Open Access Article
Open Access ArticleEfficient synthesis of spirooxindolyl oxazol-2(5H)-ones via palladium(II)-catalyzed addition of arylboronic acids to nitriles†
Hao Songa,
Na Chenga,
Li-Qin Shea,
Yi Wu*b and
Wei-Wei Liao *a
*a
aDepartment of Organic Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: wliao@jlu.edu.cn
bSchool of Pharmaceutical Science, Jilin University, Changchun 130012, P. R. China. E-mail: wuyi19651007@163.com
First published on 17th September 2019
Abstract
A versatile synthesis of spirooxindolyl oxazol-2(5H)-ones via palladium(II)-catalyzed addition of arylboronic acids to nitriles is described. A wide range of spirooxindolyl oxazol-2(5H)-ones and other spirocyclic frameworks incorporating the oxazol-2(5H)-one unit can be readily prepared in good to high yields under the optimal conditions.
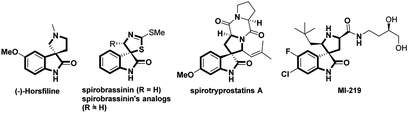
Rapid and efficient construction of pharmaceutical and biologically relevant compounds plays a very important role in modern organic synthesis, and constitutes the original impetus for the development of various novel synthetic approaches. The efficient construction of spirocyclic frameworks has been a topic of great relevance in organic synthesis due to their inherent three-dimensional architectures and the pronounced biological activities.1 In particular, the spirocyclic oxindoles have emerged as attractive synthetic targets because of their prevalence in numerous natural and unnatural products.2 Notably, the enhanced biological activities have been observed by the incorporation of a spiro five-membered azaheterocyclic ring at the C3 position of the oxindole core (Fig. 1).3 Thereby, a variety of synthetic methods have been developed to access analogous compounds possessing such privileged structure moieties.4
As one of the important N–O heterocyclic compounds, oxazolidinones and their derivatives have been widely used not only as synthetic building blocks,5 but also as pharmaceuticals6 and agrochemicals,7 owing to a diverse range of biological activities.8 Although great contributions have been made to access these valuable scaffolds,9 the construction of structurally diverse spirooxindolyl oxazol-2(5H)-ones, characterized by a spiro ring fusion at the C3 position of the oxindole core with oxazol-2(5H)-one motif, has received less attention from synthetic community,10 despite the fact that these spirocyclic heterocycles could be promising candidates possessing biological responses. In 2017, He and co-workers reported a formal [3 + 2] cycloaddition reaction of in situ generated azaoxyallyl cation with cyclic ketones for the synthesis of spiro-4-oxazolidinones.11a In 2018, Alla and co-workers described a copper-catalyzed one-pot multicomponent protocol for the synthesis of spiro(indoline-3,5′-oxazolidine)-2,2′-diones starting from ketones, arylacetylenes and isocyanates.11b
Recently, the transition-metal-catalyzed addition of organoboron reagents to nitriles has received remarkable progress,12 since the elegant works on the addition of arylpalladium species to the cyano group reported by Larock and Lu et al.,13 in which nitriles served as C building blocks and provided aryl ketones. In virtue of palladium-catalyzed tandem addition of organoboron reagents to nitriles/cyclization protocol, this approach enabled the combination of organoboron reagents and nitriles to construct a diversity of nitrogen-containing heterocycles such as 2-aminobenzophenones, benzofurans, and indoles, in which nitrile serves as C–N synthon instead and is incorporated into heterocyclic frameworks in an atom-economical fashion.14 However, the development of transition-metal-catalyzed tandem sequence involving the addition of organoboron reagents to nitriles to construct structural novel three-dimensional architectures such as spirocyclic systems is still undeveloped.
We have recently developed both intramolecular and intermolecular cyclization approaches to prepare indole and thiophene fused polycyclic derivatives via Pd-catalyzed direct C–H bond addition to nitriles.15 Given the promising biological activities of spirooxindoles-containing molecules in medicinal chemistry and our ongoing interest in the development of efficient catalytic processes to prepare diverse aza-heterocyclic frameworks, herein, we report an efficient synthetic approach to prepare spirooxindolyl oxazol-2(5H)-ones via palladium(II)-catalyzed addition of arylboronic acids to nitriles.
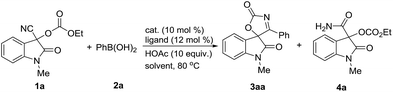
As functionalized nitriles, cyanohydrins which are readily prepared from ketones and aldehydes have demonstrated considerable synthetic potential as useful building blocks.16 We chose the Pd(II)-catalyzed reaction of 3-cyano-1-methyl-2-oxoindolin-3-yl ethyl carbonate 1a, which is readily prepared from isatin and ethyl cyanoformate, and phenylboronic acid 2a as a model reaction for the optimization of the reaction conditions (Table 1). Initially, we examined the reactions in various solvents in the presence of Pd(OAc)2 (10 mol%), 2,2′-bipyridine (L1: bpy) (12 mol%) and HOAc (10 equiv.) at 80 °C. To our delight, the desired spirooxindolyl oxazol-2(5H)-one 3aa was observed in range of solvents, in which low yield was obtained in less polar solvent along with the small amount of byproduct 4a (Table 1, entry 1), while moderate yields were obtained in polar solvents in general (Table 1, entries 2–5). Replacing Pd(OAc)2 with Pd(acac)2 afforded the cyclized product 3aa in the increased yield in NMP, while Pd(TFA)2 gave the slightly decreased yield (Table 1, entries 6 and 7). Subsequently, the effect of ligands was evaluated in the presence of Pd(acac)2. The similar results were obtained when 4,4′-dimethyl-2,2′-bipyridine (L2) and 1,10-phenanthroline (L4)were employed respectively, while 5,5′-dimethyl-2,2′-bipyridine (L3) gave the marginal reducing yield of 3a (Table 1, entries 8–10). Notably, the similar efficiency can be observed in prolonged reaction time by using Pd(OAc)2, in which 91% yield can be obtained in the presence of Pd(OAc)2 (5 mol%) and HOAc (5.0 equiv.) for 36 hours (Table 1, entry 11). The obvious decline in the yield of product 3aa was observed without HOAc (Table 1, entry 12), while HOAc cannot promote this reaction alone (Table 1, entry 15). Both ligand and Pd(II) catalyst proven to be essential to this transformation since no reaction happened without them (Table 1, entries 13 and 14). The attempt to reducing the amount of 2a resulted in the decreased yield (Table 1, entry 16). Further survey on other reaction parameters such as additives, reaction temperature and concentration did not improve the chemical outcome of this transformation (for details see the ESI†). In addition, the reaction also was evaluated with Ni(II) catalyst system. However, Ni(II)-catalyzed reaction gave the inferior to that of Pd(II) catalytic system (Table 1, entry 17), and delivered the desired product 3aa in moderate yield under the optimized reaction conditions (Ni(acac)2 (10 mol%) L2 (12 mol%) and MTBE) (for details see the ESI†).
| Entry | Cat. | Ligand | Solvent | t (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.6 mmol), catalyst (10 mol%), ligand (12 mol%) and HOAc (10 equiv.) in solvent (1 mL) at 80 °C.b Isolated yields.c Pd(OAc)2 (5 mol%) and bpy (6 mol%) were used.d HOAc (5.0 equiv.) was used.e Without HOAc.f 2a (0.4 mmol) was used.g Ni(acac)2 (10 mol%), L2 (12 mol%) and Cs2CO3 (20 mol%) in MTBE (1 mL) at 110 °C. L1: 2,2′-bipyridine; L2: 4,4′-dimethyl-2,2′-bipyridine; L3: 5,5′-dimethyl-2,2′-bipyridine; L4: 1,10-phenanthroline. | |||||
| 1 | Pd(OAc)2 | L1 | Toluene | 24 | 27 |
| 2 | Pd(OAc)2 | L1 | THF | 24 | 77 |
| 3 | Pd(OAc)2 | L1 | DMF | 24 | 79 |
| 4 | Pd(OAc)2 | L1 | DMSO | 24 | 71 |
| 5 | Pd(OAc)2 | L1 | NMP | 24 | 82 |
| 6 | Pd(TFA)2 | L1 | NMP | 24 | 77 |
| 7 | Pd(acac)2 | L1 | NMP | 24 | 88 |
| 8 | Pd(acac)2 | L2 | NMP | 24 | 86 |
| 9 | Pd(acac)2 | L3 | NMP | 24 | 73 |
| 10 | Pd(acac)2 | L4 | NMP | 24 | 88 |
| 11c,d | Pd(OAc)2 | L1 | NMP | 36 | 91 |
| 12c,e | Pd(OAc)2 | L1 | NMP | 36 | 83 |
| 13e | — | L1 | NMP | 24 | nd |
| 14e | Pd(OAc)2 | — | NMP | 24 | nd |
| 15d | — | — | NMP | 24 | nd |
| 16c,d,f | Pd(OAc)2 | L1 | NMP | 36 | 79 |
| 17g | Ni(acac)2 | L2 | MTBE | 24 | 67 |
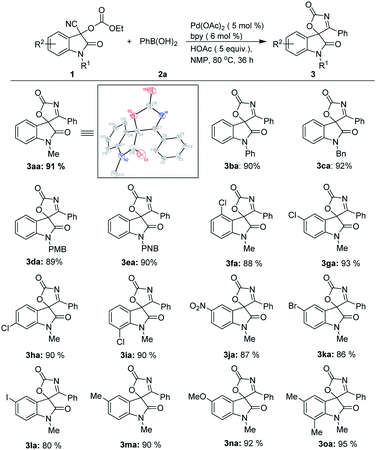
With the optimized reaction conditions in hand, the generality of the Pd-catalyzed addition/cyclization sequence for the preparation of spirooxindolyl oxazol-2(5H)-ones was evaluated by employing various isatin based-O-ethoxycarbonyl cyanohydrins 1 and phenylboronic acid 2a first (Scheme 1). Other than N-methyl substrate 1a, cyanohydrin analogues 1 bearing different N-substituents such as phenyl, benzyl, p-methoxybenzyl and p-nitrobenzyl can give the desired products 3ba–3ea in high yields. The substitution pattern at the benzene ring of cyanohydrins 1 has little influence on the results, and high yields could be obtained (3fa–3ia). In addition, the reactions between substrates possessing both electron-donating (MeO and Me) and electron-withdrawing (NO2, Br, Cl and I) substituents at the benzene ring and phenylboronic acid 2a proceeded well, and gave the corresponding products with excellent yields (3ja–3oa). The structures of spirooxindolyl oxazol-2(5H)-ones were unambiguously confirmed by the exemplification of X-ray crystal structural analysis of product 3aa.17
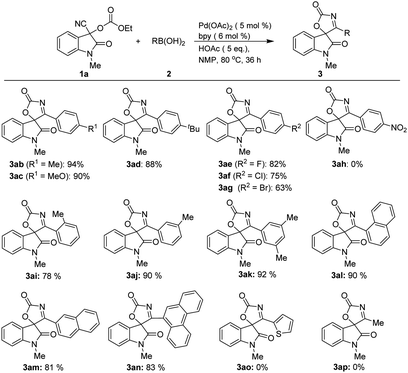
Next, the substrate scope with respect to arylboronic acids was also investigated, the results of which are summarized in Scheme 2. Arylboronic acids bearing both electron-donating (3ab–3ad) and electron-withdrawing substituents (3ae–3ag) at the benzene ring were tolerated, affording the desired products in good to high yields, exception for strong electron-withdrawing substituent such as nitro group (3ah), which did not react with 3-cyano-1-methyl-2-oxoindolin-3-yl ethyl carbonate 1a. It is noteworthy that the reaction also proceeded smoothly when a substituent was situated at the ortho position of the arylboronic acid, albeit with the slightly decreased yield (3ai). As expected, meta- and di-substituted analogues afforded products (3aj and 3ak) in high yields. Additionally, aryl boronic acids with fused ring also gave their corresponding products with high yields. For examples, treatment of both α-naphthyl and β-naphthyl boronic acids with 1a can deliver the corresponding products (3al–3am) in high yields under the optimized reaction conditions, while 9-phenanthreneboronic acid gave spirooxindolyl product 3an in 83% yield. However, hetero-aromatic boronic and alkyl boronic acid did not provided any desired products (3ao–3ap).
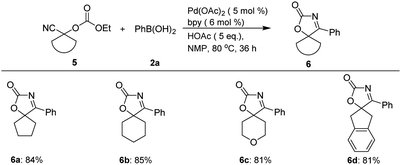
In addition, besides spirooxindolyl oxazol-2(5H)-one frameworks, this approach is also applicable to the construction of other spirocyclic frameworks incorporating oxazol-2(5H)-one unit via palladium-catalyzed tandem sequence (Scheme 3). For example, treatment of 1-cyanocyclopentyl ethyl carbonate 5a with 2a can furnish 4-phenyl-1-oxa-3-azaspiro[4.4]non-3-en-2-one 6a in 84% yield, while six-membered-ring analogues delivered the corresponding six-membered ring fused spiro-products (6b–6c) in high yields. Cyanohydrin 5d derived from 2-indanone can also serve as a suitable substrate for this tandem sequence, and provided the desired spiro-product 6d in 81% yield.
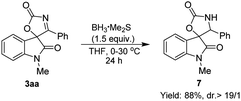
Finally, the synthetic utility of this Pd-catalyzed cyclization was demonstrated (Scheme 4). The reduction of 3aa by using BH3·SMe2 readily gave spirooxindolyl product 7 bearing the oxazolidine unit in good yield with an excellent diastereoselectivity.
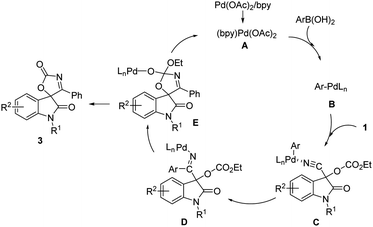
On the basis of these results and other processes involving the addition of arylpalladium species to nitrile,14,15 a plausible mechanism was illustrated in Scheme 5. First, the transmetalation of arylboronic acid by Pd(II) catalyst A generates arylpalladium species B. Then coordination of the nitrile provides intermediate C, which undergoes a carbopalladation of the cyano group to result in formation of the corresponding ketimine Pd(II) complex D. The intramolecular cyclization of the intermediate D to form palladium complex E which affords product and regenerates the Pd(II) catalyst.
In summary, we have demonstrated an efficient protocol for the synthesis of spirooxindolyl oxazol-2(5H)-ones via Pd(II)-catalyzed addition of arylboronic acids to nitriles. A diversity of functionalized spirooxindolyl oxazol-2(5H)-ones can be prepared in good to high yields under the optimal conditions. Furthermore, by the virtue of this Pd-catalyzed sequence, other five- and six-membered ring fused spiro-oxazol-2(5H)-ones can be readily prepared in good yields. Further studies on the application of this synthetic method are currently under investigation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The NSFC (No. 21772063) and the Open Project of State Key Laboratory for Supramolecular Structure and Materials (sklssm2019016) are acknowledged. This research was supported by China Scholarship Council.Notes and references
- For recent reviews, see: (a) H. Guo, J.-W. Yang and R. Ramon, Chem. Soc. Rev., 2018, 47, 5946 RSC; (b) X. Xie, C. Peng and B. Han, Adv. Synth. Catal., 2018, 360, 194 CrossRef CAS; (c) S. D. Griggs, D. T. Tape and P. A. Clarke, Org. Biomol. Chem., 2018, 16, 6620 RSC; (d) F. V. Singh, P. B. Kole, S. R Mangaonkar and S. E. Shetgaonkar, Beilstein J. Org. Chem., 2018, 14, 1778 CrossRef CAS PubMed; (e) P.-W. Xu, J.-S. Yu and J. Zhou, ACS Catal., 2019, 9, 1820 CrossRef CAS.
- (a) E. V. Mercado-Marin, R. G. Berlinck and R. Sarpong, Nature, 2014, 509, 318 CrossRef CAS; (b) Z. Bian, C. C. Marvin and S. F. Martin, J. Am. Chem. Soc., 2014, 136, 14184 CrossRef CAS; (c) Z. Bian, C. C. Marvin and S. F. Martin, J. Am. Chem. Soc., 2013, 135, 10886 CrossRef CAS; (d) A. K. Gupta, M. Bharadwaj and R. Mehrotra, Top. Curr. Chem., 2017, 375, 3 CrossRef; (e) Y.-T. Yang, J.-F. Zhu and B. Yu, Curr. Med. Chem., 2018, 25, 2233 CrossRef CAS.
- (a) A. Jossang, P. Jossang, H. A. Hadi and B. Bodo, J. Org. Chem., 1991, 56, 6527 CrossRef CAS; (b) C. B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1996, 52, 12651 CrossRef CAS; (c) M. Takasugi, K. Monde and A. Shirata, Chem. Lett., 1987, 16, 1631 CrossRef; (d) X. Jiang, Y. Cao and R. Wang, J. Am. Chem. Soc., 2010, 132, 15328 CrossRef CAS; (e) P. Zou, N. Zheng, S.-H. Yu and D.-X. Sun, J. Pharm. Pharm. Sci., 2012, 15, 265 CrossRef CAS.
- For recent reviews, see: (a) G.-J. Mei and F. Shi, Chem. Commun., 2018, 54, 6607 RSC; (b) G. M. Ziarani, R. Moradi and N. Lashgari, Tetrahedron, 2018, 74, 1323 CrossRef; (c) L.-J. Yan and Y.-C. Wang, ChemistrySelect, 2016, 1, 6948 CrossRef CAS; (d) D. Cheng, Y. Ishihara and C. F. Barbas, ACS Catal., 2014, 4, 743 CrossRef CAS.
- (a) D. J. Ager, I. Prakash, B. Tian and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS; (b) L. Aurelio, R. T. C. Brownlee and A. B. Hughes, Chem. Rev., 2004, 104, 5823 CrossRef CAS.
- K. Ando, T. Yamada and M. Shibuya, Heterocycles, 1989, 29, 2209 CrossRef CAS.
- A. Arnoldi, E. Betto, G. Farina and A. Griffini, Pestic. Sci., 1982, 13, 670 CrossRef CAS.
- (a) R. Somanathan, I. A. Rivero, G. I. Nunez and L. H. Hellberg, Synth. Commun., 1994, 24, 1483 CrossRef; (b) K. Michalska, I. Karpiuk and S. Tyski, Bioorg. Med. Chem., 2013, 21, 577 CrossRef CAS; (c) O. A. Phillips, E. E. Udo and R. Varghese, Eur. J. Med. Chem., 2013, 66, 246 CrossRef CAS; (d) X. Jiang, Y. Cao and R. Wang, J. Am. Chem. Soc., 2010, 132, 15328 CrossRef CAS PubMed; (e) T. Xue, B. Guo and Y. Yang, Bioorg. Med. Chem. Lett., 2015, 25, 2203 CrossRef CAS PubMed.
- (a) S. K. Alamsetti, A. K. Å. Persson and J.-E. Bäckvall, Org. Lett., 2014, 16, 1434 CrossRef PubMed; (b) D.-Y. Li, H.-J. Chen and P.-N. Liu, Adv. Synth. Catal., 2015, 357, 1193 CrossRef CAS; (c) M. J. Gainer, N. R. Bennett, Y. Takahashi and R. E. Looper, Angew. Chem., Int. Ed., 2011, 50, 684 CrossRef CAS PubMed; (d) H.-F. Jiang and J.-W. Zhao, Tetrahedron Lett., 2009, 50, 60 CrossRef CAS.
- (a) T. Rajasekaran, B. Sridhar and B. V. S. Reddy, Tetrahedron, 2016, 72, 2102 CrossRef CAS; (b) T. Rajasekaran, G. Karthik and B. V. S. Reddy, Org. Lett., 2013, 15, 1512 CrossRef CAS PubMed; (c) I. R. Siddiqui, P. Rai and M. A. Waseem, Tetrahedron Lett., 2015, 56, 4367 CrossRef CAS.
- (a) P.-L. Shao, Z.-R. Li and Y. He, J. Org. Chem., 2017, 82, 10680 CrossRef CAS PubMed; (b) G. R. Potuganti, D. R. Indukuri and M. Alla, J. Org. Chem., 2018, 83, 15186 CrossRef CAS PubMed.
- (a) B.-W. Zhao and X.-Y. Lu, Org. Lett., 2006, 8, 5987 CrossRef CAS PubMed; (b) Y. C. Wong, K. Parthasarathy and C.-H. Cheng, Org. Lett., 2010, 12, 1736 CrossRef CAS PubMed; (c) S. Demir, M. Yiğit and I. Özdemir, J. Organomet. Chem., 2013, 732, 21 CrossRef CAS; (d) M. Yousuf, T. Das and S. Adhikari, New J. Chem., 2015, 39, 8763 RSC; (e) J. Sävmarker, J. Rydfjord and M. Larhed, Org. Lett., 2012, 14, 2394 CrossRef.
- (a) R. C. Larock, Q. Tian and A. A. Pletnv, J. Am. Chem. Soc., 1999, 121, 3238 CrossRef CAS; (b) C. Zhou and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 2302 CrossRef CAS PubMed; (c) C. Zhou and R. C. Larock, J. Org. Chem., 2006, 71, 3551 CrossRef CAS PubMed; (d) B.-W. Zhao and X.-Y. Lu, Tetrahedron Lett., 2006, 47, 6765 CrossRef CAS.
- (a) K. Hu, J.-X. Chen and H.-Y. Wu, Green Chem., 2017, 19, 1740 RSC; (b) L. Qi, J.-X. Chen and H.-Y. Wu, Org. Lett., 2017, 19, 218 CrossRef CAS PubMed; (c) K. Hu, Q. Zhen and J.-X. Chen, Org. Lett., 2018, 20, 3083 CrossRef CAS PubMed; (d) J.-H. Zhu, Y.-L. Shao and J.-X. Chen, Org. Biomol. Chem., 2018, 16, 8596 RSC; (e) H.-H. Yu, X. Li and L.-M. Shao, Chem. Commun., 2017, 53, 9745 RSC; (f) M. Yousuf and S. Adhikari, Org. Lett., 2017, 19, 2214 CrossRef PubMed; (g) X.-C. Yang, H.-H. Yu and L.-M. Shao, J. Org. Chem., 2018, 83, 9682 CrossRef CAS PubMed.
- (a) T.-T. Wang, L. Zhao and W.-W. Liao, Org. Lett., 2016, 18, 5002 CrossRef CAS PubMed; (b) L. Zhao and W.-W. Liao, Org. Chem. Front., 2018, 5, 801 RSC; (c) T.-T. Wang, D. Zhang and W.-W. Liao, Chem. Commun., 2018, 54, 2048 RSC; (d) D. Zhang, H. Song and W.-W. Liao, Org. Lett., 2019, 21, 2745 CrossRef CAS PubMed.
- For reviews: (a) R. J. H. Gregory, Chem. Rev., 1999, 99, 3649 CrossRef CAS PubMed; (b) M. North, Tetrahedron: Asymmetry, 2003, 14, 147 CrossRef CAS; Selected examples: (c) A. Baeza, C. Najera and J. M. Sansano, Synthesis, 2005, 2787 CAS; (d) Y. Ogura, M. Akakura and K. Ishihara, Angew. Chem., Int. Ed., 2013, 52, 8299 CrossRef CAS PubMed.
- CCDC 1943540 (compound 3aa), see the ESI for details.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1943540. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra07216k |
| This journal is © The Royal Society of Chemistry 2019 |