Open Access Article
Open Access ArticleEffects of vitamin D3 release from 3D printed calcium phosphate scaffolds on osteoblast and osteoclast cell proliferation for bone tissue engineering
Ashley A. Vu and
Susmita Bose*
and
Susmita Bose*
W. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, Washington 99164, USA. E-mail: sbose@wsu.edu
First published on 29th October 2019
Abstract
Vitamin D3 is a hydrophobic micronutrient and is known for inhibiting osteoclastic bone resorption in vivo via suppression of the Receptor Activator of Nuclear factor-Kappa B (RANK ligand) expression in osteoblasts. Although vitamin D is well-known for its promotion in bone health, little is known on its effects directly on bone cells. The objective of this study was to understand the effects of vitamin D3 release from 3D printed calcium phosphate scaffolds towards bone cell proliferation. In this study, cholecalciferol, a common intake form of vitamin D3, was successfully able to release from the scaffold matrix via the use of polyethylene glycol. Results showed a decrease in osteoclast resorption pits and healthier osteoblast cellular morphology compared to the control. Additively manufactured tricalcium phosphate scaffolds with designed porosity were loaded with vitamin D3 and showed controlled release profiles in phosphate buffer and acetate buffer solutions. The release kinetics of vitamin D3 from calcium phosphate scaffolds enabling osteoblast proliferation and inhibiting osteoclastic resorption can enhance healing for low load bearing applications for bone defects or permeate voids left by tumor resection.
1. Introduction
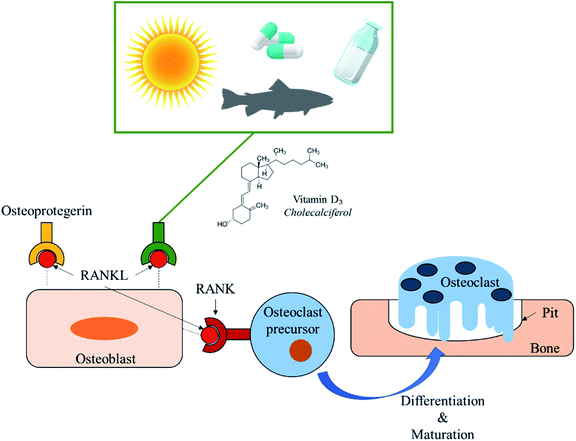
More than 8.9 million fractures occur annually due to osteoporosis.1 Osteoporosis is caused by many factors including a deficiency of calcium or vitamin D3.2 Risk of vitamin D deficiency occurs from little sun exposure and/or poor dietary intake.3 Low vitamin D levels, hypovitaminosis D, can be associated with higher secretion of parathyroid hormone, increased bone turnover, osteoporosis, osteomalacia, and higher risk for hip and other fractures.4,5 Vitamin D3 is a hydrophobic micronutrient and is known for inhibiting osteoclastic bone resorption in vivo via suppression of the Receptor Activator of Nuclear factor-Kappa B (RANK ligand) expression in osteoblasts (Fig. 1).6,7 The recommended daily intake of vitamin D3 varies from 10 to 100 μg per day. Analogs of vitamin D3 such as alfacalcidol have been used for osteoporosis since 1983 as therapeutic agents to increase bone mineral density and reduce incidences of bone fracture.8–10 Vitamin D3 and its receptors have also been reported to modulate lymphocyte and macrophage functions,11 impact the immune system,12,13 lower risk for cardiovascular mortality in haemodialysis patients,14 and even provide potential anticancer properties.15,16The objective of this study was to understand the effects of vitamin D3 release from 3D printed calcium phosphate scaffolds towards bone cell proliferation.† To the best of the authors' knowledge, the effects of vitamin D3 on osteoblast and osteoclast bone cell proliferation for bone tissue engineering applications using bioresorbable scaffolds has yet to be explored. Furthermore, limited studies have been done regarding the effects of cholecalciferol in vitro. One study successfully provided local delivery of cholecalciferol within hydroxyapatite nano-particles through the use of poly(D,L-lactide-co-glycolide).17 Another study utilized complex nanoparticles for vitamin D3 encapsulation as well as controlled release in simulated gastric fluid and simulated intestinal condition.18 Other works have shown benefits of vitamin D3 and its analogs within the body to aid in osteoporotic fractures through oral digestion.19,20
The hypothesis of this study was vitamin D3 would lower osteoclast resorption and show no cytotoxic effects towards osteoblast cells. The hydrophobic nature of vitamin D3 makes it difficult to release in an aqueous solution such as the physiological environment inside the body. Vitamin D3 enhanced hydrophilicity by using polyethylene glycol (PEG).21 PEG has been known for its use in medicine due to its various abilities such as facilitating drug absorption.22 Not only is PEG biocompatible, non-immunogenic, and highly soluble in water, it is also approved by the Food and Drug Administration (FDA).23 Polycaprolactone (PCL) is a biodegradable polymer used for drug delivery applications to modulate release kinetics.24,25 PCL is also FDA approved, biocompatible, relatively low in cost, and easy to process. In this study, a combination of both PEG and PCL was utilized for loading vitamin D3 into bone tissue engineering scaffolds. Hydroxyapatite (HA) and tricalcium phosphate (TCP) have excellent biocompatibility, biodegradability, and high osseointegration making them widely used materials for bone tissue engineering.26,27 TCP scaffolds also have the ability to be an effective, local drug delivery system when used as a bioresorbable implant.28,29 Both were utilized in vitro with osteoclast and human fetal osteoblast (hFOB) cell lines. Porous TCP scaffolds were produced using powder bed additive manufacturing to investigate in vitro release kinetics of vitamin D3. These advanced manufactured scaffolds provide the ability for patient specific implants with complex structures. The results from this study could be incorporated in low load bearing applications to provide local vitamin D3 drug delivery for bone defects or voids caused by tumor resection.
2. Materials and methods
2.1 Vitamin D3/polymer loading
Vitamin D3 (cholecalciferol, Alfa Aesar, Ward Hill, MA) was dissolved in ethanol at a concentration of 10 mg mL−1. Polymer solution of PCL (Mw = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich, MO) and PEG (Mw = 8000, Sigma-Aldrich, MO) at a 65
000, Sigma-Aldrich, MO) and PEG (Mw = 8000, Sigma-Aldrich, MO) at a 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 molar ratio was prepared. Polymer mix was dissolved in acetone at 5 wt% of PCL/PEG. Drug and polymer solutions were mixed together at a ratio of 50
35 molar ratio was prepared. Polymer mix was dissolved in acetone at 5 wt% of PCL/PEG. Drug and polymer solutions were mixed together at a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Any processing with vitamin D3 was done in the dark due to its sensitivity to light.
50. Any processing with vitamin D3 was done in the dark due to its sensitivity to light.
2.2 Effects of vitamin D3 in vitro on osteoclast and osteoblast cells
Samples used in the osteoclast cell culture study were HA (Monsanto, USA) discs and osteoblast cell culture study were synthesized TCP discs. Specific synthesis methods can be found in detail in previous works.27 Both discs were 12 mm diameter and 2.5 mm height uniaxially pressed at 165 MPa for 2 min each and sintered at 1250 °C for 2 h in a muffle furnace. All samples used in the study were sterilized in an autoclave for 1 h at 121 °C prior to any drug and polymer loading. All samples with vitamin D3 contained 20 μg of drug.2.3 Osteoclast cell protocol and resorption pit assay
Osteoclast cells (THP1 monocytes, ATCC, Manassas, VA) were seeded onto samples with a density of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sample. Growth media was comprised of Roswell Park Memorial Institute (RPMI)-1640, 0.05 mM 2-mercaptoethanol, and 10% FBS. Differentiation media was comprised of 40 ng mL−1 phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich, St. Louis, MO) and 10 ng mL−1 RANKL with RPMI-1640 and FBS. All cultures were kept at 37 °C under an atmosphere of 5% CO2 and the medium was changed every 2–3 days throughout the experiment. A timepoint of 10 days was utilized to assess resorption pits. Samples were ultrasonicated in 1 mL of 1 M NaCl solution mixed with 0.2% Triton X-100. Samples were rinsed, dehydrated, and gold coated prior to Scanning Electron Microscopy (SEM) imaging. Protocol for dehydration can be found in other work.25
000 cells per sample. Growth media was comprised of Roswell Park Memorial Institute (RPMI)-1640, 0.05 mM 2-mercaptoethanol, and 10% FBS. Differentiation media was comprised of 40 ng mL−1 phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich, St. Louis, MO) and 10 ng mL−1 RANKL with RPMI-1640 and FBS. All cultures were kept at 37 °C under an atmosphere of 5% CO2 and the medium was changed every 2–3 days throughout the experiment. A timepoint of 10 days was utilized to assess resorption pits. Samples were ultrasonicated in 1 mL of 1 M NaCl solution mixed with 0.2% Triton X-100. Samples were rinsed, dehydrated, and gold coated prior to Scanning Electron Microscopy (SEM) imaging. Protocol for dehydration can be found in other work.25
2.4 Osteoblast cell protocol and viability assays
Human fetal osteoblast cells (hFOB 1.19, ATCC, Manassas, VA) were seeded onto samples with a density of 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cells per sample. Osteoblast cell viability and morphology were analyzed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and SEM, respectively, with timepoints of 3, 7, and 11 days. Specific protocols can be referenced in other work with the exception of using ATCC recommended growth medium.26
200 cells per sample. Osteoblast cell viability and morphology were analyzed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and SEM, respectively, with timepoints of 3, 7, and 11 days. Specific protocols can be referenced in other work with the exception of using ATCC recommended growth medium.26
2.5 3D printed scaffold fabrication and characterization
Synthesized TCP scaffolds with 500 μm designed pore size were fabricated (6.7 mm diameter × 11 mm height) in a 3D powder bed printer (ExOne, Irwin, PA, USA). Fabrication and characterization methods including compressive strength testing using a screw-driven universal testing machine equipped with a load cell can be referenced in previous work.27,292.6 Vitamin D3 in vitro release study
Scaffolds were immersed in each buffer solution at 4 mL each timepoint. All samples were kept in a shaker at 37 °C with 150 rpm of constant shaking. Buffer solutions were changed periodically with fresh buffer added. Release was stopped after 7 days in ABS to eliminate erroneous data due to scaffold degradation. The concentration of vitamin D3 release at each timepoint was determined using a Biotek Synergy 2 SLFPTAD microplate reader (Biotek, Winooski, VT, USA) at an absorbance value of 260 nm wavelength. A standard curve was created using known concentrations of vitamin D3 in buffer solutions. A control of PCL/PEG loaded was subtracted from VD3/PCL/PEG release to quantify each timepoint.2.7 Statistical analysis
All compositions in each scaffold characterization test, compositions in the release study, and MTT assay were analyzed in triplicate with each measurement performed in triplicate. Data is presented as mean ± standard deviation. Comparative analysis in MTT results showed no statistical differences at a p < 0.05 confidence level.3 Results and discussion
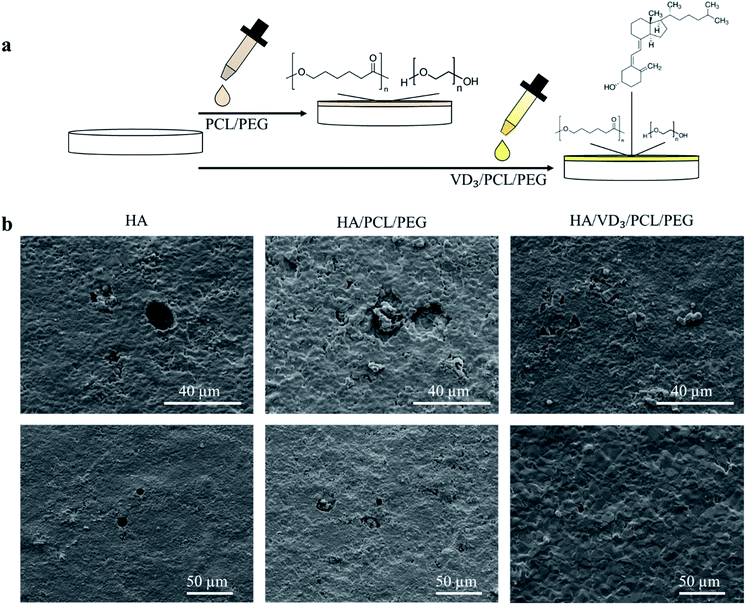
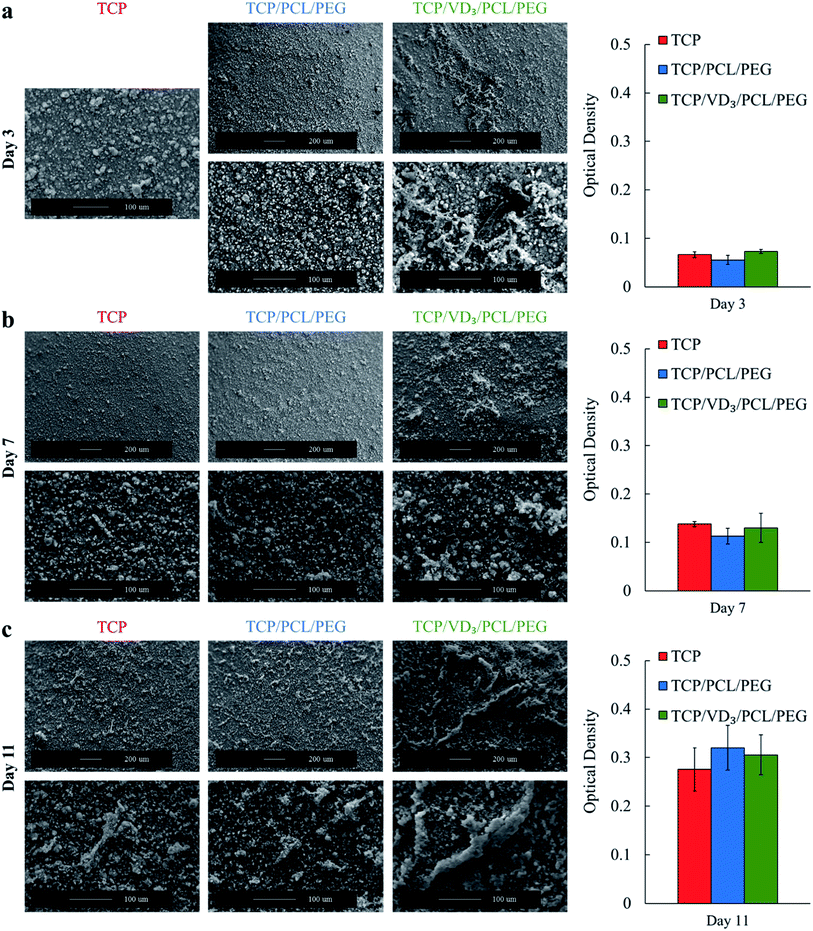
To assess bone cell materials interaction, cell cultures were performed with osteoblast cells and osteoclast cells separately followed by scanning electron microscopy (SEM) to assess morphology. The SEM images following osteoclast culture showed resorption pits of 20–30 μm size all over the surface of control HA and HA samples loaded with PCL/PEG (Fig. 2).Resorption pits were unable to be identified on samples loaded with vitamin D3/PCL/PEG indicating reduced osteoclastic activity. The use of vitamin D3 also showed no cytotoxic effects towards hFOB cells with higher cell coverage and healthier cellular morphology compared to control. MTT assay showed no statistically significant differences in cell viability for samples with PCL/PEG and VD3/PCL/PEG compared to control TCP (Fig. 3). Cytotoxicity is determined by a threshold of 70% viability in the first 24 h and neither PCL/PEG nor VD3/PCL/PEG loaded TCP samples showed cytotoxic effects in all timepoints.30 The morphology of the hFOB cells after 3 days of culture (Fig. 3a) showed mainly apatite formation with only the presence of cells on samples with vitamin D3. After 7 days of culture (Fig. 3b), the trend continued of cells being more prominent on samples loaded with vitamin D3. After 11 days of culture (Fig. 3c), cells were present on all compositions with apatite formation on top and vitamin D3 showed highest coverage of cells.
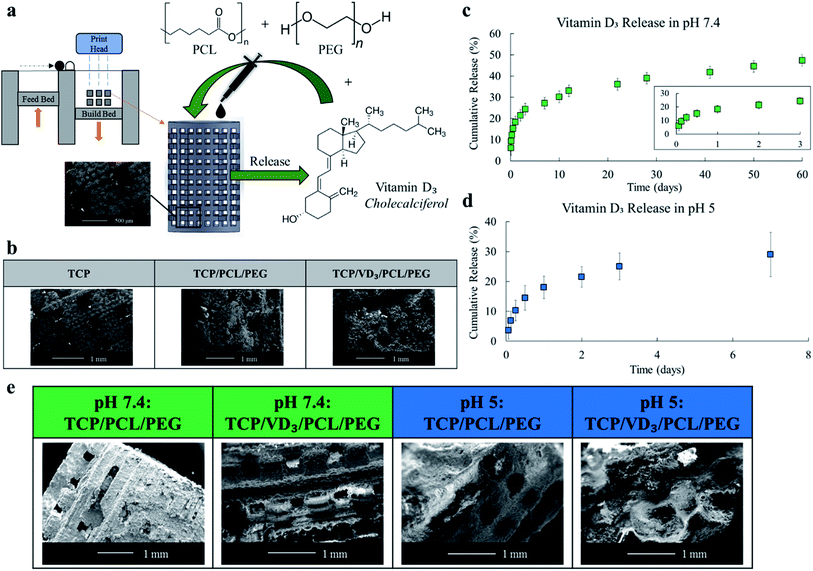
Porous scaffolds are structures that allow for the interaction of cells and extracellular matrices. Scaffolds also provide structural support for growing cells especially in circumstances involving bone defects and voids left by tumor resection. Manufacturing scaffolds using 3D printing allows for site specificity such as oral and maxillofacial defects (Fig. 4a). Pore sizes must be large enough for cell mobility, but scaffolds must also have enough structural integrity for use within the body. The 500 μm-3DP porous scaffolds used in this study have pore sizes ranging from 325 to 380 μm post sintering as assessed through SEM images (Fig. 4b). Pore sizes should be between 50 to 500 μm (ASTM F2150) where greater than 300 μm is most recommended. These scaffolds had a compressive strength of 4.0 ± 1.54 MPa. Cancellous bone, the spongy bone where these scaffolds would mostly interact with, has a compressive strength ranging from 0.22 up to 10.44 MPa.31 Bulk density, volume fraction open porosity, and relative density to TCP of the scaffolds were assessed to be 0.6 ± 0.01 g cm−3, 56.4 ± 0.31%, and 26.9 ± 0.01% respectively.
Scaffolds were loaded with 500 μg of vitamin D3 (500 μm-3DP) to investigate release kinetics in phosphate buffer solution (PBS, pH 7.4) to mimic the physiological environment and acetate buffer solution (ABS, pH 5) to mimic the micro-acidic environment post injury (Fig. 4c and d).32,33 The release of vitamin D3 depends significantly through the physicochemical interactions between the vitamin and the polymer system. Through the use of PEG, particles will aggregate via steric stabilization and increase stability during storage and application of the system.34
Additionally, PEG is a non-ionic hydrophilic polymer with stealth behavior; long circulating nanocarriers that avoid opsonization.35 The hydrophobic–hydrophilic interaction of vitamin D3 and PEG enables the release of vitamin D3 due to the more favorable solubility of the vitamin into the media compared to the binding within the scaffold matrix. With only PEG, vitamin D3 would show burst release in early timepoints, a phenomenon indicative in many drug releases.36–38 Burst release can be both pharmacologically dangerous, inefficient for long term delivery, and economically unfavorable. Minimal or eliminated burst release and prolonged release can be achieved using polymers such as PCL; a biodegradable polymer used to modulate release kinetics.23–25,39–41 PCL is degradable through hydrolysis of the ester linkages which can occur in physiological conditions.42 The hydrophobic properties of PCL limited the potentially higher burst release of vitamin D3 and enabled a more controlled, prolonged release over time. Surface degradation could be seen in all samples released in either buffer solution (Fig. 4e). Degradation of scaffolds with polymer and drug/polymer loading was comparable prior and following buffer release indicating drug loading did not worsen surface degradation (Fig. 4b and e). Scaffolds released in ABS showed heavy microstructural degradation compared to scaffolds released in PBS, as expected due to the higher acidity rapidly breaking down the scaffold matrix.
4. Conclusion
Vitamin D3 in the form of cholecalciferol showed reduced osteoclastic activity alongside no cytotoxicity effects towards osteoblast cells. Little to no resorption pits were identified with vitamin D3/PCL/PEG loaded HA samples compared to control and polymer loaded indicating a reduction in osteoclast activity. Scanning electron microscopy images showed more osteoblast cell morphology on samples with vitamin D3 compared to control TCP and TCP with PCL/PEG as well as no cytotoxicity assessed through MTT assay. A controlled release of vitamin D3 from additively manufactured porous TCP scaffolds with interconnected porosity was effectively achieved using a mixed polymer system of PCL and PEG. These results indicate vitamin D3 can be successfully released from TCP porous scaffolds to provide an effective, local drug delivery system for bone tissue engineering low load bearing applications and provide aid for bone defects or permeate voids left by tumor resection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors would like to acknowledge financial support from the National Institutes of Health under the grant number R01 AR066361. The authors would also like to thank Naboneeta Sarkar and Dishary Banerjee for their experimental help with this work. Additionally, thank you to the Franceschi Microscopy & Imaging Center at Washington State University. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.Notes and references
- O. Johnell and J. A. Kanis, Osteoporosis Int., 2006, 17, 1726 CrossRef CAS.
- J. A. Kanis, Osteoporosis Int., 1994, 4, 368 CrossRef CAS.
- R. P. Heaney, K. M. Davies, T. C. Chen, M. F. Holick and M. J. Barger-Lux, Am. J. Clin. Nutr., 2003, 77, 204 CrossRef CAS.
- A. M. Parfitt, Metabolic bone disease and clinically related disorders, 1990, p. 329 Search PubMed.
- D. P. Trivedi, R. Doll and K. T. Khaw, Br. Med. J., 2003, 326, 469 CrossRef CAS.
- T. Suda, F. Takahashi and N. Takahashi, Arch. Biochem. Biophys., 2012, 523, 22 CrossRef CAS.
- H. Wen, Y. Liu, J. Li, D. Wei, D. Liu and F. Zhao, Clin. Exp. Rheumatol., 2018, 36, 798 Search PubMed.
- Y. Hayashi, T. Fujita and T. Inoue, J. Bone Miner. Metab., 1992, 10, 184 CrossRef.
- H. Orimo, M. Shiraki, Y. Hayashi, T. Hoshino, T. Onaya, S. Miyazaki, H. Kurosawa, T. Nakamura and N. Ogawa, Calcif. Tissue Int., 1994, 54, 370 CrossRef CAS.
- M. W. Tilyard, G. F. Spears, J. Thomson and S. Dovey, N. Engl. J. Med., 1992, 326, 357 CrossRef CAS.
- K. Muller and K. Bendtzen, J. Invest. Dermatol., 1996, 1, 68 CAS.
- L. Piemonti, P. Monti, M. Sironi, P. Fraticelli, B. E. Leone, E. D. Cin, P. Allavena and V. D. Carlo, J. Immunol., 2000, 164, 4443 CrossRef CAS.
- R. Lin, Y. Nagai, R. Sladek, Y. Bastien, J. Ho, K. Petrecca, G. Sotiropoulou, E. P. Diamandis, T. J. Hudson and J. H. White, Mol. Endocrinol., 2002, 16, 1243 CrossRef CAS.
- T. Shoji, K. Shinohara, E. Kimoto, M. Emoto, H. Tahara, H. Koyama, M. Inaba, S. Fukumoto, E. Ishimura, T. M. T. Tabata and Y. Nishizawa, Nephrol., Dial., Transplant., 2004, 19, 179 CrossRef CAS.
- R. J. Skowronski, D. M. Peehl and D. Feldman, Endocrinology, 1995, 136, 20 CrossRef CAS.
- M. Hao, S. Hou, L. Xue, H. Yuan, L. Zhu, C. Wang, B. Wang, C. Tang and C. Zhang, J. Med. Chem., 2018, 61, 3059 CrossRef CAS.
- N. Ignjatović, V. Uskoković, Z. Ajduković and D. Uskoković, Mater. Sci. Eng. Carbon, 2013, 33, 943 CrossRef.
- Z. Teng, Y. Luo and Q. Wang, Food Chem., 2013, 141, 524 CrossRef CAS.
- T. Matsumoto, M. Ito, Y. Hayashi, T. Hirota, Y. Tanigawara, T. Sone, M. Fukunaga, M. Shiraki and T. Nakamura, Bone, 2011, 49, 605 CrossRef CAS.
- U. Lange, J. Teichmann, J. Strunk, U. Muller-Ladner and K. L. Schmidt, Osteoporosis Int., 2005, 16, 1999 CrossRef CAS.
- A. A. Vu and S. Bose, Ann. Biomed. Eng., 2019, 1, 1–9 Search PubMed.
- T. Hsu and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 15816 CrossRef CAS.
- F. M. Veronese and G. Pasut, Drug Discovery Today, 2005, 10, 1451 CrossRef CAS.
- W. Xue, A. Bandyopadhyay and S. Bose, J. Biomed. Mater. Res., Part B, 2009, 91, 831 CrossRef.
- S. Tarafder, K. Nansen and S. Bose, Mater. Sci. Eng. Carbon, 2013, 33, 3121 CrossRef CAS.
- S. Bose, N. Sarkar and D. Banerjee, Mater. Today Chem., 2018, 8, 110 CrossRef CAS.
- D. Ke, W. Dernell, A. Bandyopadhyay and S. Bose, J. Biomed. Mater. Res., Part B, 2015, 103, 1549 CrossRef CAS.
- H. Roussiere, F. Fayon, B. Alonso, T. Rouillon, V. Schnitzler, E. Verron, J. Guicheux, M. Petit, D. Massiot, P. Janvier, J. Bouler and B. Bujoli, Chem. Mater., 2008, 20, 182 CrossRef CAS.
- S. Bose, D. Banerjee, S. Robertson and S. Vahabzadeh, Ann. Biomed. Eng., 2018, 46, 1241 CrossRef PubMed.
- International Organization for Standardization, Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity, 2009, ISO No. 10993 Search PubMed.
- C. E. Misch, Z. Qu and M. W. Bidez, J. Oral Maxillofac. Surg., 1999, 57, 700 CrossRef CAS.
- D. Shiino, Y. Murata, A. Kubo, Y. J. Kim, K. Kataoka, Y. Koyama, A. Kikuchi, M. Yokoyama, Y. Sakurai and T. Okano, J. Controlled Release, 1995, 37, 269 CrossRef CAS.
- T. Yoneda, K. Hata, M. Nakanishi, M. Nagae, T. Nagayama, H. Wakabayashi, T. Nishisho, T. Sakurai and T. Hiraga, Bone, 2011, 48, 100 CrossRef CAS.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288 CrossRef CAS.
- S. Salmaso and P. Caliceti, J. Drug Delivery, 2013, 2013 Search PubMed.
- X. Huang and C. S. Brazel, J. Controlled Release, 2001, 73, 121 CrossRef CAS.
- M. L. Hans and A. M. Lowman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 319 CrossRef CAS.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993 CrossRef CAS.
- S. Bose, A. A. Vu, K. Emshadi and A. Bandyopadhyay, Mater. Sci. Eng. Carbon, 2018, 88, 166 CrossRef CAS.
- S. Vahabzadeh, J. Edgington and S. Bose, Mater. Sci. Eng. Carbon, 2013, 33, 3576 CrossRef CAS.
- A. G. A. Coombes, S. C. Rizzi, M. Williamson, J. E. Barralet, S. Downes and W. A. Wallace, Biomaterials, 2004, 25, 315 CrossRef CAS.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1 CrossRef CAS.
Footnote |
| † Vitamin D3 (cholecalciferol) was obtained from Alfa Aesar, Ward Hill, MA. |
| This journal is © The Royal Society of Chemistry 2019 |