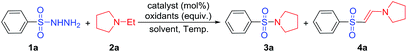Open Access Article
Open Access ArticleIodine-catalyzed sulfonylation of sulfonyl hydrazides with tert-amines: a green and efficient protocol for the synthesis of sulfonamides†
Jinyang
Chen
 a,
Xiaoran
Han
a,
Lan
Mei
a,
Jinchuan
Liu
*a,
Kui
Du
a,
Tuanwu
Cao
*a and
Qiang
Li
a,
Xiaoran
Han
a,
Lan
Mei
a,
Jinchuan
Liu
*a,
Kui
Du
a,
Tuanwu
Cao
*a and
Qiang
Li
 b
b
aCollege of Chemistry and Chemical Engineering, Yangtze Normal University, Fuling, Chongqing, 408000, P. R. China. E-mail: liujinchuan123@yeah.net; caotw2013@163.com
bInstitution of Functional Organic Molecules and Materials, School of Chemistry and Chemical Engineering, Liaocheng University, No. 1, Hunan Street, Liaocheng, Shandong 252059, P. R. China
First published on 2nd October 2019
Abstract
This study provides a direct, sustainable and eco-friendly method for the synthesis of various sulfonamides via the sulfonylation of sulfonyl hydrazides with tert-amines. The method utilizes sulfonyl hydrazides to oxidize and couple with tertiary amines through selective cleavage of C–N bonds. In this reaction, molecular iodine was used as the catalyst and t-butyl hydroperoxide was used as the oxidant.
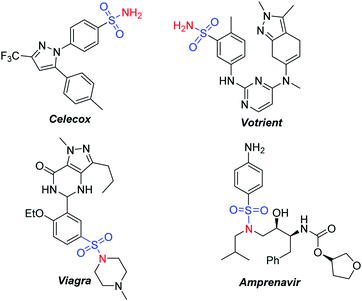
Sulfonamides commonly serve as synthetic intermediates to produce various drugs and industrial compounds (Scheme 1).1 They also commonly act as a N-sulfonyl protecting group for easy removal under mild conditions.2 There have been many efforts devoted to synthesizing these compounds. Among the methods developed, nucleophilic substitution of an amine with a sulfonyl chloride or sulfonamides with organic halides in the presence of a base is frequently utilized.3 Over the past few years, transition metal catalysis has been proved a powerful tool to synthesize sulfonamides. For example, a cross-coupling reaction of primary sulfonamides with aryl halide or boronic acids,4 a Chan-Lam type coupling reaction of sulfonyl azides with boronic acids,5 or an oxidative coupling reaction of sulfinate salts with amines were developed.6 However, the use of non-stable, hazardous and mutagenic starting materials and toxic high boiling polar solvents in these reactions resulted in a larger amount of toxic waste. Furthermore, the use of stoichiometric amounts of bases or transition metals makes reactions with a slow reactivity and poor functional group tolerability. Therefore, a novel, sustainable, efficient, and eco-friendly method is desired to synthesize sulfonamides.
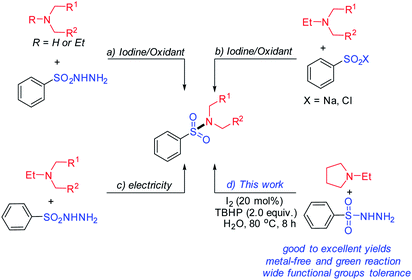
Iodine and its salts have been reported as very efficient catalysts in CDC reactions in which transition metals are used as catalysts.7 In recent years, many methods have been reported for synthesis of sulfonamides under metal-free conditions.8 As a source of sulfonyl groups, sulfonyl hydrazides are readily accessible solid. They are stable in air and under moisture conditions, and can be easily prepared and stored. Most importantly, only water and nitrogen were obtained as by-products during the reactions using sulfonyl hydrazides as starting materials. Iodine catalyzed oxidative coupling of sulfonyl hydrazides with secondary amines have been developed (Scheme 2(a)).9Tert-amines can donate an amine group via a C–N cleavage in place of primary or secondary amines. Compared to the high reactivity of primary or secondary amines, tert-amines are less nucleophilic and non-destructive for some amine-sensitive functional groups. Recently, Yuan et al. and Gui's have developed a new method to sulfonamides using I2-mediated or catalyzed C–N bond cleavage of tert-amines (Scheme 2(b)).10 Meanwhile, Sheykhan et al. reported a novel electrochemical oxidative sulfonylation11 of tert-amines (Scheme 2(c)).12 Moreover, catalytic reactions in the aqueous phase have also been recently developed,13 and sulfonylation of sulfonyl hydrazides has caused wide interest recently.14 In this study, we will report a new method to synthesize sulfonamides using iodine-catalyzed oxidative coupling of sulfonyl hydrazides with tert-amines (Scheme 2(d)). This approach avoids use of metal catalysts and hazardous regents; the materials, sulfonyl hydrazides and tert-amines, are versatile intermediates in commercial.
Commercially available sulfonyl hydrazide 1a was selected as a sulfonyl source to synthesize sulfonamides. When 1a was mixed with 1 equiv. of N-ethyl pyrrolidine under various solvents at 80 °C, an 80% yield of the corresponding sulfonamide 3a was obtained after 4 hours in the aqueous phase (Table 1, entries 1–6). We found that both iodine as the catalyst and t-butyl hydro-peroxide (TBHP) as the oxidant are essential to convert tert-amines to secondary amines efficiently in this reaction (Table 1, entries 7 and 8). When TBHP was replaced by H2O2, oxone, or O2, we found that the reaction was not able to be completed to produce the desired sulfonamide (Table 1, entries 9–11). The amounts of catalyst (I2) and oxidant were also examined, and results shown that 20 mol% of I2 and TBHP were most suitable for this reaction for giving desired product in 80% yield (Table 1, entry 3). We attempted to derive the reaction to be completed by increasing the temperature but it was not successful and there was no improvement in yield (Table 1, entry 15). Interestingly, temperature decrease has decreased the product yield rapidly (Table 1, entry 16). When iodine was replaced by tetrabutylammonium iodide (TBAI) or ammonium iodide (NH4I) as the catalyst, only trace amount or 0% of the desired sulfonamide 3a was achieved, respectively (Table 1, entries 17 and 18). Furthermore, the sulfonylation was affect inapparently by the atmosphere of air or nitrogen (Table 1, entry 19). After extensive screening, we were glad to find that the reaction of benzenesulfonohydrazide (1a) with 1-ethylpyrrolidine (2a) in H2O catalyzed by 20 mol% of I2 and in the presence of 2.0 equiv. of TBHP provided the desired product (3a) with an excellent yield of 80% at 80 °C for 8 h (Table 1, entry 3). And the standard process was shown as follows (Scheme 3).
| Entry | I2 (mol%) | Oxidant (equiv.) | Solvent | Temp. (°C) | Yieldsb (3a/4a%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.3 mmol), I2 (20 mol%), H2O (3 mL), 8 h, 80 °C. TBHP: tert-butyl hydroperoxide, 5.0–6.0 M in decane. b Isolated yield. c The reaction was performed under nitrogen atmosphere. | |||||
| 1 | I2 (20 mol%) | TBHP (2.0 equiv.) | CH3CN | 80 | 0/50 |
| 2 | I2 (20 mol%) | TBHP (2.0 equiv.) | THF | 80 | 5/29 |
| 3 | I 2 (20 mol%) | TBHP (2.0 equiv.) | H 2 O | 80 | 80/0 |
| 4 | I2 (20 mol%) | TBHP (2.0 equiv.) | DMF | 80 | NR |
| 5 | I2 (20 mol%) | TBHP (2.0 equiv.) | Toluene | 80 | NR |
| 6 | I2 (20 mol%) | TBHP (2.0 equiv.) | EtOH | 80 | 31/27 |
| 7 | — | TBHP (2.0 equiv.) | H2O | 80 | 0 |
| 8 | I2 (20 mol%) | — | H2O | 80 | NR |
| 9 | I2 (20 mol%) | H2O2 (2.0 equiv.) | H2O | 80 | NR |
| 10 | I2 (20 mol%) | Oxone (2.0 equiv.) | H2O | 80 | NR |
| 11 | I2 (20 mol%) | O2 | H2O | 80 | NR |
| 12 | I2 (10 mol%) | TBHP (2.0 equiv.) | H2O | 80 | 56/0 |
| 13 | I2 (5 mol%) | TBHP (2.0 equiv.) | H2O | 80 | 43/0 |
| 14 | I2 (20 mol%) | TBHP (1.0 equiv.) | H2O | 80 | 62/0 |
| 15 | I2 (20 mol%) | TBHP (2.0 equiv.) | H2O | 100 | 61/0 |
| 16 | I2 (20 mol%) | TBHP (2.0 equiv.) | H2O | 60 | 40/0 |
| 17 | NH4I | TBHP (2.0 equiv.) | H2O | 80 | Trace |
| 18 | TBAI | TBHP (2.0 equiv.) | H2O | 80 | NR |
| 19c | I2 (20 mol%) | TBHP (2.0 equiv.) | H2O | 80 | 80/0 |
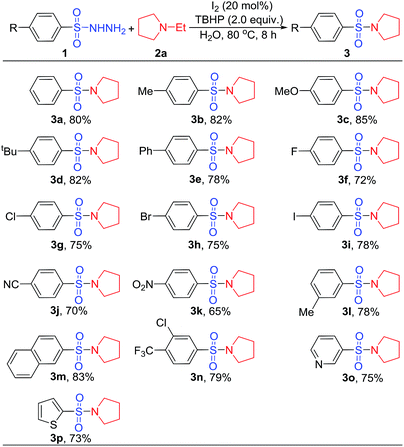
In sulfonylation of amines, it is essential to maintain the process intact by methyl, methoxy, tertiary butyl, phenyl, halo, cyan and nitro substituents presenting at the aromatic ring. In all cases, the yields of the corresponding sulfonamides 3b–3k were in the range of 65–85%. In this series, the lowest yields were obtained for electron-deficient substituted products, which could be attributed to electron factors affecting the catalysis. Notably, arylsulfonyl hydrazides bearing substituents at the metal position could be converted to the sulfonamides in yields of 78% (3l). The yield of 2-naphthalene sulfonamides was 83% (3m). The reaction of 3-chloro-4-(trifluoromethyl)benzene-1-sulfonyl hydrazide with amine produced 3p in a good yield, which could be leveraged for consequent coupling reaction. Because most sulfonamides with relevance for crop protection or medical application contain heterocycles, we included such substrates in our work. Fortunately, heteroaromatic sulfonyl hydrazides could be tolerated in this reaction, achieving the desired product in better yields (3o and 3p). Thus, this method has been demonstrated as a practical and efficient way to synthesize sulfonamides (Table 2).
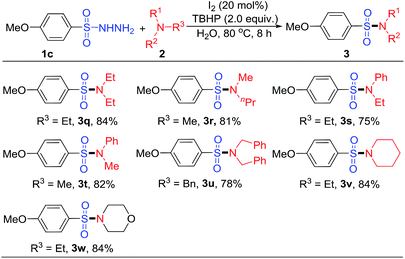
In subsequent studies, we examined the sulfonylation of sulfonyl hydrazides with various tertiary amines under the optimal conditions, and results were summarized in Table 3. Analyzing Table 3, we can see that various tertiary amines were compatible with the standard conditions, affording the corresponding sulfonamides in moderate to excellent yields. Using 4-methoxybenzenesulfonyl hydrazides (1c) as sulfonating reagent, we found that both aliphatic and aromatic tertiary amines were able to react smoothly with 1c to produce the desired products with a yield from 75% to 84%. When the tertiary amine with two different substituent groups were involved, such as N,N-triethylamine, N,N-dimethyl-N-propylamine, N,N-diethylaniline and N,N-dimethylaniline, the desired products were also achieved and the yield is of over 75% (3q–3t). It was found that tribenzylamine was able to react smoothly with 1c to produce the target product 3u in 78% yield. In addition, more cyclic or heterocyclic tertiary amines, such as 1-ethylpiperidine and 4-ethylmorpholine were used as the substrates, and the corresponding sulfonamides were obtained with yields of 84% (3v and 3w).
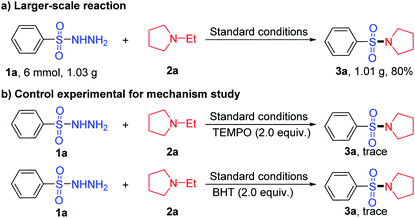
The sulfonylation can also be carried out on a larger scale reaction, and the desired product (3a) was obtained in the yield of 80%, when 6 mmol of benzenesulfonohydrazide (1a) was treated with 6 mmol of 1-ethylpyrrolidine (2a) under the standard conditions (Scheme 4(a)). To shed light on the mechanism of the reaction, benzenesulfonohydrazide (1a) was treated with 1-ethylpyrrolidine (2a) under standard conditions by using 2.0 equiv. of TEMPO or BHT as radical scavengers (Scheme 4(b)), and desired product 3a was obtained in trace yields, suggesting that a single-electron transfer process was involved through the whole reaction.
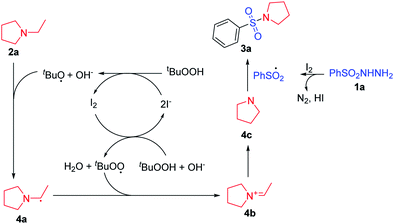
On the basis of the above experimental results and previous works,9a,10b a possible mechanism has been depicted in Scheme 5. Firstly, the transformation presumably involves an initial reaction of I2 with TBHP to create a reactive tert-butoxyl or tert-butyl peroxy radical. Then, tert-butoxyl or tert-butyl peroxy radical abstract a hydrogen from N-ethyl pyrrolidine to form radical 4a. After an electron transformation, it is converted to an intermediate aninium ion 4b. This intermediate 4b was then hydrolyzed by the elimination of an aldehyde to result in a secondary amine 4c. Finally, 4c reacts with a sulfonyl radical to generate the desired sulfonamide product 3a.
Conclusions
In conclusion, we have developed a novel method to synthesize sulfonamides through iodine catalyzed C–N bond cleavage of tertiary amines in the aqueous phase. The method is simple and easy in operation, along with inexpensive and accessible substrates. In addition, nitrogen and acetaldehyde were the major by-products. Importantly, iodine/TBHP was first time used as the terminal oxidant in preparation of various sulfonamides from sulfonyl hydrazides and tert-amines.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21902014) and the Basic and Frontier Research Project of Chongqing (Cstc2018jcyjAX0051, Cstc2016jcyjA0056) for the funding support.Notes and references
- (a) C. T. Supuran, A. Casini and A. Scozzafava, Med. Res. Rev., 2003, 5, 535 CrossRef; (b) A. Scozzafava, T. Owa, A. Masttolorenzo and C. T. Supuran, Curr. Med. Chem., 2003, 10, 925 CrossRef CAS; (c) J. D. Wilden, J. Chem. Res., 2010, 34, 541 CrossRef CAS; (d) Q. Liang, Y. Zhang, M. Zeng, L. Guan, Y. Xiao and F. Xiao, Toxicol. Res., 2018, 7, 521 RSC.
- (a) W. Yuan, K. Fearson and M. H. Gelb, J. Org. Chem., 1989, 54, 906 CrossRef CAS; (b) S. Chandrasekhar and S. Mohapatra, Tetrahedron Lett., 1988, 39, 695 CrossRef; (c) S. P. Fritz, A. Mumtaz, M. Yar, E. M. McGarrigle and V. K. Aggarwal, Eur. J. Org. Chem., 2011, 3156 CrossRef CAS.
- (a) S. W. Wright and K. N. Hallstrom, J. Org. Chem., 2006, 71, 1080 CrossRef CAS; (b) A. R. Katritzky, A. A. A. Abdel-Fattah, A. V. Vakulenko and H. Tao, J. Org. Chem., 2005, 70, 9191 CrossRef CAS PubMed; (c) S. Caddick, J. D. Wilden and D. B. Judd, J. Am. Chem. Soc., 2004, 126, 1024 CrossRef CAS; (d) R. Pandya, T. Murashima, L. Tedeschi and A. G. M. Barrett, J. Org. Chem., 2003, 68, 8274 CrossRef CAS; (e) J. W. Lee, Y. Q. Louie, D. P. Walsh and Y.-T. Chang, J. Comb. Chem., 2003, 5, 330 CrossRef CAS; (f) C. G. Frost, J. P. Hartley and D. Griffin, Synlett, 2002, 1928 CrossRef CAS; (g) M. N. S. Rad, A. Khalafi-Nezhad, Z. Asrari, S. Behrouz, Z. Amini and M. Behrouz, Synthesis, 2009, 3983 CAS; (h) S. Lakrout, H. Ktir, A. Amira, M. Berredjem and N.-E. Aouf, RSC Adv., 2014, 4, 16027 RSC.
- (a) J. Yin and S. L. Buchwald, Org. Lett., 2000, 2, 1101 CrossRef CAS; (b) J. Yin and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 6043 CrossRef CAS; (c) H. He and Y.-J. Wu, Tetrahedron Lett., 2003, 44, 3385 CrossRef CAS; (d) J. Baffoe, M. Y. Hoe and B. B. Toure, Org. Lett., 2010, 12, 1532 CrossRef CAS PubMed; (e) B. R. Rosen, J. C. Ruble, T. J. Beauchamp and A. Navarro, Org. Lett., 2011, 13, 2564 CrossRef CAS; (f) D. Audisio, S. Messaoudi, J. F. Peyrat, J. D. Brion and M. Alami, J. Org. Chem., 2011, 76, 4995 CrossRef CAS PubMed; (g) K. S. Rao and T.-S. Wu, Tetrahedron, 2012, 68, 7735 CrossRef.
- S.-Y. Moon, J. Nam, K. Rathwell and W.-S. Kim, Org. Lett., 2014, 16, 338 CrossRef CAS.
- For selected examples: (a) X. Tang, L. Huang, C. Qi, X. Wu, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 6102 RSC; (b) H. Zhu, Y. Shen, Q. Deng and T. Tu, Chem. Commun., 2015, 51, 16573 RSC; (c) J. Ji, Z. Liu, P. Liu and P. Sun, Org. Biomol. Chem., 2016, 14, 7018 RSC; (d) M. Chen, Z.-T. Huang and Q.-Y. Zheng, Org. Biomol. Chem., 2014, 12, 9337 RSC; (e) W. Zhang, J. Xie, B. Rao and M. Luo, J. Org. Chem., 2015, 80, 3504 CrossRef CAS.
- For selected examples: (a) P. T. Parvatkar, R. Manetsch and B. Banik, Chem.–Asian J., 2019, 14, 6 CrossRef CAS; (b) A. Yoshimura and V. V. Zhdankin, Chem. Rev., 2016, 116, 3328 CrossRef CAS; (c) X. Wang and A. Studer, Acc. Chem. Res., 2017, 50, 1712 CrossRef CAS.
- For selected examples: (a) S. Sohrabnezhad, K. Bahrami and F. Hakimpoor, J. Sulfur Chem., 2019, 40, 256 CrossRef CAS; (b) K. Bahrami, M. M. Khodaei and M. Soheilizad, J. Org. Chem., 2009, 74, 9287 CrossRef CAS; (c) X. Pan, J. Gao, J. Liu, J. Lai, H. Jiang and G. Yuan, Green Chem., 2015, 17, 1400 RSC; (d) J.-B. Feng and X.-F. Wu, Org. Biomol. Chem., 2016, 14, 6951 RSC; (e) A. S. Tsai, J. M. Curto, B. N. Rocke, A. M. R. Dechert-Schmitt, G. K. Ingle and V. Mascitti, Org. Lett., 2016, 18, 508 CrossRef CAS; (f) D. L. Poeira, J. Macara, H. Faustino, J. A. S. Coelho, P. M. P. Gois and M. M. B. Marques, Eur. J. Org. Chem., 2019, 2695 CrossRef CAS; (g) H. Veisi, R. Ghorbani-Vaghei, S. Hemmati and J. Mahmoodi, Synlett, 2011, 2315 CrossRef CAS; (h) W. Wei, C. Liu, D. Yang, J. Wen, J. You and H. Wang, Adv. Synth. Catal., 2015, 357, 987 CrossRef CAS; (i) M. Zhu, W. Wei, D. Yang, H. Cui, L. Wang, G. Meng and H. Wang, Org. Biomol. Chem., 2017, 15, 4789 RSC.
- (a) S. K. R. Parumala and R. K. Peddinti, Tetrahedron Lett., 2016, 57, 1232 CrossRef CAS; (b) S. Yotphan, L. Sumunnee, D. Beukeaw, C. Buathongjan and V. Reutrakul, Org. Biomol. Chem., 2016, 14, 590 RSC; (c) H. Yu and Y. Zhang, Chin. J. Chem., 2016, 34, 359 CrossRef CAS.
- (a) J. Lai, L. Chang and G. Yuan, Org. Lett., 2016, 18, 3194 CrossRef CAS; (b) H. Jiang, X. Tang, Z. Xu, H. Wang, K. Han, X. Yang, Y. Zhou, Y.-L. Feng, X.-Y. Yu and Q. Gui, Org. Biomol. Chem., 2019, 17, 2715 RSC.
- (a) Y. Yang, Y. Bao, Q. Guan, Q. Sun, Z. Zha and Z. Wang, Green Chem., 2017, 19, 112 RSC; (b) K. Xu, L. Li, W. Yan, Y. Wu, Z. Wang and S. Zhang, Green Chem., 2017, 19, 4494 RSC; (c) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (d) C. Wu, L.-H. Lu, A.-Z. Peng, G.-K. Jia, C. Peng, Z. Cao, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20, 3683 RSC; (e) L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604 RSC; (f) L.-H. Lu, Z. Wang, W. Xia, P. Cheng, B. Zhang, Z. Cao and W.-M. He, Chin. Chem. Lett., 2019, 30, 1237 CrossRef CAS; (g) B. Wang, Z. Yan, L. Liu, J. Wang, Z. Zha and Z. Wang, Green Chem., 2019, 21, 205 RSC; (h) M. Sun, J. Jiang, J. Chen, Q. Yang and X. Yu, Tetrahedron, 2019 DOI:10.1016/j.tet.2019.07.014; (i) S. Peng, Y.-X. Song, J.-Y. He, S.-S. Tang, J.-X. Tan, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.08.002; (j) L. Wang, M. Zhang, Y. Zhang, Q. Liu, X. Zhao, J.-S. Li, Z. Luo and W. Wei, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.05.041; (k) L.-Y. Xie, T.-G. Fang, J.-X. Tan, B. Zhang, Z. Cao, L.-H. Yang and W.-M. He, Green Chem., 2019, 21, 3858 RSC.
- M. Sheykhan, S. Khani, M. Abbasnia, S. Shaabanzadeh and M. Joafshan, Green Chem., 2017, 19, 5940 RSC.
- For selected examples: (a) D.-Q. Dong, X. Gao, L.-X. Li, S.-H. Hao and Z.-L. Wang, Res. Chem. Intermed., 2018, 44, 7557 CrossRef CAS; (b) C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (c) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; (d) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (e) L.-Y. Xie, S. Peng, F. Liu, Y.-F. Liu, M. Sun, Z.-L. Tang, S. Jiang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 7193 CrossRef CAS; (f) Z. Cao, Q. Zhu, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.09.041; (g) C. Wu, X. Xin, Z.-M. Fu, L.-Y. Xie, K.-J. Liu, Z. Wang, W. Li, Z.-H. Yuan and W.-M. He, Green Chem., 2017, 19, 1983 RSC; (h) W. Li, G. Yin, L. Huang, Y. Xiao, Z. Fu, X. Xin, F. Liu, Z. Li and W. He, Green Chem., 2016, 18, 4879 RSC; (i) D.-Q. Dong, S.-H. Hao, H. Zhang and Z.-L. Wang, Chin. Chem. Lett., 2017, 28, 1597 CrossRef CAS; (j) L.-Y. Xie, Y. Duan, L.-H. Lu, Y.-J. Li, S. Peng, C. Wu, K.-J. Liu, Z. Wang and W.-M. He, ACS Sustainable Chem. Eng., 2017, 5, 10407 CrossRef CAS.
- (a) B. Wang, Z. Yan, L. Liu, J. Wang, Z. Zha and Z. Wang, Green Chem., 2019, 21, 205 RSC; (b) Y. Yang, Y. Bao, Q. Guan, Q. Sun, Z. Zha and Z. Wang, Green Chem., 2017, 19, 112 RSC; (c) K. Xu, L. Li, W. Yan, Y. Wu, Z. Wang and S. Zhang, Green Chem., 2017, 19, 4494 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07361b |
| This journal is © The Royal Society of Chemistry 2019 |