Open Access Article
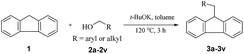
Open Access Articlet-BuOK-catalysed alkylation of fluorene with alcohols: a highly green route to 9-monoalkylfluorene derivatives†
Jiang-Tao Fanab,
Xin-Heng Fan *a,
Cai-Yan Gaoa,
Zhenpeng Wangc and
Lian-Ming Yang
*a,
Cai-Yan Gaoa,
Zhenpeng Wangc and
Lian-Ming Yang *a
*a
aBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Green Printing, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yanglm@iccas.ac.cn; xinxin9968@iccas.ac.cn; Fax: +8610-62559373
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cNational Centre for Mass Spectrometry in Beijing, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
First published on 4th November 2019
Abstract
A simple, mild and efficient protocol was developed for the alkylation of fluorene with alcohols in the presence of t-BuOK as catalyst, affording the desired 9-monoalkylfluorenes with near quantitative yields in most cases.
Introduction
Fluorene motifs are a very useful class of building blocks for the construction of organic materials potentially applicable in optoelectronics, semiconductors, and solar cells.1 Given their importance, chemical modifications or transformations of the parent fluorene have been an intriguing and valuable subject of research in organic synthesis. Among them is fluorene alkylation. Typically, 9-monoalkylated fluorenes were prepared from the condensation of fluorene and aldehydes and their subsequent hydrogenation2 or via a classical SN2 reaction of 9-lithofluorene with haloalkanes.3 These traditional methods commonly suffer from harsh reaction conditions, a complex product distribution, and difficult experimental operations. A simple alternative to 9-monoalkylfluorenes is the alkylation of fluorene with alcohols using bases as the promoting agents.4 However, this approach required both extremely high temperatures (150–210 °C) and highly concentrated strong bases (more than stoichiometric quantities), resulting in the occurrence of many side reactions. A very recent publication disclosed a greener method for the aldehyde/ketone-catalysed alkylation of fluorene,5 where significant improvements were made on reaction conditions, selectivity, efficiency, and substrate scope. But an evident limitation of the method lies in the fact that individual substrates require a corresponding, specific aldehyde/ketone catalyst. Accordingly, to further improve this synthetic reaction remains a requirement. Herein, we wish to report a green protocol for t-BuOK-catalysed 9-alkylation of fluorene with alcohols.Results and discussion
This work was initiated by some new recognitions from our own studies on alcohol activation termed as the “borrowing hydrogen” reaction:6 upon following a base-promoted reaction of fluorene and an aliphatic alcohol, we found that both the type and the quantity of bases dramatically affected reaction conditions, conversions, as well as product distributions, and interestingly the alkylated product increased with reducing the amount of the base, contrary to what we usually believed.Then, a more detailed survey of the base-catalysed alkylation reaction was performed by choosing fluorene and p-methoxybenzyl alcohol as model substrates, and the results are summarized in Table 1. After some experimentation, our standard reaction conditions (i.e., in the presence of 50 mol% t-BuOK in toluene at 120 °C under N2 for 3 h) were determined, where a complete conversion and near quantitative yield were achieved (entry 1). The role of bases has been examined: no conversion occurred in the absence of the base (entry 2); KOH (entry 3) can give a high yield of 85%, and thus will be an optional catalyst when a large-scale preparation is considered; and other bases, such as t-BuONa (entry 4), NaOH (entry 5), CsOH·H2O (entry 6) and K2CO3 (entry 7), seemed to be inferior or ineffective. The reaction temperature also was crucial for this reaction since a modest drop of reaction temperatures from 120 °C to 100 °C led to an incomplete conversion, an extremely low yield, and a substantial quantity of 9-benzylidenefluorene byproduct 4 (entry 8).7 Toluene (entry 1) and dioxane (entry 9) were the choice of solvents, but THF (entry 10) was not suitable for the reaction. Subsequently, a systematic investigation was made on the amount of t-BuOK used, suggesting that with increasing the base from 50 mol% (entry 1) to 100 mol% (entry 11), 150 mol% (entry 12) and 200 mol% (entry 13), the desired product decreased and the byproduct 9-fluorenone increased gradually; when it was reduced from 50 mol% (entry 1) to 25 mol% (entry 14), and 10 mol% (entry 15), the reaction could proceed to completion with no side reaction as long as the reaction lasted long enough.
| Entry | Variation from the standard conditions | 3cb | 1b | 4b | Fluorenoneb |
|---|---|---|---|---|---|
| a The standard conditions: fluorene (0.5 mmol), 4-methoxybenzyl alcohol (1.5 mmol), base (0.25 mmol, 50 mol% relative to fluorene), solvent (4 mL), 120 °C, in N2, 3 h.b The crude product examined quantitatively by 1H NMR and qualitatively by TLC; NMR yields determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard.c Reaction time: 24 h.d Reaction time: 48 h. | |||||
| 1 | None | 99 | 0 | 0 | 0 |
| 2 | With no use of base | 0 | 100 | 0 | 0 |
| 3 | KOH instead of t-BuOK | 85 | 10 | 0 | 0 |
| 4 | t-BuONa instead of t-BuOK | ∼5 | 71 | 21 | 0 |
| 5 | NaOH instead of t-BuOK | Trace | 72 | 25 | 0 |
| 6 | CsOH·H2O instead of t-BuOK | 26 | 54 | ∼5 | 0 |
| 7 | K2CO3 instead of t-BuOK | 0 | 100 | 0 | 0 |
| 8 | 100 °C instead of 120 °C | 10 | 48 | 40 | 0 |
| 9 | 1,4-Dioxane instead of toluene | 92 | ∼5 | 0 | 0 |
| 10 | THF instead of toluene | Trace | 88 | 10 | 0 |
| 11 | 0.5 mmol (100 mol%) t-BuOK | 90 | ∼5 | ∼5 | Trace |
| 12 | 0.75 mmol (150 mol%) t-BuOK | 60 | ∼5 | ∼5 | 28 |
| 13 | 1.0 mmol (200 mol%) t-BuOK | 50 | ∼5 | 0 | 40 |
| 14c | 0.125 mmol (25 mol%) t-BuOK | 99 | 0 | 0 | 0 |
| 15d | 0.05 mmol (10 mol%) t-BuOK | 99 | 0 | 0 | 0 |
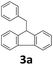
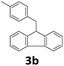
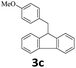
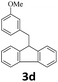
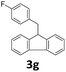
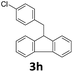
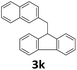
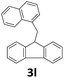
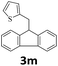
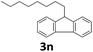
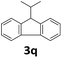
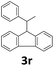
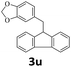
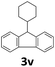
Next, fluorene reacted with a range of representative alcohols to determine the generality of this protocol (Table 2). Generally, benzylic alcohols, whether the nonactivated (3a, 3f, 3k, and 3l), activated (3g, 3h, 3i, and 3j) or deactivated (3b, 3c, and 3d), smoothly underwent the reaction to afford the desired products with almost quantitative conversions and yields. Ortho-substituted benzylic alcohol (3e) needed a prolonged reaction time due to its steric effect, giving an excellent yield of 95%. Additionally, the mild reaction conditions tolerated some functional groups like the fluoro (3g), chloro (3h), bromo (3i and 3s), iodo (3j) or trifluoromethyl (3t) group. As we know, such halogen-containing derivatives would be very useful in organic synthesis as they might be further transformed and compounds containing trifluoromethyl functional groups are important pharmaceutical intermediates. Likewise, the reaction of fused aryl (3k and 3l), heteroaryl (3m) carbinols and piperonyl alcohol (3u) proceeded smoothly in near quantitative conversions under the moderately modified reaction conditions. Although aliphatic alcohols are much less reactive as alkylating reagents than benzylic alcohols,5 that isn't the case in our reaction. Primary aliphatic alcohols (3n, 3o and 3p) were quantitatively converted to the desired 9-monoalkylfluorenes; even sterically congested secondary alcohols such as isopropanol (3q), 1-phenyl ethanol (3r) and cyclohexanol (3v) furnished the corresponding products in high yields at a more elevated temperature of 140 °C.
| Entry | Alcohol | Product | Isolated yield (%) |
|---|---|---|---|
| a Reaction conditions: fluorene (0.5 mmol), alcohols (1.5 mmol), t-BuOK (0.25 mmol), toluene (4 mL), in N2.b 24 h.c t-BuOK (0.375 mmol).d t-BuOK (0.50 mmol).e t-BuOK (0.75 mmol).f 140 °C.g 2-Bromo-9-fluorene was used. | |||
| 1 | 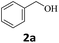 |
 |
98 |
| 2 | 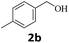 |
 |
99 |
| 3 | 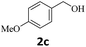 |
 |
96 |
| 4 | 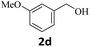 |
 |
99 |
| 5b |  |
 |
95 |
| 6 |  |
 |
99 |
| 7 | 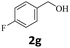 |
 |
99 |
| 8 | 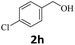 |
 |
99 |
| 9 | 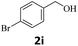 |
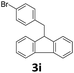 |
99 |
| 10 | 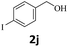 |
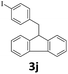 |
99 |
| 11b,c | 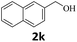 |
 |
99 |
| 12b,c | 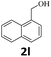 |
 |
99 |
| 13b,d | 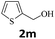 |
 |
97 |
| 14b,c | 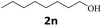 |
 |
99 |
| 15b,c |  |
 |
99 |
| 16b,d |  |
 |
99 |
| 17b,e,f | 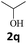 |
 |
89 |
| 18b,e,f | 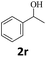 |
 |
90 |
| 19g | 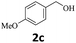 |
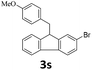 |
99 |
| 20b,e,f | 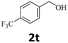 |
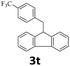 |
85 |
| 21c | 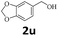 |
 |
99 |
| 22b,e,f | 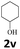 |
 |
81 |
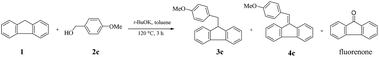
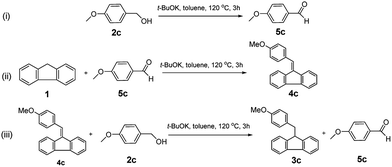
To ascertain the mechanism of the reaction, several additional control experiments were designed and carried out (Scheme 1). In a blank experiment, the freshly distilled p-methoxybenzyl alcohol was treated with potassium tert-butoxide (0.5 equivalents) at 120 °C in N2 for 3 h, affording an around 5% yield of anisaldehyde 5c (Scheme 1-i). 9-Benzylidenefluorene 4c was readily obtained in 72% isolated yield from the reaction of fluorene and benzaldehyde under the standard conditions (Scheme 1-ii). In the process of transfer hydrogenation of 4c with 2c, the target product 3c and equimolar aldehyde 5c (5c/3c = 0.98/1.00 mol mol−1 by 1H NMR analysis of the reaction mixture) would be generated simultaneously (Scheme 1-iii).
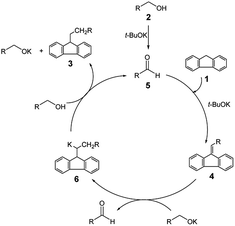
Combining our own experimentation with the relevant publications,4,5 we proposed a plausible mechanistic path for this reaction (Scheme 2). As shown in Scheme 2, a small amount of aldehyde 5 corresponding to alcohol 2 would first occur under the reaction conditions given. It was well established that an aldehyde 5 condenses with fluorene 1 to give a dibenzofulvene 4 in the presence of the base.8 Next, potassium alkoxide reduces the exo-double bond with attendant formation of a molecule of aldehyde. This step may formally be regarded as a type of Meerwein–Ponndorf–Verley reduction9 where the exo-double bond of the dipolar fulvene plays the role of the hydrogen acceptor. Finally, the potassium derivative of the product 6 reacts with the alcohol to give 9-alkylfluorene 3.
Conclusions
In summary, we have developed a simple, mild and efficient protocol for the alkylation of fluorene with alcohols in the presence of potassium tert-butoxide as catalyst. This method affords a highly green access to 9-monoalkylfluorenes. Further study to expand the scope of substrates is ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank National Natural Science Foundation of China (Project No. 21503234 and 21572235) for financial support of this work.Notes and references
- (a) N. R. Evans, L. S. Devi, C. S. K. Mak, S. E. Watkins, S. I. Pascu, A. Köhler, R. H. Friend, C. K. Williams and A. B. Holmes, J. Am. Chem. Soc., 2006, 128, 6647 CrossRef CAS; (b) G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC; (c) U. Scherf and E. J. W. List, Adv. Mater., 2002, 14, 477 CrossRef CAS; (d) M. T. Bernius, M. Inbasekaran, J. O Brien and W. Wu, Adv. Mater., 2000, 12, 1737 CrossRef CAS; (e) K. Miyatake, B. Bae and M. Watanabe, Polym. Chem., 2011, 2, 1919 RSC; (f) O. Inganäs, F. Zhang and M. R. Andersson, Acc. Chem. Res., 2009, 42, 1731 CrossRef; (g) G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323 CrossRef CAS.
- The formation and hydrogenation of 9-alkylidenefluorenes: (a) G. B. Bachman and S. Polansky, J. Org. Chem., 1951, 16, 1690 CrossRef CAS; (b) C. A. Fleckenstein, R. Kadyrov and H. Plenio, Org. Process Res. Dev., 2008, 12, 475 CrossRef CAS.
- The SN2 reaction of 9-lithofluorene with haloalkanes: (a) G. S. Hamilton, R. E. Mewshaw, C. M. Bryant, Y. Feng, G. Endemann, K. S. Madden, J. E. Janczak, J. Perumattam, L. W. Stanton, X. Yang, Z. Yin, B. Venkataramen and D. Y. Liu, J. Med. Chem., 1995, 38, 1650 CrossRef CAS; (b) Q. Pei and Y. Yang, J. Am. Chem. Soc., 1996, 118, 7416 CrossRef CAS; (c) C. A. Fleckenstein and H. Plenio, Chem. – Eur. J., 2007, 13, 2701 CrossRef CAS; (d) F. Turhan, F. Pak, A. Yesildag, Z. Kudas and D. Ekinci, Polym. Bull., 2012, 68, 1677 CrossRef CAS.
- The base-catalyzed 9-monoalkylation of fluorene with alcohols: (a) K. L. Schoen and E. I. Becker, J. Am. Chem. Soc., 1955, 77, 6030 CrossRef CAS; (b) K. L. Schoen and E. I. Becker, Organic Synthesis, Wiley, New York, 1963, Collect. vol. IV, p. 623 Search PubMed; (c) Also see: ref. 2b.
- The aldehyde/ketone-catalyzed 9-monoalkylation of fluorene with alcohols: J. Chen, Y. Li, S. Li, J. Liu, F. Zheng, Z. Zhang and Q. Xu, Green Chem., 2017, 19, 623 RSC.
- For selected reviews on the use of alcohols as alkylating reagents, see: (a) A. J. A. Watson and J. M. J. Williams, Science, 2010, 329, 635 CrossRef CAS; (b) T. D. Nixon, M. K. Whittlesey and J. M. J. Williams, Dalton Trans., 2009, 753 RSC; (c) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853 CrossRef; (d) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS; (e) G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611 CrossRef CAS; (f) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410 CrossRef CAS; (g) G. Chelucci, Coord. Chem. Rev., 2017, 331, 1 CrossRef CAS; (h) C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef.
- It was found that the 9-alkylidene fluorene 4 would become a major product under the same conditions as for the 9-monoalkyl fluorene synthesis except at a lower reaction temperature of about 70 °C. Also see: Table S1 in ESI.†.
- R. F. Schultz and C. F. Smullin, J. Am. Chem. Soc., 1940, 62, 2904 CrossRef.
- For selected Meerwein–Ponndorf–Verley reduction reactions, see: (a) J. Lee, T. Ryu, S. Park and P. H. Lee, J. Org. Chem., 2012, 77, 4821 CrossRef CAS; (b) C. Battilocchio, J. M. Hawkins and S. V. Ley, Org. Lett., 2013, 15, 2278 CrossRef CAS; (c) J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu and B. Han, Angew. Chem., Int. Ed., 2015, 54, 9399 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07557g |
| This journal is © The Royal Society of Chemistry 2019 |