Open Access Article
Open Access ArticleEnhancing the Vickers hardness, melting point and thermodynamic properties of hafnium dodecaboride†
Yong Pan *a,
Shuang Chena and
Yanlin Jia*bc
*a,
Shuang Chena and
Yanlin Jia*bc
aSchool of Materials Science and Engineering, Southwest Petroleum University, Chengdu, 610500, China. E-mail: y_pan@ipm.com.cn
bCollege of Materials Science and Engineering, Central South University, Changsha, 410083, China. E-mail: jiayanlin@126.com
cCollege of Materials Science and Engineering, Beijing University of Technology, 100 Ping Le Yuan, Chaoyang District, Beijing 100124, China
First published on 18th October 2019
Abstract
Although HfB12 is a promising surperhard material because of the boron cuboctahedron cage, the Vickers hardness of HfB12 remains controversial. We apply first-principles calculations to investigate the influence of a transition metal on the structural stability, Vickers hardness and thermodynamic properties of HfB12. The Vickers hardness of HfB12 is 39.3 GPa. In particular, the Vickers hardness of TM-doped HfB12, which are novel superhard materials, is larger than 40 GPa. The Vickers hardness of Re-doped HfB12 is up to 47.6 GPa. The improvement of Vickers hardness is that the introduction of an alloying element improves the localized hybridization between B and Hf, and then enhances the bond strength of the B–B covalent bond and the Hf–B bond. In addition, these alloying elements enhance the melting-point and Debye temperature of the HfB12. Therefore, we believe that alloying is an effective method to improve the Vickers hardness and thermodynamic properties of HfB12 superhard material.
1. Introduction
Compared to diamond and boron nitride superhard materials, transition metal borides (TMBs) are attractive superhard materials because of their fascinating mechanical, electronic and thermodynamic properties etc.1–9 However, hardness is a complex phenomenon, which is related to the structural configuration, chemical bonding, electronic structure, deformation, defects, etc.10–14 For TMBs superhard materials, previous work has shown that the Vickers hardness is determined by the network and shorter B–B covalent bonds.15,16 Namely, the boron concentration plays a crucial role in hardness. Therefore, research has focused on the boron-rich borides such as TMB3, TMB4 and TMB12 in recent years.17–21 However, the investigation of boron-rich boride faces two key problems: one is that the boron-rich boride has difficulty meeting stable conditions. For example, the published TMB12 mainly focuses on the ZrB12 and HfB12.22 The investigation of other TMB12 is scarce. Another important factor is that the Vickers hardness of TMBs is also influenced by the TM–B bond, in addition to the B–B covalent bond.Thus, alloying is an effective approach to improve the bond strength of TM–B bond and then enhance the Vickers hardness of TMBs superhard material.23–26 For example, Kaner et al. work has shown that the measured Vickers hardness of WB4 with alloyed 20 at% Ti, 10 at% Zr and 6 at% Hf is up to 50.9 ± 2.2 GPa, 55.9 ± 2.7 GPa and 51.6 ± 2.8 GPa, respectively.27 The WB is not a superhard material. However, Ta addition markedly improves the Vickers hardness of WB, while the measured Vickers hardness of W0.5Ta0.5B is up to 42.8 GPa.28 Similarly, the Y and Sc additions enhance Vickers hardness of ZrB12, while the measured Vickers hardness of Zr0.5Y0.5B12 and Zr0.5Sc0.5B12 is up to 45.8 GPa and 48.0 GPa,29 respectively. Based on the design principle, we believe that the alloying is to improve the bonding state (TM–B bond) and Vickers hardness of TMBs superhard materials.
Among these TMBs, HfB12 with cubic structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) is an attractive superhard material because this dodecaboride is composed of the boron cuboctahedron cage (24 B atoms).30,31 The boron cage can enhance the Vickers hardness and elastic modulus of HfB12. However, the superhard characteristic of HfB12 remains controversy. For example, the recent work has shown that the calculated Vickers hardness of HfB12 is 39.1 GPa,32 which is not a superhard material (≥40 GPa). Therefore, the great challenge of TMBs superhard material is how to enhance the Vickers hardness of HfB12. On the other hand, the thermodynamic properties must be considered as the important factor because the thermal stability plays a crucial role in industrial applications. For instance, diamond does not cut the ferrous material because of the formation of iron when the temperature is above than 600 °C.33 As a result, an important work is how to improve the melting point and Debye temperature of superhard materials.
m) is an attractive superhard material because this dodecaboride is composed of the boron cuboctahedron cage (24 B atoms).30,31 The boron cage can enhance the Vickers hardness and elastic modulus of HfB12. However, the superhard characteristic of HfB12 remains controversy. For example, the recent work has shown that the calculated Vickers hardness of HfB12 is 39.1 GPa,32 which is not a superhard material (≥40 GPa). Therefore, the great challenge of TMBs superhard material is how to enhance the Vickers hardness of HfB12. On the other hand, the thermodynamic properties must be considered as the important factor because the thermal stability plays a crucial role in industrial applications. For instance, diamond does not cut the ferrous material because of the formation of iron when the temperature is above than 600 °C.33 As a result, an important work is how to improve the melting point and Debye temperature of superhard materials.
In the present work, we apply the first-principles approach to investigate the influence of alloying elements on the Vickers hardness, elastic modulus and thermodynamic properties of HfB12. We consider five possible transition metals: Nb(4d-), Mo(4d-), W(5d-), Re(5d-) and Os(5d-), respectively. Our work shows that these alloying elements not only enhance the Vickers hardness, but also improve the melting point and Debye temperature of HfB12. Therefore, we predict that TM-doped HfB12 is a novel superhard material.
2. Theoretical methods
To explore the TMBs superhard materials, we mainly focus on the boron-rich TMBs. Therefore, HfB12 with the cubic structure is likely to a potential superhard material. The experimental lattice parameter of HfB12 is a = 7.377 Å. HfB12 with a unit-cell has 52 atoms, which are composed of B cuboctahedron cage. The structural configuration of HfB12 is shown in ESI.† It is obvious that the network B–B covalent bond is the origin of high hardness of HfB12. However, the Vickers hardness of HfB12 is also affected by the bond strength of Hf–B bond, in addition to the network B–B covalent bonds. To improve the bond strength of Hf–B bond and enhance the Vickers hardness, one Hf atom in a unit-cell is replaced by the 4d- and 5d-transition metal.In this paper, the Vickers hardness of HfB12 with alloying elements is calculated by the semiempirical model.34 On the other hand, the Vickers hardness of a solid is indirectly estimated by the elastic modulus and brittle behavior. Here, the elastic constants of TM-doped HfB12 are calculated by the stress vs. stain method.35,36 The elastic modulus of TM-doped HfB12 is obtained by the elastic constants. Here, we consider three elastic parameters: bulk modulus, shear modulus and Young modulus,37–41 respectively. The bulk modulus and shear modulus of TM-doped HfB12 are calculated by the Voigt–Reuss–Hill (VRH) approximation.42,43 In addition, the Vickers hardness of TMBs is indirectly demonstrated by the B/G ratio.44,45 The general trend is, the lower the B/G ratio, the high Vickers hardness for the TMBs.46,47
In addition, we should be considered the thermodynamic stability of alloying elements in HfB12. Here, the thermodynamic stability of TM-doped HfB12 is estimated by the impurity formation energy (Ef),48 which is given by:
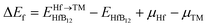 | (1) |
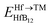 and EHfB12 are the total energy of TM-doped HfB12 and the HfB12, respectively. μHf and μTM are the chemical potential of Hf and TM (TM = Nb, Mo, W, Re and Os) elements. The chemical potential of a solid is estimated by the electronic energy difference. When considering the elemental phases, the chemical potential is simply proportional to the Gibbs energy of the system.49,50
and EHfB12 are the total energy of TM-doped HfB12 and the HfB12, respectively. μHf and μTM are the chemical potential of Hf and TM (TM = Nb, Mo, W, Re and Os) elements. The chemical potential of a solid is estimated by the electronic energy difference. When considering the elemental phases, the chemical potential is simply proportional to the Gibbs energy of the system.49,50
About thermodynamic properties, we consider two important parameters: the melting point and Debye temperature, respectively. The melting point of TM-doped HfB12 is calculated by:51,52
| Tm = 354 + 4.5(2C11 + C33)/3 | (2) |
The Debye temperature of TM-doped HfB12 is calculated by the average sound velocity, which is obtained by:53,54
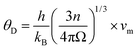 | (3) |
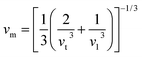 | (4) |
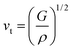 | (5) |
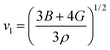 | (6) |
All calculations in this paper were calculated by the first-principles calculations, as implemented in the CASTEP code.55,56 The exchange-correlation-function was treated by the generalized gradient correction(GGA) within Perdew–Burke–Ernzerhof functional (PBE) functional.57,58 The cutoff energy of HfB12 with alloying element was 400 eV for the plane-wave expansion. The integration in the Brillouin zone was carried out by the k-point grid of 5 × 5 × 5. The interaction between the ionic and valence electron was treated by the ultrasoft pseudopotential.59,60 The SCF tolerance was smaller than 1.0 × 10−6 eV/atom and the maximal displacement was lower than 0.001 Å. During the process of structural optimization, all systems were relaxed.61–63
3. Results and discussions
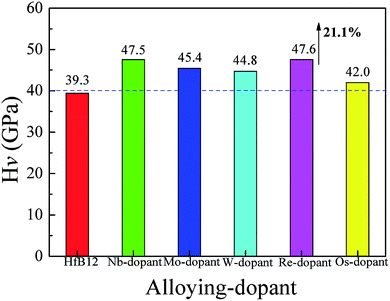
To investigate the influence of alloying elements on the Vickers hardness of HfB12, Fig. 1 shows the calculated Vickers hardness of TM-doped HfB12, and the HfB12 for comparison. It can be seen that the calculated Vickers hardness of HfB12 is 39.3 GPa, which is in good agreement with the Korozlu et al. work (39.1 GPa).64 When TM atom is introduced, it is found that these alloying elements markedly enhance the Vickers hardness of HfB12. In particular, the calculated Vickers hardness of Re-doped HfB12 is up to 47.6 GPa, which is 21.1% larger than that of the HfB12. It must be mentioned that the calculated Vickers hardness of TM-doped HfB12 is above 40 GPa, indicating that TM-doped HfB12 are novel superhard materials. Therefore, we believe that alloying is an effective method to enhance the Vickers hardness of TMBs.To demonstrate the influence of transition metal on the hardness of HfB12, Fig. 2 shows the calculated bulk modulus, shear modulus and Young's modulus of TM-doped HfB12 and the HfB12. Here, the calculated bulk modulus, shear modulus and Young's modulus of HfB12 are 238.2 GPa, 188.6 GPa and 447.6 GPa, respectively, which are in good agreement with the other theoretical results.64 When Hf atom is substituted by the TM atom, although the calculated bulk modulus of TM-doped HfB12 is smaller than that of the HfB12, the calculated shear modulus and Young's modulus of TM-doped HfB12 are larger than that of the HfB12. As mentioned above, it is concluded that these alloying elements enhance the shear deformation resistance and elastic stiffness of the HfB12. Here, the calculated shear modulus and Young's modulus of Nb-doped HfB12 are 210.0 GPa and 487.3 GPa, respectively, which are larger than that of the other TM-doped HfB12. Namely, the 4d-Nb enhances the shear deformation resistance and elastic stiffness of HfB12 in comparison to 5d-TM. The increasing of shear deformation is that these alloying elements improve the localized hybridization between B and Hf along the shear direction. This result is demonstrated by the variation of chemical bonding (see charge density distribution).
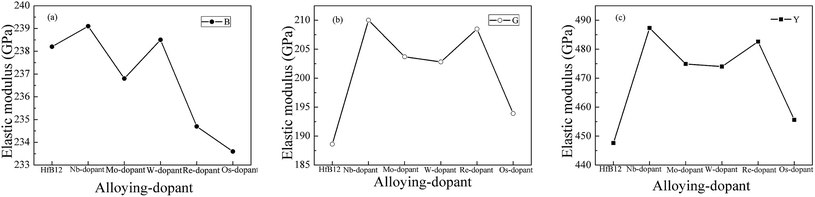 | ||
| Fig. 2 Calculated elastic modulus of TM-doped HfB12 and the HfB12, (a) bulk modulus, (b) shear modulus and (c) Young's modulus, respectively. | ||
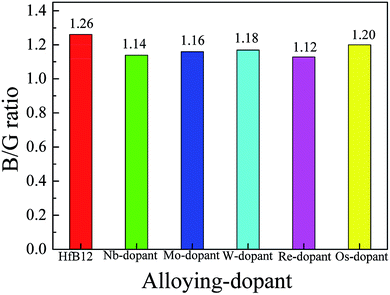
As mentioned above, we know that the Vickers hardness of TMBs is indirectly demonstrated by the B/G ratio. To demonstrate the variation of hardness, Fig. 3 displays the calculated B/G ratio of TM-doped HfB12 and the HfB12. The calculated B/G ratio of the HfB12 is 1.26, indicating that HfB12 is a brittle material. Note that the calculated B/G ratio of TM-doped HfB12 is smaller than that of the HfB12. This result indicates that these alloying additions enhance the Vickers hardness of the HfB12. The trend of B/G ratio is consistent with the variation of Vickers hardness of TM-doped HfB12.
Based on the analysis of mechanical properties, we believe that these alloying elements enhance the Vickers hardness of the HfB12. Following, it is necessary to study the stability of alloying elements in HfB12. Table 1 lists the calculated lattice parameter, density, volume and impurity formation energy of TM-doped HfB12. It is found that these alloying elements are thermodynamic stability in HfB12 because the calculated impurity formation energy of TM-doped HfB12 is smaller than zero. It is worth noticing that the calculated impurity formation energy of TM-doped HfB12 increases gradually with increasing the atomic number. This trend may be related to the valence electronic configuration of TM atom. Here, the thermodynamic stability of TM-doped HfB12 follows the order of Nb-dopant > Mo-dopant > W-dopant > Re-dopant > Os-dopant.
Naturally, the trend of thermodynamic stability is related to the electronic interaction between atoms, which are demonstrated by the variation of lattice parameter. As listed in Table 1, firstly, we find that the calculated lattice parameter of the HfB12 is a = 7.389 Å, which is in good agreement with the other theoretical result and experimental data.64,65 However, the alloying additions lead to lattice shrinkage of HfB12 because the calculated lattice parameter of TM-doped HfB12 is smaller than that of the parent HfB12. This result also demonstrates that these alloying elements enhance the localized hybridization between B and Hf. As a result, alloying addition effectively enhances the bond strength of B–B covalent bond and Hf–B bond. This is why the Vickers hardness of TM-doped HfB12 is larger than that of the HfB12.
It is well known that the structural stability is related not only to the thermodynamic stability but also to the dynamic stability.66,67 Generally, the dynamic stability of a solid is measured by the phonon frequency. The imaginary phonon frequency means the dynamic instability, and vice versa. To examine the dynamic stability, Fig. 4 shows the calculated phonon dispersion curves of TM-doped HfB12 and the HfB12 along the high symmetry direction. It can be seen that HfB12 is a dynamic stability because no imaginary phonon frequencies are found in HfB12. However, we observed the imaginary phonon frequency in TM-doped HfB12, indicating that TM-dopant is a dynamic instability in HfB12. That is to say, these alloying additions will form the solid solution in HfB12. These results are similar to the Kaner et al. report.27,68
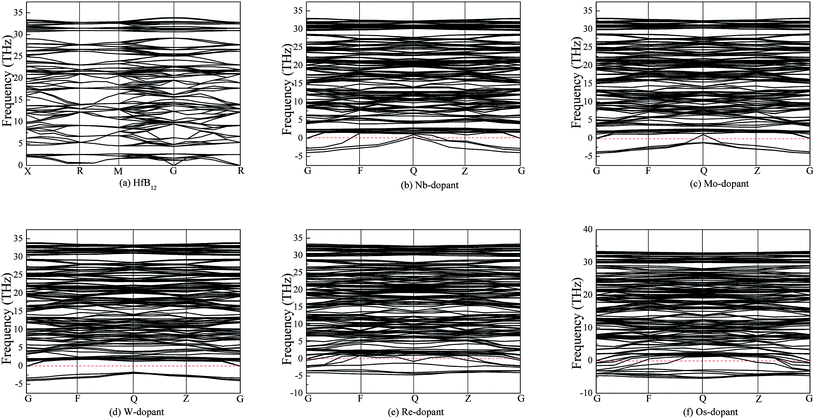 | ||
| Fig. 4 . Calculated phonon dispersion curves of TM-doped HfB12 and the HfB12, (a) the HfB12, (b) Nb-dopant, (c) Mo-dopant, (d) W-dopant, (e) Re-dopant and (f) Os-dopant, respectively. | ||
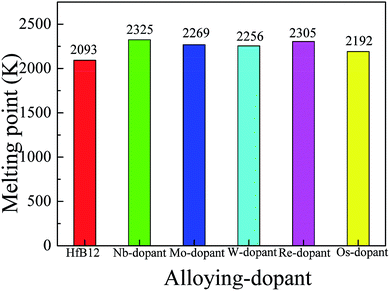
To explore the influence of alloying elements on the thermodynamic properties of HfB12 superhard material, here, we consider two thermodynamic parameters: melting point and Debye temperature, respectively. Fig. 5 shows the calculated melting point of TM-doped HfB12 and the HfB12. It can be seen that the calculated melting point of the HfB12 is 2093 K. In particular, we note that the calculated melting point of TM-doped HfB12 is much larger than that of the parent HfB12. Here, the calculated melting point follows the order of Nb-dopant > Re-dopant > Mo-dopant > W-dopant > Os-dopant > HfB12.
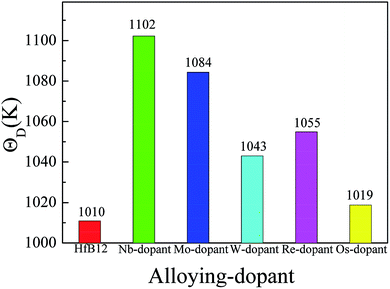
Fig. 6 shows the calculated Debye temperature of TM-doped HfB12 and the HfB12 for comparison. Here, the calculated Debye temperature of the HfB12 is 1010 K. Similarly, the calculated Debye temperature of TM-doped HfB12 is larger than that of the HfB12. In our work, the trend of Debye temperature is similar to the variation of melting point. In particular, the calculated Debye temperature of Nb-doped HfB12 is 1102 K, which is larger than that of the other TM-doped HfB12 and the HfB12. Therefore, we believed that these alloying elements improve the thermodynamic properties of HfB12. As mentioned above, it is concluded that Nb is the best element to enhance the thermodynamic properties of HfB12 in comparison to the other alloying elements.
Fig. 7 shows the calculated total and partial density of state (DOS) of TM-doped HfB12 and the HfB12. It is found that the DOS profile of the HfB12 is composed of the B-2s state, B-2p state and Hf-5d state. In particular, the formation of B–B covalent bond is attributed to the localized hybridization between B and B. Based on the structural configuration, we suggest that the high hardness of HfB12 is attributed to the network B–B covalent bonds. The bond characteristic of B–B covalent bond is demonstrated by the charge density distribution.
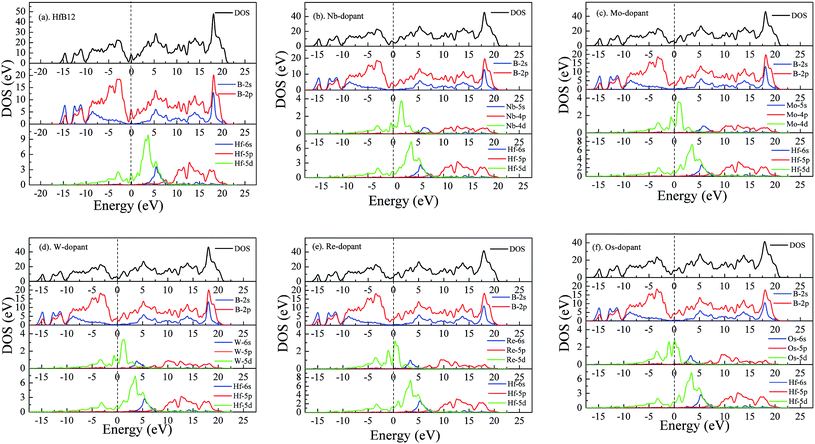 | ||
| Fig. 7 Calculated density of state of TM-doped HfB12 and the HfB12, (a) the HfB12, (b) Nb-dopant, (c) Mo-dopant, (d) W-dopant, (e) Re-dopant and (f) Os-dopant, respectively. | ||
When Hf atom is replaced by TM atom, it is found that the introduction of alloying element gives rise to B-2p state migration from the high energy region to the low energy region. This phenomenon will enhance the localized hybridization between B and B, and then improve the bond strength of B–B covalent bond. In addition, the band migration of B-2p state also changes the localized hybridization between Hf and B. As a result, the formation of B–B covalent bond and Hf–B bond enhances the Vickers hardness of the HfB12. This result is well affirmed by the charge density distribution.
To reveal the nature of Vickers hardness, Fig. 8 displays the calculated charge density distribution in (001) plane for TM-doped HfB12 and the HfB12. For charge density distribution, the red color means the maximum localization of electrons and the blue color implies the maximum delocalization of electrons.69 Compared to the other alloying elements, it can be seen that the alloying element of Re markedly improves the Vickers hardness of the HfB12. Hence, we will focus on the charge density distribution between HfB12 and Re-doped HfB12.
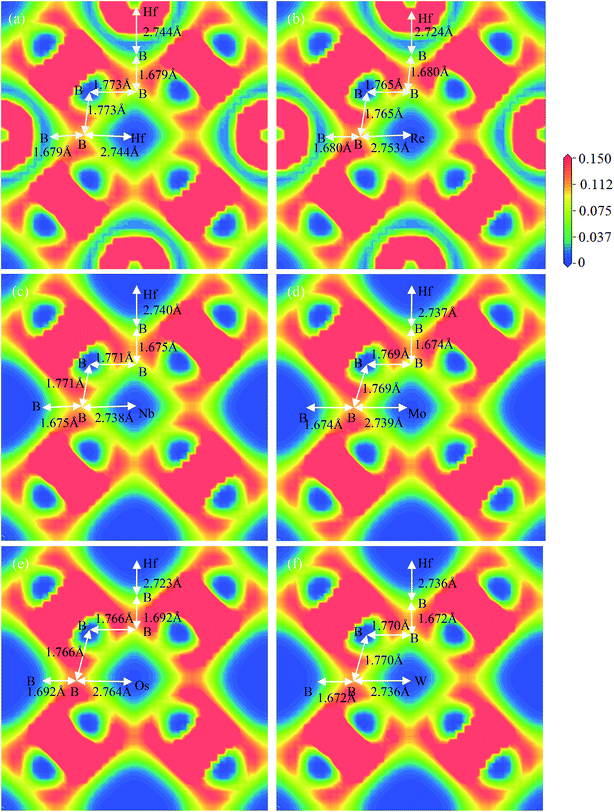 | ||
| Fig. 8 Calculated charge density distribution along the (001) plane, (a) the HfB12, (b) Re-dopant, (c) Nb-dopant, (d) Mo-dopant, (e) Os-dopant and (f) W-dopant, respectively. | ||
From Fig. 8, firstly, we observe the formation of directional B–B covalent bonds because of the strong localized hybridization between B and Hf. The B icosahedrons cage will form two different B–B covalent bonds, which are the origin of high hardness. For the parent HfB12(see Fig. 8(a)), in our work, the calculated bond length of B–B covalent bond is 1.679 Å and 1.773 Å, respectively, which are in good agreement with the other theoretical result (1.682 Å).65 In addition we find that the calculated bond length of Hf–B bond is 2.744 Å. In particular, the B–B covalent bonds will form the 3D-network bond, which can improve the mechanical properties of HfB12. Therefore, it is concluded that the Vickers hardness of HfB12 is also affected by the bond strength of Hf–B bond in addition to the B–B covalent bonds.
However, we find that the introduction of alloying element can improve the localize hybridization between B and the near B, which is demonstrated by the bond length of B–B covalent bond. From Fig. 8, it can be seen that the bond length of B–B covalent bond of TM-doped HfB12(near TM atom) is shorter than that of the corresponding B–B covalent bond of HfB12(1.773 Å). On the other hand, it is find that the calculated bond length of Hf–B bond for TM-doped HfB12 is also shorter than that of the corresponding Hf–B bond for HfB12. Namely, the alloying addition can improve the electronic interaction between Hf and B atoms. This is why the Vickers hardness of TM-doped HfB12 is larger than that of the HfB12.
For example, we find that the introduction of alloying element (Re) improves the localized hybridization between B and Hf. From Fig. 7 and 8, the introduction of alloying element (Re) changes the charge interaction between B and Hf, and then improves the bonding state. Here, the calculated bond length of the corresponding B–B covalent bond is 1.680 Å along the a–c plane and 1.765 Å along the shear direction, respectively. There is well explained why the shear deformation resistance of TM-doped HfB12 is stronger than that of the parent HfB12. On the other hand, the calculated bond length of Hf–B bond is 2.724 Å, which is shorter than the corresponding Hf–B bond for the HfB12. As mentioned above, it is concluded that alloying is an effective method to improve the Vickers hardness of TMBs superhard material.
4. Conclusions
In summary, we apply the first-principles calculations to investigate the improvement of Vickers hardness, melting point and Debye temperature of HfB12 with alloying element, together with the HfB12 for comparison. We consider five transition metals: Nb(4d-), Mo(4d-), W(5d-), Re(5d-) and Os(5d-) respectively. The results show that the calculated Vickers hardness of HfB12 is 39.3 GPa, which is in good agreement with the other theoretical result. In particular, the calculated Vickers hardness of TM-doped HfB12 is bigger than 40 GPa, indicating that TM-doped HfB12 is a novel superhard material. The calculated Vickers hardness of Re-doped HfB12 is up to 47.6 GPa, which is 21.1% larger than that of the HfB12.The variation of Vickers hardness is demonstrated by the elastic modulus and brittleness. The calculated shear modulus and Young's modulus of TM-doped HfB12 are larger than that of the HfB12. Interestingly, the calculated shear modulus and Young's modulus of Nb(4d)-doped HfB12 are bigger than that of the Re(5d)-doped HfB12. The calculated B/G ratio of TM-doped HfB12 is smaller than that of the HfB12, indirectly demonstrates that the Vickers hardness of TM-doped HfB12 is bigger than that of the HfB12. In addition, the calculated melting point and Debye temperature of TM-doped HfB12 are larger than that of the HfB12. In particular, the calculated melting point and Debye temperature of Nb-doped HfB12 are 2325 K and 1102 K, which are higher than that of the other TM-doped HfB12. The improvement of Vickers hardness is that the introduction of alloying element improves the localized hybridization between B and Hf, and then enhances the bond strength of B–B bond and Hf–B bond.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by grants from the National Natural Science Foundation of China (No. 51274170) and State Key Laboratory of Advanced Technology for Comprehensive Utilization of Platinum Metals (grant no. SKL-SPM-201816). We acknowledge the discussion from Lady Yun Zheng and Runxi Pan.References
- P. R. Jothi, K. Yubuta and B. P. T. Fokwa, Adv. Mater., 2018, 30, 1704181 CrossRef.
- Y. Pan and Y. Lin, J. Phys. Chem. C, 2015, 119, 23175–23183 CrossRef CAS.
- S. Ma, K. Bao, Q. Tao, C. Xu, X. Feng, X. Zhao, Y. Ge, P. Zhu and T. Cui, Phys. Chem. Chem. Phys., 2019, 21, 2697–2705 RSC.
- Y. Pan, W. M. Guan and Y. Q. Li, Phys. Chem. Chem. Phys., 2018, 20, 15863–15870 RSC.
- P. J. Robinson, G. Liu, S. Ciborowski, C. M. Martinez, J. R. Chamorro, X. Zhang, T. M. Mcqueen, K. H. Bowen and A. N. Alexandroca, Chem. Mater., 2017, 29, 9892–9896 CrossRef CAS.
- Y. Pan, S. Wang, X. Zhang and L. Jia, Ceram. Int., 2018, 44, 1744–1750 CrossRef CAS.
- C. Zhao, Y. Duan, J. Gao, W. Liu, H. Dong, H. Dong, D. Zhang and A. R. Oganov, Phys. Chem. Chem. Phys., 2018, 20, 24665–24670 RSC.
- Y. Pan and B. Zhou, Ceram. Int., 2017, 43, 8763–8768 CrossRef CAS.
- A. G. Kvashnin, H. A. Zakaryan, C. Zhao, Y. Duan, Y. A. Kvashnina, C. Xie, H. Dong and A. R. Oganov, J. Phys. Chem. Lett., 2018, 9, 3470–3477 CrossRef CAS.
- Y. Pan and W. M. Guan, Phys. Chem. Chem. Phys., 2017, 19, 19427–19433 RSC.
- P. Wang, Z. Y. Gong, J. Hu, J. Pu and W. J. Cao, Surf. Eng., 2019, 35, 627–634 CrossRef CAS.
- Y. Pan, P. Mao, H. Jiang, Y. Wan and W. Guan, Ceram. Int., 2017, 43, 5274–5282 CrossRef CAS.
- P. Wang, T. Wu, Y. T. Xiao, L. Zhang, J. Pu, W. J. Cao and X. M. ZHong, Vacuum, 2017, 142, 21–28 CrossRef CAS.
- Y. Pan and C. Jing, Ceram. Int., 2019, 45, 21373–21378 CrossRef CAS.
- G. Akopov, M. T. Yeung and R. B. Kaner, Adv. Mater., 2017, 29, 1604506 CrossRef.
- Y. Pan, Y. Lin and C. Tong, J. Phys. Chem. C, 2016, 120, 21762–21769 CrossRef CAS.
- L. H. Huang, Y. R. Zhao, G. T. Zhang, M. G. Zhang, P. Y. Li and Y. F. Hu, Mol. Phys., 2019, 117, 547–556 CrossRef CAS.
- G. Zhang, R. Gao, Y. Zhao, T. Bai and Y. Hu, J. Alloys Compd., 2017, 723, 802–810 CrossRef CAS.
- G. Zhang, T. Bai, Y. Zhao and Y. Hu, Materials, 2016, 9, 703 CrossRef.
- Y. Pan, X. Wang, S. Li, Y. Li and M. Wen, RSC Adv., 2018, 8, 18008–18015 RSC.
- X. Li, Y. Tao and F. Peng, J. Alloys Compd., 2016, 687, 579–585 CrossRef CAS.
- Z. Q. Chen, Y. S. Peng, M. Hu, C. M. Li and Y. T. Luo, Ceram. Int., 2016, 42, 6624–6631 CrossRef CAS.
- Y. Pan and Y. Lin, JOM, 2017, 69, 2009–2013 CrossRef CAS.
- R. Mohammadi, C. L. Turner, M. Xie, M. T. Yeung, A. T. Lech, S. H. Tolbert and R. B. Kaner, Chem. Mater., 2016, 28, 632–637 CrossRef CAS.
- P. Wang, T. Wu, Y. T. Xiao, J. Pu, X. Y. Guo, J. Huang and C. L. Xiang, J. Mater. Eng. Perform., 2016, 25, 3972–3976 CrossRef CAS.
- P. Wang, T. Wu, Y. T. Xiao, J. Pu and X. Y. Guo, Mater. Lett., 2016, 182, 27–31 CrossRef CAS.
- G. Akopov, M. T. Yeung, C. L. Turner, R. Mohammadi and R. B. Kaner, J. Am. Chem. Soc., 2016, 138, 5714–5721 CrossRef CAS.
- M. T. Yeung, J. Lei, R. Mohammadi, C. L. Turner, Y. Wang, S. H. Tolbert and R. B. Kaner, Adv. Mater., 2016, 28, 6993–6998 CrossRef CAS.
- G. Akopov, M. T. Yeung, Z. C. Sobell, C. L. Turner, C. W. Lin and R. B. Kaner, Chem. Mater., 2016, 28, 6605–6612 CrossRef CAS.
- A. G. V. D. Geest and A. N. Kolmogorov, Calphad, 2014, 46, 184–204 CrossRef.
- G. Akopov, I. Roh, Z. C. Sobell, M. T. Yeung and R. B. Kaner, Dalton Trans., 2018, 47, 6683–6691 RSC.
- Y. Liang, Y. Zhang, H. Jiang, L. Wu, W. Zhang, K. Heckenberger, K. Hofmann, A. Reitz, F. C. Stober and B. Albert, Chem. Mater., 2019, 31, 1075–1083 CrossRef CAS.
- A. Latini, L. V. Rau, D. Ferro, R. Teghil, V. R. Albertini and S. Barinov, Chem. Mater., 2008, 20, 4507–4511 CrossRef CAS.
- N. H. Miao, B. S. Sa, J. Zhou and Z. M. Sun, Comput. Mater. Sci., 2011, 50, 1559–1566 CrossRef CAS.
- Y. Pan, P. Wang and C. Zhang, Ceram. Int., 2018, 44, 12357–12362 CrossRef CAS.
- D. Li, X. Zhang, J. Li, L. Zhao, F. Wang and X. Chen, Vacuum, 2019, 169, 108883 CrossRef CAS.
- Y. Pan, Mater. Res. Bull., 2017, 93, 56–62 CrossRef CAS.
- S. Wang, Y. Pan, Y. Lin and C. Tong, Comput. Mater. Sci., 2018, 146, 18–25 CrossRef CAS.
- Y. Pan, S.-L. Wang and C.-M. Zhang, Vacuum, 2018, 151, 205–208 CrossRef CAS.
- W. Ping, W. Ting, P. Hao and G. X. Yang, Mater. Lett., 2016, 170, 171–174 CrossRef CAS.
- Y. Pan and C. Jin, Vacuum, 2017, 143, 165–168 CrossRef CAS.
- R. Hill, Proc. Phys. Soc. A., 1952, 65, 349 CrossRef.
- Y. Pan and M. Wen, Thin Solid Films, 2018, 664, 46–51 CrossRef CAS.
- S. F. Pugh, Philos. Mag. A, 1954, 45, 823–843 CrossRef CAS.
- Y. Pan, Y. Li and Q. Zheng, J. Alloys Compd., 2019, 789, 860–866 CrossRef CAS.
- S. Zhu, X. Zhang, J. Chen, C. Liu, D. Li, H. Yu and F. Wang, Vacuum, 2019, 165, 118–126 CrossRef CAS.
- Y. Pan, C. Jing and Y. P. Wu, Vacuum, 2019, 167, 374–381 CrossRef CAS.
- Y. Pan, J. Alloys Compd., 2019, 779, 813–820 CrossRef CAS.
- H. P. Komsa and A. V. Krasheninnikov, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 125304 CrossRef.
- S. Cristol, J. F. Paul, E. Payen, D. Bougeard, S. Clemendot and F. Hutschka, J. Phys. Chem. B, 2002, 106, 5659–5667 CrossRef CAS.
- M. Alouani and R. C. Albers, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 6500–6509 CrossRef CAS.
- S. Wang and Y. Pan, J. Am. Ceram. Soc., 2019, 102, 4822–4834 CrossRef CAS.
- Y. Pan, Y. Lin, H. Wang and C. Zhang, Mater. Des., 2015, 86, 259–265 CrossRef CAS.
- D. Connétable and O. Thomas, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 094101 CrossRef.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717–2744 CrossRef CAS.
- Y. Pan, Int. J. Hydrogen Energy, 2018, 43, 3087–3091 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- Y. Pan, Ceram. Int., 2019, 45, 18315–18319 CrossRef CAS.
- W. A. Harrison, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 6008–6019 CrossRef CAS.
- Y. Pan and M. Wen, Int. J. Hydrogen Energy, 2018, 43, 22055–22063 CrossRef CAS.
- Y. Pan and W. M. Guan, Ceram. Int., 2018, 44, 9893–9898 CrossRef CAS.
- X. Bi, X. Hu and Q. Li, Results Phys., 2019, 15, 102607 CrossRef.
- Y. Pan, Int. J. Hydrogen Energy, 2019, 44, 18153–18158 CrossRef CAS.
- N. Korozlu, K. Colakoglu, E. Deligoz and S. Aydin, J. Alloys Compd., 2013, 546, 157–164 CrossRef CAS.
- H. Werheit, V. Filipov, K. Shirai, H. Dekura, N. Shitsevalova, U. Schwarz and M. Armbruster, J. Phys.: Condens. Matter, 2011, 23, 065403 CrossRef CAS.
- Y. Pan, Y. Q. Li, Q. H. Zheng and Y. Xu, J. Alloys Compd., 2019, 786, 621–626 CrossRef CAS.
- Y. Pan and W. M. Guan, Inorg. Chem., 2018, 57, 6617–6623 CrossRef CAS.
- G. Akopov, M. T. Yeung, C. L. Turner, R. L. Li and R. B. Kaner, Inorg. Chem., 2016, 55, 5051–5055 CrossRef CAS.
- Y. Pan and W. Guan, J. Power Sources, 2016, 325, 246–251 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07702b |
| This journal is © The Royal Society of Chemistry 2019 |