 2.7 Hz), 131.6 (d, J = 97.8 Hz), 131.0 (d, J = 97.8 Hz), 129.3 (d, J = 6.5 Hz), 128.6, 128.21, 128.17 (d, J = 11.4 Hz), 128.0 (d, J = 11.9 Hz), 127.0, 68.8 (d, J = 64.4 Hz), 32.8 (d, J = 3.7 Hz), 32.7, 28.3, 25.4 (d, J = 5.5 Hz); 31P NMR (162 MHz, CDCl3) δ 35.80, 34.60; IR (CH2Cl2): 3378, 3058, 2960, 2925, 2854, 1699, 1437, 1179, 1112, 750, 722, 696, 543 cm−1; MS (EI): m/z (%) = 400(24)[M+], 359(42), 358(100), 281(16), 219(24), 202(47), 201(98), 182(45), 167(53), 155(40), 128(34), 115(25), 105(21), 91(36), 77(63), 51(33), 43(65); HRMS (EI): m/z calcd for C26H25O2P 400.1592; found 400.1587.
Dimethyl (2E)-2-(4-fluorobenzylidene)cyclopentane-1,1-dicarboxylate (55)
Prepared in reaction of dimethyl 4-pentenylmalonate and 1-chloro-4-fluorobenzene following modified general procedure (run at 80 °C) (74 mg, yield 63%). Product was isolated as oil after column chromatography on silica gel (15 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (400 MHz, CDCl3) δ 7.33–7.27 (m, 2H), 7.04–6.97 (m, 2H), 6.66 (t, J = 2.7 Hz, 1H), 3.76 (s, 6H), 2.66 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 162.8, 161.5 (d, J = 246.8 Hz), 140.6 (d, J = 2.1 Hz), 133.7 (d, J = 3.3 Hz), 130.2 (d, J = 8.0 Hz), 126.3, 115.0 (d, J = 21.5 Hz), 65.3, 52.7, 35.7, 31.8, 24.7; 19F NMR (376 MHz, CDCl3) δ −114.96; IR (CH2Cl2): 2954, 2879, 2842, 1733, 1603, 1508, 1434, 1227, 1190, 1159, 1098, 1065, 1014, 929, 885, 827, 773, 523 cm−1. MS (EI): m/z (%) = 293(6)[M+], 292(32), 260(13), 233(32), 232(44), 201(65), 200(21), 173(100), 146(23), 133(18), 109(44), 77(7), 59(16), 43(4); HRMS (EI): m/z calcd for C16H17O4F 292.1111; found 292.1117.
10). 1H NMR (400 MHz, CDCl3) δ 7.33–7.27 (m, 2H), 7.04–6.97 (m, 2H), 6.66 (t, J = 2.7 Hz, 1H), 3.76 (s, 6H), 2.66 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 6.9 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.3, 162.8, 161.5 (d, J = 246.8 Hz), 140.6 (d, J = 2.1 Hz), 133.7 (d, J = 3.3 Hz), 130.2 (d, J = 8.0 Hz), 126.3, 115.0 (d, J = 21.5 Hz), 65.3, 52.7, 35.7, 31.8, 24.7; 19F NMR (376 MHz, CDCl3) δ −114.96; IR (CH2Cl2): 2954, 2879, 2842, 1733, 1603, 1508, 1434, 1227, 1190, 1159, 1098, 1065, 1014, 929, 885, 827, 773, 523 cm−1. MS (EI): m/z (%) = 293(6)[M+], 292(32), 260(13), 233(32), 232(44), 201(65), 200(21), 173(100), 146(23), 133(18), 109(44), 77(7), 59(16), 43(4); HRMS (EI): m/z calcd for C16H17O4F 292.1111; found 292.1117.
Dimethyl (2E)-2-(4-(methoxycarbonyl)benzylidene)cyclopentane-1,1-dicarboxylate (56)
Prepared in reaction of dimethyl 4-pentenylmalonate and methyl 4-chlorobenzoate following modified general procedure (run at 80 °C) (78 mg, yield 59%, isomer E/Z 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90
20). Product was isolated as oil after column chromatography on silica gel (25 g, hex/AcOEt 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 → 85
10 → 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15). 1H NMR (400 MHz, CDCl3) δ 7.99–7.95 (m, 2H), 7.40–7.36 (m, 2H), 6.72 (t, J = 2.6 Hz, 1H), 3.88 (s, 3H), 3.76 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 7.0 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.0, 166.8, 143.7, 142.0, 129.4, 128.5, 128.3, 126.7, 65.5, 52.8, 51.9, 35.6, 32.1, 24.7; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.95–7.89 (m, 2H), 7.35 (s, 1H), 6.65 (d, J = 2.3 Hz, 1H), 3.87 (s, 3H), 3.43 (s, 5H), 2.67–2.62 (m, 2H), 2.42 (t, J = 7.0 Hz, 2H), 1.78–1.70 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 170.7, 143.5, 141.4, 129.0, 128.3, 128.2, 125.8, 63.8, 52.4, 39.4, 34.8, 22.5, 14.0; IR (CH2Cl2): 2963, 2843, 1724, 1606, 1565, 1435, 1279, 1183, 1156, 1111, 1066, 1017, 966, 890, 777, 700, 522 cm−1; MS (EI): m/z (%) = 333(9)[M+], 332(39), 301(24), 273(24), 272(43), 242(32), 241(100), 240(48), 214(30), 213(98), 181(32), 155(35), 154(39), 153(48), 129(44), 128(19), 115(16), 105(6), 91(9), 77(12), 59(41), 41(4); HRMS (EI): m/z calcd for C18H20O6 332.1260; found 332.1266.
15). 1H NMR (400 MHz, CDCl3) δ 7.99–7.95 (m, 2H), 7.40–7.36 (m, 2H), 6.72 (t, J = 2.6 Hz, 1H), 3.88 (s, 3H), 3.76 (s, 6H), 2.70 (td, J = 7.2, 2.6 Hz, 2H), 2.38 (t, J = 7.0 Hz, 2H), 1.83 (p, J = 7.1 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 171.0, 166.8, 143.7, 142.0, 129.4, 128.5, 128.3, 126.7, 65.5, 52.8, 51.9, 35.6, 32.1, 24.7; indicative signals of minor isomer (Z): 1H NMR (400 MHz, CDCl3) δ 7.95–7.89 (m, 2H), 7.35 (s, 1H), 6.65 (d, J = 2.3 Hz, 1H), 3.87 (s, 3H), 3.43 (s, 5H), 2.67–2.62 (m, 2H), 2.42 (t, J = 7.0 Hz, 2H), 1.78–1.70 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 170.7, 143.5, 141.4, 129.0, 128.3, 128.2, 125.8, 63.8, 52.4, 39.4, 34.8, 22.5, 14.0; IR (CH2Cl2): 2963, 2843, 1724, 1606, 1565, 1435, 1279, 1183, 1156, 1111, 1066, 1017, 966, 890, 777, 700, 522 cm−1; MS (EI): m/z (%) = 333(9)[M+], 332(39), 301(24), 273(24), 272(43), 242(32), 241(100), 240(48), 214(30), 213(98), 181(32), 155(35), 154(39), 153(48), 129(44), 128(19), 115(16), 105(6), 91(9), 77(12), 59(41), 41(4); HRMS (EI): m/z calcd for C18H20O6 332.1260; found 332.1266.
Conclusions
In summary, we developed an efficient protocol for tandem Pd-catalyzed intramolecular addition of active methylene compounds to alkynes, followed by subsequent cross-coupling with (hetero)aryl bromides and chlorides. The methodology features exceptional tolerance to functional groups (including unprotected OH, NH2, or enolizable ketones), broad applicability of aryl and heteroaryl bromides of different electronic properties, as well as a range of active methylene partners, including acetylenic derivatives of malonates, cyanomalonates, β-ketoesters, β-diketones, cyanoacetates, and organophosphorus compounds. Mechanistic studies revealed a plausible mechanism comprising oxidative addition of haloarene, nucleophilic addition to alkyne activated by coordination to aryl–Pd(II), and reductive elimination. However, for the transformations of less C–H acidic substrates (e.g. β-ketoesters, β-diketones) and electron-deficient haloarenes, an alternative path involving syn-carbometallation may operate in parallel.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Polish National Science Centre (Grant decision DEC-2016/22/E/ST5/00537) is gratefully acknowledged.Notes and references
- X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed.
- (a) D. E. Fogg and E. N. dos Santos, Coord. Chem. Rev., 2004, 248, 2365–2379 CrossRef CAS; (b) R. P. Herrera and E. Marqués-López, Multicomponent Reactions: Concepts and Applications for Design and Synthesis, Wiley, 1st edn, 2015 Search PubMed.
- (a) S. Cacchi, J. Organomet. Chem., 1999, 576, 42–64 CrossRef CAS; (b) G. Balme, E. Bossharth and N. Monteiro, Eur. J. Org. Chem., 2003, 2003, 4101–4111 CrossRef; (c) G. Balme, D. Bouyssi, T. Lomberget and N. Monteiro, Synthesis, 2003, 2115–2134 CrossRef CAS; (d) F. Alonso, I. P. Beletskaya and M. Yus, Chem. Rev., 2004, 104, 3079–3160 CrossRef CAS PubMed; (e) G. Balme, D. Bouyssi and N. Monteiro, Pure Appl. Chem., 2006, 78, 231–239 CAS; (f) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS PubMed; (g) F. Dénès, A. Pérez-Luna and F. Chemla, Chem. Rev., 2010, 110, 2366–2447 CrossRef PubMed; (h) H. Ohno, Asian J. Org. Chem., 2013, 2, 18–28 CrossRef CAS; (i) R. Chinchilla and C. Nájera, Chem. Rev., 2014, 114, 1783–1826 CrossRef CAS PubMed.
- (a) G. Fournet, G. Balme and J. Gore, Tetrahedron Lett., 1987, 28, 4533–4536 CrossRef CAS; (b) G. Fournet, G. Balme and J. Gore, Tetrahedron Lett., 1989, 30, 69–70 CrossRef CAS.
- (a) G. Fournet, G. Balme, B. Van Hemelryck and J. Gore, Tetrahedron Lett., 1990, 31, 5147–5150 CrossRef CAS; (b) G. Fournet, G. Balme and J. Gore, Tetrahedron, 1991, 47, 6293–6304 CrossRef CAS.
- D. Bouyssi, G. Balme and J. Gore, Tetrahedron Lett., 1991, 32, 6541–6544 CrossRef CAS.
- G. Liu and X. Lu, Tetrahedron Lett., 2002, 43, 6791–6794 CrossRef CAS.
- W. Chaładaj and S. Domański, Adv. Synth. Catal., 2016, 358, 1820–1825 CrossRef.
- D. Fujino, H. Yorimitsu and A. Osuka, Org. Lett., 2012, 14, 2914–2917 CrossRef CAS PubMed.
- (a) X.-H. Duan, L. Guo, H.-P. Bi, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2006, 8, 3053–3056 CrossRef CAS PubMed; (b) L.-N. Guo, X.-H. Duan, H.-P. Bi, X.-Y. Liu and Y.-M. Liang, J. Org. Chem., 2006, 71, 3325–3327 CrossRef CAS PubMed; (c) D. Zhang, Z. Liu, E. K. Yum and R. C. Larock, J. Org. Chem., 2007, 72, 251–262 CrossRef CAS PubMed; (d) Z.-H. Guan, Z.-H. Ren, L.-B. Zhao and Y.-M. Liang, Org. Biomol. Chem., 2008, 6, 1040–1045 RSC.
- D. Fujino, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2011, 133, 9682–9685 CrossRef CAS PubMed.
- (a) A. Arcadi, S. Cacchi, R. C. Larock and F. Marinelli, Tetrahedron Lett., 1993, 34, 2813–2816 CrossRef CAS; (b) A. Arcadi and E. Rossi, Tetrahedron Lett., 1996, 37, 6811–6814 CrossRef CAS; (c) A. Arcadi, S. Cacchi, G. Fabrizi, F. Marinelli and L. M. Parisi, Tetrahedron, 2003, 59, 4661–4671 CrossRef CAS; (d) A. Kołodziejczyk and W. Chaładaj, Eur. J. Org. Chem., 2018, 2018, 2554–2560 CrossRef.
- A. Kołodziejczyk, S. Domański and W. Chaładaj, J. Org. Chem., 2018, 83, 12887–12896 CrossRef PubMed.
- See the ESI† for more details.
- N. C. Bruno, M. T. Tudge and S. L. Buchwald, Chem. Sci., 2013, 4, 916–920 RSC.
- (a) J. M. Fox, X. Huang, A. Chieffi and S. L. Buchwald, J. Am. Chem. Soc., 2000, 122, 1360–1370 CrossRef CAS; (b) H. Tanaka, K. Isobe, S. Kawaguchi and S. Okeya, Bull. Chem. Soc. Jpn., 1984, 57, 1850–1855 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08002c |
| This journal is © The Royal Society of Chemistry 2019 |