Open Access Article
Open Access ArticleEffect of Cu2+ on the nucleation kinetics and crystallization of rod-shaped CaSO4·2H2O in aqueous solution†
Xiangbin Sun a,
Xianshun Wanga,
Genlei Zhang
a,
Xianshun Wanga,
Genlei Zhang *abc,
Peng Cui*a and
Hao Shenc
*abc,
Peng Cui*a and
Hao Shenc
aSchool of Chemistry and Chemical Engineering, Anhui Province Key Laboratory of Controllable Chemistry Reaction and Material Chemical Engineering, Hefei University of Technology, Hefei 230009, China. E-mail: genleizhang@hfut.edu.cn; cuipeng@hfut.edu.cn
bSchool of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China
cAnhui Liuguo Chemical Co. Ltd, Tongling 244021, China
First published on 5th November 2019
Abstract
In this study, a simple and efficient strategy is developed to synthesize rod-like CaSO4·2H2O (DH) crystals with tunable aspect ratio in aqueous solution using Cu2+ as modifier. The aspect ratio and length of the DH crystals are effectively reduced to 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and about 35 μm in the presence of Cu2+, respectively. The interfacial tension value (γ) in the aqueous solution is improved significantly with the assistance of Cu2+, yet the nucleation rate (J) of the DH crystal is decreased sharply. The interfacial tension value (γ) in the aqueous solution is improved and the nucleation rate (J) of the DH crystal is drastically decreased due to the introduction of Cu2+, leading to the induction time of the DH crystallization being extended from 4 min to 25 min. The diversification of morphology for the DH crystals is incited by the changes of nucleation kinetics and Cu2+ incorporated into the crystal lattice, affecting the crystal growth habit, and finally controlling the growth of DH crystals in aqueous solution.
1 and about 35 μm in the presence of Cu2+, respectively. The interfacial tension value (γ) in the aqueous solution is improved significantly with the assistance of Cu2+, yet the nucleation rate (J) of the DH crystal is decreased sharply. The interfacial tension value (γ) in the aqueous solution is improved and the nucleation rate (J) of the DH crystal is drastically decreased due to the introduction of Cu2+, leading to the induction time of the DH crystallization being extended from 4 min to 25 min. The diversification of morphology for the DH crystals is incited by the changes of nucleation kinetics and Cu2+ incorporated into the crystal lattice, affecting the crystal growth habit, and finally controlling the growth of DH crystals in aqueous solution.
1. Introduction
Calcium sulfate is a common inorganic compound which is of huge environmental and industrial importance. In nature, it is present as large-scale deposits of CaSO4·2H2O (DH), CaSO4·0.5H2O (HH) and CaSO4 (AH),1–4 and is the source material for the preparation of high volumes of compounds including plaster and cement and for use in agricultural applications.5–7 In particular, DH, as a crucial mineral, is widely used in industry for bone tissue regeneration, as a medical carrier and in 3D-printing.8–11 However, in addition to these highly profitable applications, undesirable DH precipitation can also induce huge damage in industrial processes that depend on water handling systems such as waste water treatment, desalination plants and oil/gas production, where the accumulation of its precipitates can seriously impede flow and reduce production efficiency.12–14 The size, morphology and nucleation rate of DH crystals are closely related, it is thus not surprising that a plethora of studies focused on elucidating the nucleation and growth mechanism of DH.15–20Controlling the crystallization induction time (tind) and crystal morphology of the DH crystal can be achieved by changing the crystallization chemistry of the system. The most effective and widely used strategy is the introduction of soluble additives, where these can effectively affect nucleation period and growth processes, and resulting in variation for particle phases, sizes as well as morphology. Numerous investigations on the inorganic and organic additives affect the crystallization have been well documented.21–26 Organic acids and carboxyl-rich polymers such as fulvic acid (FA),27 sodium dodecyl sulfonate (SDS),28,29 and polyacrylic acid (PAA)30 have been shown to be particularly effective in inhibiting induction period for crystallization. The mechanism of action of organic additives is mainly to complex the active groups of organic substances or to adsorb Ca2+ in aqueous solution, reduce the supersaturation of the system to prolong the induction time of DH crystals, and form a shell-like structure on the surface of the crystal. The directional migration of Ca2+ is hindered in aqueous solution. Thus, the DH crystal growth suppressed and formed a smaller size.
The crystallization time of the DH crystal in the aqueous phase can be significantly extended in the presence of inorganic ions such as K+, Na+, Li+ and Mg2+.31,32 K+ and Na+ have little effect on the DH product, and the DH crystal size and morphology are basically unchanged. However, Li+ and Mg2+ can double the length of the DH crystal, but the crystal width changes slightly compared to the blank.33 The nucleation surface energy of the DH crystal is reduced in the presence of Mg2+, which leads to prolonged DH crystal induction time.34 Mg2+ ionic radius is smaller than Ca2+, the XRD peak of the DH crystal shifted to higher 2θ angles and the unit cell volume decreases in presence of Mg2+, which suggested Mg2+ acts by substituting the Ca2+ ions in the DH crystals lattice.35 Cu2+, as a typical divalent metal cation, has been reported as an inorganic additive for the regulation of HH morphology.31 However, there is few reports on the Cu2+ used as an inorganic additive for the regulation of DH morphology and its effect in the crystallization process has not been revealed clearly.
In this study, we developed a simple strategy for the synthesis of controllable aspect ratio rod-shaped DH crystals, and Cu2+ is widely significance in controlling the morphology of DH crystals. The influence of dynamic parameters such as interfacial tension values and nucleation rates were investigated, the diversification of morphology for the crystallization of DH crystals is incited by the changes of nucleation kinetics and Cu2+ incorporated into the crystal lattice, affecting the crystal growth habit in aqueous solution.
2. Experimental
2.1 Experimental procedure
Analytical grade chemicals used and purchased from the Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China) in this nucleation experiment. All crystallization tests were executed in a 500 dm3 thermostatic double-walled glass vessel equipped with a Teflon stirrer, a cooled condenser and a Thermocouple. The constant temperature of the reactor was stably controlled by the oil bath circulation between the double walls and oil bath. 100 dm3 of CaCl2 solution and CuCl2 (0–20 mM) mixed with deionized water was preheated to working temperature (70 °C) in the reactor, and stirring constantly at 100 rpm. Equal volume (100 dm3) of equimolar Na2SO4 was also preheated to the same experimental temperature (70 °C), and quickly mixing it into the reactor. Once mixed, solution was supersaturated, and as the experiment progresses DH will be produced. At the end of each test, the slurry was hot-filtered by a 0.45 μm cellulose membrane, and the sample was washed three times with boiling water and rinsed with ethanol then dried for 2 h at 45 °C in a vacuum drying oven before further analyses.The effect of Cu2+ on DH solubility were evaluated by adding CuCl2 (0–20 mM) to supersaturated DH slurry, and stirred at 70 °C for 48 hours. 1 mL of solution was filtered by a syringe filter with a 0.45 μm cellulose membrane and quickly diluted with deionized water in a 100 mL of volumetric flask for measure the concentration of Ca2+. Each experimental set was executed three times to ensure the accuracy of the data.
2.2 Characterization
The Atomic Absorption Spectroscopy (AAS) used in this study to determine total Ca2+ concentration in the filtrate by AA-6300C AAS instrument (Shimadzu; Japan). Induction time of the DH crystals were monitored by measure the ionic conductivity using a LE703 conductivity meter (METTLER TOLEDO; Switzerland). The scanning electronic microscopy (SEM) images of DH crystals were investigated using a SU8020 scanning electron microscope (Hitachi; Japan) with an accelerating voltage of 10 kV. The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) equipped with selected area electron diffraction (SAED) were performed using JEOL 2100F microscope (JEOL; Japan) operated at 200 kV. The energy dispersive X-ray spectroscopy (EDS) analysis was conducted using the TEM to examine the chemical composition of samples. The crystallographic characteristics of the DH crystals were investigated utilizing powder X-ray diffraction (XRD) using a D/max-γB X-ray diffractometer (Rigaku; Japan) with Cu Kα radiation (λ = 0.15406 nm) operated at 40 kV and 80 mA. The X-ray photoelectron spectroscopy (XPS) data of DH crystals were recorded using a ESCALAB250Xi X-ray photoelectron spectrometer (Thermo Scientific; USA) with using a monochromatized Al Kα (h = 1486.6 eV) X-ray source.2.3 Experimental procedure
The interfacial tension gap the supersaturated aqueous and the crystals (γ, J m−2) is a crucial factor at the rate of nucleation and crystal growth. The interfacial tension could be estimated from the induction time of crystals as a function of supersaturation. Based on classical nucleation theory,36 the induction procedure of DH nucleation, defined as the period time between the generation of nuclei and the initial supersaturated solution which caused changes in physical parameters. The induction time and supersaturation correlated by the following equation:37,38
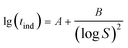 | (1) |
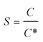 | (2) |
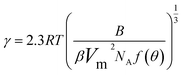 | (3) |
The nucleation rate (J) defined as the number of nucleation per unit time per unit volume (nuclei per m3 per s), can be calculated from the following equation:
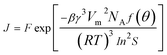 | (4) |
3. Results and discussion
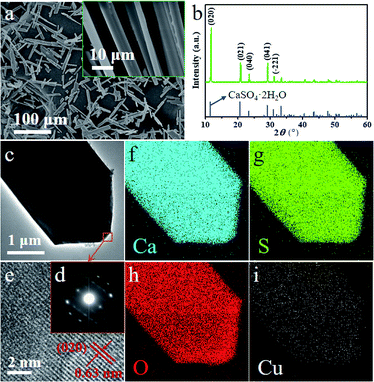
The scanning electronic microscopy (SEM) images of end-product crystals prepared with the aid of 20 mM Cu2+ in aqueous solution is shown in Fig. 1a, which exhibits the obtained crystal in the morphology of the long rod having an average length of 35 ± 5 μm (which is shown in Fig. 2d) and an average aspect ratio of about 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S1†). The X-ray diffraction (XRD) pattern of the obtained crystals is shown in Fig. 1b and the main diffraction peaks about at 11.67°, 20.79°, 23.42°, 29.16° and 31.20° are assigned to CaSO4·2H2O (020), (021), (040), (041) and (−221) facets (JCPDS 33-0311),40 respectively.
1 (Fig. S1†). The X-ray diffraction (XRD) pattern of the obtained crystals is shown in Fig. 1b and the main diffraction peaks about at 11.67°, 20.79°, 23.42°, 29.16° and 31.20° are assigned to CaSO4·2H2O (020), (021), (040), (041) and (−221) facets (JCPDS 33-0311),40 respectively.
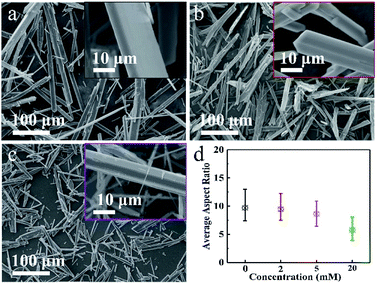 | ||
| Fig. 2 SEM images of (a) DH-0, (b) DH-2 and (c) DH-5 crystals prepared assisted by 0 mM, 2 mM and 5 mM Cu2+, respectively; (d) average aspect ratios of DH-0, DH-2, DH-5 and DH-20. | ||
XRD result shows that the rod-like crystals obtained is CaSO4·2H2O in the presence of 20 mM Cu2+ and are noted as DH-20 in this work.
Transmission electron microscopic (TEM), selected area electron diffraction (SAED), high resolution electron microscopy (HRTEM) and energy dispersive X-ray spectroscopy (EDS) have been performed to investigate the morphology and structure of DH-20 further. The TEM image (Fig. 1c) shows that circular edge apparent in the top of the DH-20 crystal, and the indexes of the spots in the SAED pattern (Fig. 1d) corresponding to the orange square region in Fig. 1c indicate that the DH-20 crystal is single-crystalline.41 The fringe spacing of 0.63 nm in the HRTEM image (Fig. 1e) corresponds to the (020) plane, indicating the preferential growth of the DH-20 along the (020) direction.17,19 Ca, S and O elements are homogeneously distributed throughout the while DH-20 crystal, which is confirmed by EDS elemental mapping images (Fig. 1f–h). Besides, Cu signal is also detected and its distribution is similar to the above three elements (Fig. 1i). The Cu signal detected in the obtained DH-20 indicates that Cu2+ may be adsorbed on the surface or doped into the lattice of DH.
In order to study the effect of Cu2+ on the morphology of DH, series of concentration-dependent experiments were conducted, and DH-0, DH-2 and DH-5 were prepared assisting by 0 mM, 2 mM and 5 mM Cu2+, respectively. The above three samples were characterized by SEM shown in Fig. 2a–c, and the average length, width and aspect ratio were displayed in Fig. 2d and S1†, respectively. As combining indicated by Fig. 1a and 2a–c, the long rod shape of DH is hardly affected by Cu2+ besides the length, width and aspect ratio. The length, width and aspect ratio of DH crystals were depended on the concentration of Cu2+, as displayed in Fig. 2d and S1†, when the concentrations of Cu2+ were 0 mM, 2 mM, 5 mM and 20 mM, respectively, the average aspect ratios were 9.7/1, 9.4/1, 8.3/1 and 5.7/1, and the average lengths were about 197 μm, 153 μm, 82 μm and 35 μm in turn. According to the theoretical fundamentals of crystal growth, the final external shape of a crystal depends on the relative growth rates of different crystal faces, which could be controlled by modifying the thermodynamic or kinetic parameters of the crystallization environment.17,42 In this study, the addition of Cu2+ significantly inhibits the growth of the DH crystal along the length and width directions and more finely divided DH crystals with a smaller aspect ratio are formed.
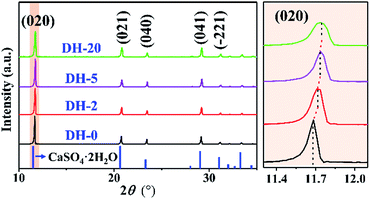
The crystals noted as DH-20 is CaSO4·2H2O in nature, which is indicated by its XRD pattern (Fig. 1b). The XRD patterns of DH-0, DH-2 and DH-5 are presented in Fig. 3 and S2a,† and obviously, these XRD patterns are all similar to that of DH-20 (green curve in Fig. 1 and 3), which indicates the DH-0, DH-2 and DH-5 synthesized are all CaSO4·2H2O crystals in the aqueous solution in the absence (black curve in Fig. 3) or presence (red, purple and green curves in Fig. 3) of Cu2+. In addition, it is noted that the (020) peak shifts to larger angles with increasing the Cu2+ concentration (Fig. 3), as well as the other XRD peaks (Fig. S2b†). The above phenomenon indicates that Cu atoms have been successfully incorporated into the lattice of CaSO4·2H2O.33,35 Beyond that, the gradual decrease of peak intensity and the slight increase in the peak width with the introduction of Cu2+ indicates the grain size is minimal and the crystallinity is decrease after Cu is doped into CaSO4·2H2O. This indicates that the doping of Cu into CaSO4·2H2O lattice inhibit crystal growth, agreeing with the results of the length and width (Fig. S1†). The unit cell parameters calculated from XRD patterns of DH crystals listed in Table 1. With the addition of Cu2+, the value of a for the unit cell is increased and the unit cell volume is expanded, although the value of c is reduced. The data from the Table 1 indicate that the unit cell of DH is distorted when Cu2+ is added during DH crystallization.
| Cu2+ concentration (mM) | a (Å) | b (Å) | c (Å) | β (deg) | V (Å3) |
|---|---|---|---|---|---|
| 0 | 6.1965 | 15.2443 | 5.7300 | 114.2731 | 493.4027 |
| 2 | 6.2351 | 15.2221 | 5.7295 | 114.4721 | 494.9321 |
| 5 | 6.2406 | 15.2537 | 5.7218 | 114.3853 | 496.0781 |
| 20 | 6.2586 | 15.2496 | 5.7182 | 114.1812 | 497.8629 |
The influence behavior of Cu2+ on the obtained DH crystals was also illustrated by X-ray photoelectron spectroscopy (XPS), and all of the binding energies (BEs) were calibrated using C 1s as the reference energy at 284.5 eV. Fig. 4a and b show the XPS spectra of the S 2p and Cu LMM regions of DH-0 prepared without CuCl2, respectively. For comparison, the XPS spectra of the S 2p and Cu LMM regions for DH-2, DH-5 and DH-20 are also shown in Fig. 4a and b, respectively. Compared to the S 2p1/2 spectrum of DH-0, an obvious negative shift (about 0.2 eV) in the binding energy was observed for DH-2, DH-5 and DH-20 (Fig. 4a), and the specific values of the XPS peak positions are listed in Table 2. The Cu signal is observed in DH-2, DH-5 and DH-20 except DH-0 (Fig. 4b), indicating that Cu element is doped into the DH crystal when CuCl2 is present in the reaction system. The shifts in the BE for S 2p1/2 and the appearance of the Cu signal in Cu LLM indicate an interaction between SO42− and Cu2+ in DH-2, DH-5 and DH-20.43 The above evidence indicates speculate the possible Cu2+ doping into the lattice gap of the DH unit cell structure and affecting the crystal growth habit which leaded the controlling the crystal morphology in aqueous solution ultimately.
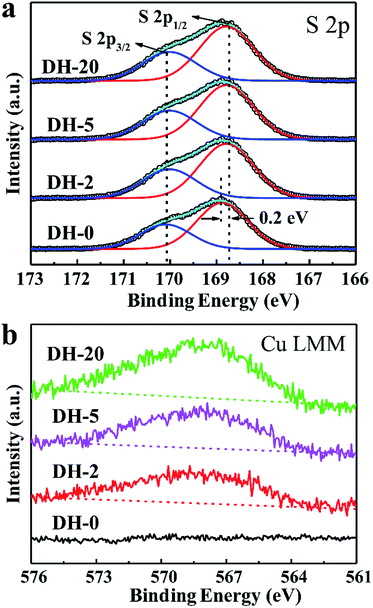 | ||
| Fig. 4 (a) S 2p and (b) Cu LLM XPS spectra of DH-0, DH-2, DH-5 and DH-20 crystals prepared with the aid of CuCl2 with different concentration. | ||
| Sample | S 2p1/2 (eV) | S 2p3/2 (eV) | Cu LMM (eV) |
|---|---|---|---|
| DH-0 | 168.9 | 170.1 | — |
| DH-2 | 168.7 | 170.1 | 568.1 |
| DH-5 | 168.7 | 170.1 | 568.1 |
| DH-20 | 168.7 | 170.1 | 568.1 |
The nucleation and growth rates of the DH crystal are determined by its equilibrium solubility (C*), and according to several previous reports,44–47 the value of C* in this system can be measured by a seeded growth equilibration method and the data are displayed in Table 3. The C* value of saturated gypsum solution can be increased effectively by the introduction of Cu2+, and as the concentration of Cu2+ increases, the C* value is increased gradually. The above data in Table 3 provides evidence for the calculation of the relative supersaturation (S) and the interfacial tension (γ) during the subsequent DH crystallization.
| Cu2+ concentration (mM) | C* (mM) |
|---|---|
| 0 | 14.31 |
| 2 | 14.56 |
| 5 | 15.13 |
| 20 | 17.42 |
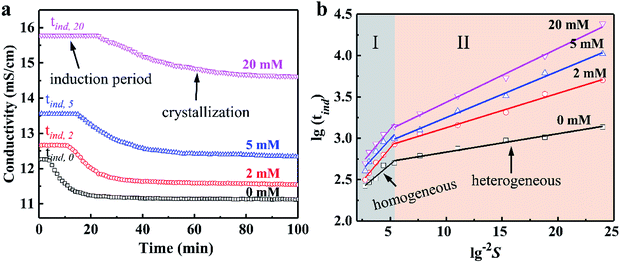
The effect of concentration of Cu2+ on the DH crystallization is shown in Fig. 5a. Induction time (tind) is defined as the time required for the commencement of crystallization or it is the time elapsed until the moment at which the onset of crystallization can be detected. The commencement of crystallization in our experiments was detected by drop in conductivity. When the conductivity decreases rapidly, crystallization is visible. It is apparent from Fig. 5a that the tind increases with the increase in concentration of Cu2+. The tind is extended from 4 min to 11 min, 15 min and 25 min when the concentration of Cu2+ are 2 mM, 5 mM and 20 mM, respectively. Later on the crystal growth recommences with a measurable rate observed by a drop in conductivity. The linear change of conductivity for gypsum nucleation in the presence of Cu2+ at 70 °C was employed to calculate the values of interfacial tension and the rate of nucleation process. The effect of increasing the supersaturation on reducing the induction period was showed in Fig. 5b. The straight lines with different slopes suggest that two nucleation mechanisms may exist for DH crystallization. The behavior of high supersaturation crystallization is more consistent with a homogeneous nucleation mechanism (region I) in contrast to lower supersaturation where heterogeneous nucleation mechanism (region II) is more likely. The slope of the induction period plot is used to distinguish between nucleation mechanisms,48–51 and the relatively gentle slope is used to calculate the values of γ (listed in Table 4) according eqn (3).
| Cu2+ concentration (mM) | γ (mJ m−2) | J (×1028 nuclei per cm3 per s) |
|---|---|---|
| 0 | 6.32 | 60.11 |
| 2 | 8.07 | 34.65 |
| 5 | 8.75 | 25.90 |
| 20 | 9.46 | 18.14 |
As shown in Table 4, in the presence of Cu2+, the γ is preceded by an induction time of several minutes to several thousand minutes. Obviously, the γ increases sharply in the presence of Cu2+ (8.07 mJ m−2 for 2 mM Cu2+ vs. 6.32 mJ m−2 for 0 mM Cu2+), and the γ increases slowly as the Cu2+ concentration continues to increase. The γ value of 9.46 mJ m−2 was obtained when the Cu2+ concentration is 20 mM, corresponding to a significant increase of about 49% with respect to the γ value for the additive-free series. Higher nucleation energy needs to be overcome to form a critical nucleus in a nucleation process when the γ value is large, which reflects extension of the tind for crystallization period in the presence of Cu2+. Theoretically, controlling the crystal morphology and diameter is closely related to the nucleation rates.52–54 The rate of spherical DH nucleation can be estimated from eqn (4), and the date are listed in Table 4. The nucleation rate of DH would be decreased due to the introduction of Cu2+, and when the of Cu2+ concentration is increased to 20 mM, the corresponding nucleation rate is reduced to 1/3 of that in the absence of Cu2+ (18.14 × 1028 nuclei per cm3 per s vs. 60.11 × 1028 nuclei per cm3 per s). The data of EDS (Fig. 1), XRD (Fig. 3) and XPS (Fig. 4) indicate that the Cu2+ doping into the crystal lattice, similar to other metal cations (such as Mg2+ and Al3+) on the morphology of DH,35,55 thereby controlling DH crystallization rate and crystal size. Therefore, the nucleation rate of DH can be effectively inhibited by Cu2+, which results in a significant change in crystal morphology and size.
4. Conclusions
In summary, we have developed a simple strategy for the synthesis of controllable aspect ratio rod-shaped DH crystals, and Cu2+ is widely significance in controlling the morphology of DH crystals. With the concentration of Cu2+ increased from 0 mM to 20 mM, the average aspect ratios decreased sharply from 9.7/1 to 5.7/1, and the corresponding average lengths decreased from about 197 μm to 35 μm. The nucleation kinetic parameters of the DH crystals crystallization that act as critical roles in controlling DH nucleation rate and induction time have been modified in the presence of Cu2+. The induction time of the DH crystallization is extended from 4 min to 25 min using Cu2+ as a modifier. The diversification of morphology for the crystallization of DH crystals is incited by the changes of nucleation kinetics and Cu2+ incorporated into the crystal lattice, affecting the crystal growth habit in aqueous solution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by Natural Science Foundation of China (21806065) and Doctoral Special Research Foundation of HFUT (JZ2018HGBZ0122 and JZ2018HGBZ0087). We also thank the support from Anhui Province Key Laboratory of Advanced Catalytic Materials and Reaction Engineering (45000-411104/007).References
- D. R. Govender, W. W. Focke, S. M. Tichapondwa and W. E. Cloete, ACS Appl. Mater. Interfaces, 2018, 10, 20679–20687 CrossRef CAS.
- G. Sirokman, J. Chem. Educ., 2014, 91, 557–559 CrossRef CAS.
- T. Tyner and J. Francis, ACS Reagent Chemicals: Specifications and Procedures for Reagents and Standard-Grade Reference Materials, ACS Publications, 2016 Search PubMed.
- H. Tan, F. Dong and J. Liu, J. Phys. Chem. Solids, 2018, 112, 239–245 CrossRef CAS.
- D. C. Engbrecht and D. A. Hirschfeld, Thermochim. Acta, 2016, 639, 173–185 CrossRef CAS.
- I. Koh, A. López, B. Helgason and S. J. Ferguson, J. Mech. Behav. Biomed. Mater., 2014, 34, 187–198 CrossRef CAS.
- N. Jiang, D. Cai, L. He, N. Zhong, H. Wen, X. Zhang and Z. Wu, ACS Sustainable Chem. Eng., 2015, 3, 374–380 CrossRef CAS.
- J. Baek, H. Lee, T. S. Jang, J. Song, H. E. Kim and H. D. Jung, ACS Biomater. Sci. Eng., 2018, 4, 846–856 CrossRef CAS.
- Z. Zhou, F. Buchanan, C. Mitchell and N. Dunne, Mater. Sci. Eng., C, 2014, 38, 1–10 CrossRef CAS.
- S. Grudén, M. Sandelin, V. Rasanen, P. Micke, M. Hedeland, N. Axén and M. Jeansson, Eur. J. Pharm. Biopharm., 2017, 114, 186–193 CrossRef.
- Y. Shen, S. Yang, J. Liu, H. Xu, Z. Shi, Z. Lin, X. Ying, P. Guo, T. Lin and S. Yan, ACS Appl. Mater. Interfaces, 2014, 6, 12177–12188 CrossRef CAS.
- W.-Y. Shih, A. Rahardianto, R.-W. Lee and Y. Cohen, J. Membr. Sci., 2005, 252, 253–263 CrossRef CAS.
- T. A. Hoang, M. Ang and A. L. Rohl, Chem. Eng. Technol., 2011, 34, 1003–1009 CrossRef CAS.
- A. E. S. V. Driessche, J. M. GarcíA-Ruiz, J. M. Delgado-LóPez and G. Sazaki, Cryst. Growth Des., 2010, 10, 3909–3916 CrossRef.
- E. H. Byrne, P. Raiteri and J. D. Gale, J. Phys. Chem. C, 2017, 121, 25956–25966 CrossRef CAS.
- T. M. Stawski, A. D. Van, M. Ossorio, R. B. Diego, R. Besselink and L. G. Benning, Nat. Commun., 2016, 7, 1–19 Search PubMed.
- T. M. Stawski, H. M. Freeman, A. E. S. Van Driessche, J. Hövelmann, R. Besselink, R. Wirth and L. G. Benning, Cryst. Growth Des., 2019, 19, 3714–3721 CrossRef CAS.
- K. Gupta, S. Singh and M. R. Rao, Cryst. Growth Des., 2016, 16, 3256–3261 CrossRef CAS.
- S. Leukel, M. Panthöfer, M. Mondeshki, W. Schärtl, S. Plana-Ruiz and W. Tremel, Langmuir, 2018, 34, 7096–7105 CrossRef CAS.
- E. R. Ravenhill, P. M. Kirkman and P. R. Unwin, Cryst. Growth Des., 2016, 16, 5887–5895 CrossRef CAS.
- X. Mu, G. Zhu, X. Li, S. Li, X. Gong, H. Li and G. Sun, ACS Omega, 2019, 4, 12702–12710 CrossRef.
- H. Wang, G. Liu, J. Huang, Y. Zhou, Q. Yao, S. Ma, C. Ke, Y. Liu, W. Wu and S. Wei, Desalin. Water Treat., 2015, 53, 8–14 CrossRef CAS.
- C. Fan and R. M. Pashley, Chem. Eng. Sci., 2016, 142, 23–31 CrossRef CAS.
- H. Wang, Y. Zhou, Q. Yao and W. Sun, Polym. Bull., 2015, 72, 2171–2188 CrossRef CAS.
- M. G. Lioliou, C. A. Paraskeva, P. G. Koutsoukos and A. C. Payatakes, J. Colloid Interface Sci., 2006, 303, 164–170 CrossRef CAS.
- L. Shen, H. Sippola, X. Li, D. Lindberg and P. Taskinen, J. Chem. Eng. Data, 2019, 64, 2697–2709 CrossRef CAS.
- Y. Xu, Y. Liao, Z. Lin, J. Lin, Q. Li, J. Lin and Z. Jin, Chem. Eng. J., 2019, 361, 1078–1088 CrossRef CAS.
- X. Mao, X. Song, G. Lu, Y. Xu, Y. Sun and J. Yu, Chem. Eng. J., 2015, 278, 320–327 CrossRef CAS.
- X. Mao, X. Song, G. Lu, Y. Sun, Y. Xu and J. Yu, Ind. Eng. Chem. Res., 2015, 54, 4781–4787 CrossRef CAS.
- T. Rabizadeh, D. J. Morgan, C. L. Peacock and L. G. Benning, Ind. Eng. Chem. Res., 2019, 58, 1561–1569 CrossRef CAS.
- G. Jiang, H. Fu, K. Savino, J. Qian, Z. Wu and B. Guan, Cryst. Growth Des., 2013, 13, 5128–5134 CrossRef CAS.
- T. Feldmann and G. P. Demopoulos, Ind. Eng. Chem. Res., 2013, 52, 6540–6549 CrossRef CAS.
- T. Rabizadeh, T. M. Stawski, D. J. Morgan, C. L. Peacock and L. G. Benning, Cryst. Growth Des., 2017, 17, 582–589 CrossRef CAS.
- M. M. Rashad, M. H. H. Mahmoud, I. A. Ibrahim and E. A. Abdel-Aal, J. Cryst. Growth, 2004, 267, 372–379 CrossRef CAS.
- S. B. Ahmed, M. M. Tlili, M. Amami and M. B. Amor, Ind. Eng. Chem. Res., 2014, 53, 9554–9560 CrossRef.
- J. C. Brice and P. Rudolph, Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- M. Prisciandaro, A. Lancia and D. Musmarra, Ind. Eng. Chem. Res., 2003, 42, 6647–6652 CrossRef CAS.
- B. Guan, L. Yang and Z. Wu, Ind. Eng. Chem. Res., 2010, 49, 5569–5574 CrossRef CAS.
- F. Wang, T. E. Davis and V. V. Tarabara, Ind. Eng. Chem. Res., 2010, 49, 11344–11350 CrossRef CAS.
- X. Sun, G. Zhang and P. Cui, RSC Adv., 2019, 9, 21601–21607 RSC.
- T. Lehnert, M. K. Kinyanjui, A. Ladenburger, D. Rommel, K. Wörle, F. Börrnert, K. Leopold and U. Kaiser, ACS Nano, 2017, 11, 7967–7973 CrossRef CAS.
- L. Zhang, L. Yu, Y. Liu, C. Liu, H. Li and J. Wu, Mater. Sci. Eng., A, 2017, 695, 66–73 CrossRef CAS.
- K. Liu, J. Yang, H. Hou, S. Liang, Y. Chen, J. Wang, B. Liu, K. Xiao, J. Hu and H. Deng, Environ. Sci. Technol., 2019, 53, 2748–2757 CrossRef CAS PubMed.
- T. J. Trivedi, J. Shukla and A. Kumar, J. Chem. Eng. Data, 2013, 58, 773–779 CrossRef CAS.
- J. Shukla, M. J. Mehta and A. Kumar, J. Chem. Eng. Data, 2019, 64, 536–544 CrossRef CAS.
- J. Sun, L. Wang and G. Yu, J. Chem. Eng. Data, 2015, 60, 2559–2566 CrossRef CAS.
- J. Shukla, M. J. Mehta and A. Kumar, J. Chem. Eng. Data, 2018, 63, 2743–2752 CrossRef CAS.
- X. Liu, D. Xu, M. Ren, G. Zhang, X. Wei and J. Wang, Cryst. Growth Des., 2010, 10, 3442–3447 CrossRef CAS.
- D. Zhang, Y. Wang, S. Ma, S. Wu and H. Hao, J. Chem. Eng. Data, 2012, 58, 176–182 CrossRef.
- H. Fu, C. Jia, Q. Chen and G. Jiang, J. Cryst. Growth, 2017, 470, 143–148 CrossRef CAS.
- M. Kobari, N. Kubota and I. Hirasawa, CrystEngComm, 2012, 14, 5255–5261 RSC.
- G. Di Profio, E. Curcio, S. Ferraro, C. Stabile and E. Drioli, Cryst. Growth Des., 2009, 9, 2179–2186 CrossRef CAS.
- E. Abdel-Aal, M. Rashad and H. El-Shall, Cryst. Res. Technol., 2004, 39, 313–321 CrossRef CAS.
- H. Fu, B. Guan, G. Jiang, M. Z. Yates and Z. Wu, Cryst. Growth Des., 2012, 12, 1388–1394 CrossRef CAS.
- W. Zhao, C. Gao, G. Zhang, J. Xu, C. Wang and Y. Wu, New J. Chem., 2016, 40, 3104–3108 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07640a |
| This journal is © The Royal Society of Chemistry 2019 |