Open Access Article
Open Access ArticleStructural tuning enables piezochromic and photochemical properties in N-aryl-β-enaminones†
Wan-Chi Hsieh,
Kiran B. Manjappa * and
Ding-Yah Yang
* and
Ding-Yah Yang *
*
Department of Chemistry, Tunghai University, No. 1727, Sec. 4, Taiwan Boulevard, Xitun District, Taichung 40704, Taiwan. E-mail: kiran@thu.edu.tw; yang@thu.edu.tw
First published on 23rd October 2019
Abstract
An efficient synthesis of N-aryl-β-enaminones via Et3N-mediated, one-pot three-component reaction of 4-hydroxycoumarin/dimedone, β-nitrostyrene/2-(2-nitrovinyl)thiophene, and arylamine in toluene under refluxed conditions is herein presented. Some prepared compounds were found to exhibit piezochromic properties. The XRD and SEM measurements of the piezochromic compound showed substantial crystal packing and morphology changes before and after grinding. Further, one prepared compound was found to be light-sensitive and can be converted to a furo[3,2-b]pyridin-2(4H)-one derivative upon UV irradiation. A plausible mechanism for this photochemical reaction was proposed.
1 Introduction
The β-enaminones represent a crucial molecular skeleton that is present in various natural products.1 They were found to be associated with a wide range of pharmacological activities, including antimicrobial,2 antibacterial,3 anti-inflammatory,4 and anti-leukemia5 properties. Owing to their diverse biological activities, quite a few methodologies for the synthesis of β-enaminones have been reported in the literature.6 Most of them focused on the preparation of N-alkyl-β-enaminones; the synthesis of N-aryl-β-enaminones is much less explored. As a result, the photochemical and functional properties of N-aryl-β-enaminones have never been reported. Scheme 1 lists the previous syntheses of N-aryl-β-enaminones. While they can be readily prepared by either condensation7 of dimedone, orthoester, and aniline or dehydration8 of 3-acetyl-4-hydroxycoumarin and arylamine, the scope of these reactions is rather limited. Recently, Banerjee9 and coworkers reported a highly atom-economical and efficient method for the preparation of N-aryl-β-enaminones via CuO–ZnO NPs-catalyzed, one-pot three-component reaction of 4-hydroxycoumarin/dimedone, β-nitrostyrene, and arylamine. Nevertheless, the CuO-doped ZnO nanoparticles are currently not commercially available, and the self-prepared nanomaterials need to be analyzed by sophisticated spectroscopic and analytical techniques. Thus, the development of the simple methodology for the preparation of N-aryl-β-enaminones with readily available reagents remains desirable. Here, we report the modified preparation of N-aryl-β-enaminones via base-mediated, one-pot three-component reaction of 4-hydroxycoumarin/dimedone, β-nitrostyrene/2-(2-nitrovinyl)thiophene, and arylamine in toluene under refluxed conditions. The functional and photochemical properties of the prepared compounds such as piezochromism10 are also investigated.2 Results and discussion
Our initial efforts toward the preparation of N-aryl-β-enaminones called for the base-mediated, one-pot three-component reaction of 4-hydroxycoumarin, aldehyde, and arylamine in the presence of DABCO as a base in nitromethane under refluxed conditions.11 Although the desired product was indeed isolated, this reaction suffered from moderate to low yields. Further, the reaction scope for the substrates was somewhat limited. Alternatively, we pursued the target compound synthesis by modifying the three-component reaction conditions reported by Wang.6c Table 1 lists the optimization for condensation of 4-hydroxycoumarin (1), β-nitrostyrene (2), and aniline (3) under the influence of different organic bases and solvents. To our delight, we found that the reaction can proceed smoothly in the presence of one equiv. of triethylamine as a base in toluene under refluxed conditions for 1.5 h (Entry 4, Table 1). These optimization reaction conditions were then subsequently employed to the preparation of other N-aryl-β-enaminones with different substituents.| Entry | Base (equiv.) | Solvent | Time (h) | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | K2CO3 (1.0) | Toluene | 1.5 | 31 |
| 2 | DABCO (1.0) | Toluene | 1.5 | 68 |
| 3 | DMAP (1.0) | Toluene | 1.5 | 20 |
| 4 | Et3N (1.0) | Toluene | 1.5 | 76 |
| 5 | Et3N (0.5) | Toluene | 1.5 | 53 |
| 6 | Et3N (1.0) | EtOH | 1.5 | 21 |
| 7 | Et3N (1.0) | CH3CN | 1.5 | 30 |
| 8 | Et3N (1.0) | DCE | 1.5 | 44 |
| 9 | Et3N (1.0) | Toluene | 3.0 | 76 |
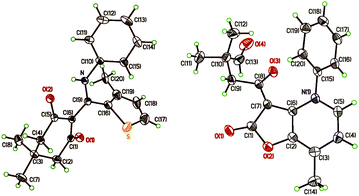
Fig. 1 lists the structures of the prepared N-aryl-β-enaminones and their yields. The molecular structures of 4a–s were elucidated by 1H and 13C NMR spectroscopy. Compounds 4g and 4j were further verified by X-ray crystallography, as depicted in Fig. 2.12 An intramolecular hydrogen bonding between the amine hydrogen and carbonyl oxygen atom is clearly observed for both of the compounds. Generally, the N-aryl-β-enaminones with electron-donating group substituted at benzene or coumarin moiety gave better yields compared to their unsubstituted counterparts. The opposite was true for N-aryl-β-enaminones with electron-withdrawing group substituted at benzene or coumarin moiety. Further, when the substrate 4-hydroxycoumarin (1) was replaced by dimedone, the yield of the corresponding product (4q, 56%) was less than that of unsubstituted coumarin counterpart (4a, 76%), indicating that 4-hydroxycoumarin is a better substrate than that of dimedone for this multicomponent reaction.
With the availability of N-aryl-β-enaminones 4a–s, their functional properties were then investigated. Interestingly, compounds 4g–j were found to be sensitive to pressure, especially for 4g. Upon grinding, compound 4g changes from yellow to red. It can be swiftly reverted to the original color after exposed to methylene chloride vapor, as shown in Fig. 3.
 | ||
| Fig. 3 Color transition of 4g (a) before grinding; (b) after grinding; (c) after exposed to fuming CH2Cl2. | ||
To investigate the phase transition during the piezochromic process, the X-ray diffraction (XRD) patterns of 4g in different solid states were recorded, as depicted in Fig. 4. Several sharp and strong peaks were observed for the crystal sample of 4g, illustrating the presence of a crystalline structure (Fig. 4a). Nevertheless, the diffraction peaks of 4g became substantially weaker after grinding (Fig. 4b). The sharp and strong diffraction peaks were recovered when the ground powder of 4g was exposed to CH2Cl2 vapor for 10 s (Fig. 4c).
To obtain more information about the morphology of different aggregated states of 4g prior to and after grinding, the scanning electron microscopy (SEM) images were also recorded, as illustrated in Fig. 5. Before grinding, the prepared 4g showed regular plate crystals with a relatively smooth surface (Fig. 5a). However, the smooth surface became rough after grinding (Fig. 5b). This external stimuli-induced damage to the surface topography was swiftly repaired by exposing to CH2Cl2 vapor (Fig. 5c). While the detailed piezochromic mechanism of 4g–j remains to be investigated, the presence of an N,N-dimethylamino substituent on β-nitrostyrene substrate seems to be required for their color change upon grinding. The discovery of piezochromism from the prepared N-aryl-β-enaminones 4g–j suggests that multicomponent reactions may serve as a useful tool not only to prepare compounds with potential biological and pharmaceutical activities but also to unearth molecules with novel functional properties.
As for their photochemical properties, the prepared N-aryl-β-enaminones 4a–r were all found to be light-insensitive even after prolonged (more than 30 min) irradiation with UV light, except for compound 4s. Upon UV irradiation in acetonitrile under aerobic conditions for half an hour, compound 4s was converted to furo[3,2-b]pyridin-2(4H)-one 5 in 56% yield (Scheme 2). Both of the molecular structures of 4s and 5 were confirmed by the X-ray crystallography, as shown in Fig. 6.12 While the detailed photochemical mechanism for this transformation is currently unclear, this photo-oxidation substantially alters the molecular structure of 4s by opening up two rings, that is, thiophene and dimedone, and forming of a new fused ring, that is, furopyridinone. To gain insights into the mechanism of this photo-oxidation, compound 4s was subjected to EPR measurement under UV irradiation, and the result turned out to be EPR-silent. Further, the yield of the photoreaction was not affected by the presence of a radical scavenger like TEMPO. These observations suggest the photo-oxidation might not proceed with the radical mechanism. Scheme 3 depicts the plausible intermediates involved in this photo-oxidation. It presumably starts with light-mediated ring-opening of thiophene ring to give the thiol 6, which undergoes enol–keto tautomerization to yield the thiolactone 7. The keto–enol tautomerization of ketone 7 and the subsequent intramolecular cyclization furnish the hemithioketal 8. The elimination of hydrogen sulfide from 8 yields benzofuropyridinone 9. Final oxidative cleavage of the double bond of vinyl ether 9 affords the furopyridinone 5. The fact that compound 4s is light-sensitive and compound 4r is not implies that the methyl substituent on the thiophene moiety of 4s plays a crucial role in its photochemical properties.
3 Conclusions
In summary, we have demonstrated that N-aryl-β-enaminones 4a–s can be efficiently synthesized via one-pot three-component reaction of 4-hydroxycoumarin/dimedone, β-nitrostyrene/2-(2-nitrovinyl)thiophene, and arylamine in the presence of trimethylamine as a base in toluene under refluxed conditions. Moreover, compounds 4g–j were found to exhibit piezochromic properties, whereas compound 4s was found to be sensitive to light and could be converted to the furo[3,2-b]pyridin-2(4H)-one upon UV irradiation. Further exploration of other functional properties such as photochromism via structural tuning of N-aryl-β-enaminones is currently underway and will be reported in due course.4 Experimental
4.1 General
Melting points were determined on a Mel-Temp melting point apparatus in open capillaries and are uncorrected. Infrared (IR) spectra were recorded using 1725XFT-IR spectrophotometer. High-resolution mass spectra (HRMS) were obtained on a Thermo Fisher Scientific Finnigan MAT95XL spectrometer using magnetic sector analyzer. X-ray diffraction (XRD) spectra were recorded using PHILIPS X’PERT Pro MPD. Scanning electron microscopy (SEM) images were recorded on a JEOL JSM-6500F. 1H NMR (400 MHz) and 13C NMR (100 or 150 MHz) spectra were recorded on a Bruker 400 or Agilent Technologies DD2 600 spectrometer. Chemical shifts were reported in parts per million on the δ scale relative to an internal standard (tetramethylsilane, or appropriate solvent peaks) with coupling constants given in hertz. 1H NMR multiplicity data are denoted by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel 60G-254 plates (25 mm) and developed with the solvents mentioned. Visualization was accomplished by using portable UV light, ninhydrin spray, or iodine chamber. Flash chromatography was performed in columns of various diameters with Merck silica gel (230–400 mesh ASTM 9385 kieselgel 60H) by elution with the solvent systems. Solvents, unless otherwise specified, were reagent grade and distilled once prior to use. All new compounds exhibited satisfactory spectroscopic and analytical data.4.2 General procedure for the synthesis of β-nitrostyrene derivatives
To a solution of NH4OAc (10.4 g, 135 mmol, 2.4 equiv.) and acetic acid (100 mL) was added nitromethane (23.8 g, 389 mmol, 6.9 equiv.) and appropriate aldehyde (56.4 mmol, 1 equiv.) at room temperature. The resulting mixture was then heated at 100 °C for 6 h. After cooled down to room temperature, the reaction mixture was poured into water (300 mL). The pH of the solution was adjusted to 7 with 2 M NaOH(aq) and the product was then extracted with EtOAc (5 × 150 mL). The organic layers were combined, washed with brine (1 × 150 mL), dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (EtOAc/hexanes) to obtain the title compound.4.3 General procedure for the synthesis of 4
To a mixture of 4-hydroxycoumarin (1.00 mmol, 1 equiv.) in toluene (10 mL) was added β-nitrostyrene (1 equiv.), amine (1 equiv.), and Et3N (1 equiv.) at room temperature. The resulting mixture was then refluxed for 1.5 h. The progress of the reaction was monitored by TLC. After cooled down to room temperature, the filtrate was concentrated in vacuo. The crude product was purified by column chromatography (EtOAc/hexanes) to give the desired compound 4.4.4 Photoirradiation of N-aryl-β-enaminone 4s
The solution of compound 4s (50 mg) in acetonitrile (1000 mL) was irradiated under aerobic conditions using a photochemical reactor (PR-2000, 352 nm × 10 lamps) for 30 min. The solution was then concentrated in vacuo and the crude product was purified by column chromatography (40% EtOAc/hexanes) to obtain the brown solid furopyridinone 5.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Ministry of Science and Technology of the Republic of China, Taiwan, for financially supporting this research under Grant No. MOST 108-2113-M-029-001.Notes and references
- (a) J. P. Michael, C. B. Koning, G. D. Hosken and T. V. Stanbury, Tetrahedron, 2001, 57, 9635–9648 CrossRef CAS; (b) C. J. Valduga, H. S. Braibante and M. E. F. Braibante, J. Heterocycl. Chem., 1998, 35, 189–192 CrossRef CAS; (c) H. M. C. Ferraz, E. O. de Oliveira, M. E. Payret-Arrua and C. A. Brandt, J. Org. Chem., 1995, 60, 7357–7359 CrossRef CAS; (d) J. D. White and D. C. Lhle, Org. Lett., 2006, 8, 1081–1084 CrossRef CAS; (e) G. Li, K. Watson, R. W. Buckheit and Y. Zhang, Org. Lett., 2007, 9, 2043–2046 CrossRef CAS.
- M. Mladenovic, N. Vukovic, N. Niciforovic, S. Sukdolak and S. Solujic, Molecules, 2009, 14, 1495–1512 CrossRef CAS PubMed.
- N. Vukovic, S. Sukdolak, S. Solujic and N. Niciforovic, Food Chem., 2010, 120, 1011–1018 CrossRef CAS.
- D. L. Garmaise, D. T. W. Chu, E. Bernstein and M. Inaba, J. Med. Chem., 1979, 22, 559–564 CrossRef CAS.
- E. Badzisz, E. Brzezinska, U. Krajewska and M. Rozalski, Eur. J. Med. Chem., 2003, 38, 597–603 CrossRef.
- (a) P. Y. Kuo, R. R. Chuang and D. Y. Yang, Mol. Diversity, 2009, 13, 253–260 CrossRef CAS; (b) E. Ghabraie, M. Bararjanian, S. Balalaie, F. Rominger and H. R. Bijanzadeh, Helv. Chim. Acta, 2011, 94, 1440–1446 CrossRef CAS; (c) W. J. Ye, Y. Li, L. X. Zhou, J. J. Liu and C. D. Wang, Green Chem., 2015, 17, 188–192 RSC.
- A. V. Komkov, M. A. Prezent, A. V. Ignatenko, I. P. Yakovlev and V. A. Dorokhov, Russ. Chem. Bull., 2006, 55, 2085–2090 CrossRef CAS.
- E. H. Avdović, D. Milenković, J. M. D. Marković, J. Đorović, N. Vuković, M. D. Vukić, V. V. Jevtić, S. R. Trifunović, I. Potočňák and Z. Marković, Spectrochim. Acta, Part A, 2018, 195, 31–40 CrossRef.
- A. Saha, S. Payra, S. Akhtar and S. Banerjee, ChemistrySelect, 2017, 2, 7319–7324 CrossRef CAS.
- (a) Z. Gao, K. Wang, F. Liu, C. Feng, X. He, J. Li, B. Yang, B. Zou and P. Lu, Chem.–Eur. J., 2017, 23, 773–778 CrossRef CAS; (b) C. Feng, K. Wang, Y. Xu, L. Liu, B. Zou and P. Lu, Chem. Commun., 2016, 52, 3836–3839 RSC.
- K. B. Manjappa, Y. A. Yang, S. S. Mysore and D. Y. Yang, ChemistrySelect, 2018, 3, 10701–10705 CrossRef CAS.
- Crystallographic data (excluding structure factors) for 4g, 4j, 4s, and 5 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC-1913301, 1913302, 1953598, and 1953599 respectively..
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1913301, 1913302, 1953598, and 1953599. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra07598d |
| This journal is © The Royal Society of Chemistry 2019 |