Open Access Article
Open Access ArticleIn-chain functionalized syndiotactic 1,2-polybutadiene by a Ziegler–Natta iron(III) catalytic system†
Shanshan Liang ab,
Huaqiang Zhangc,
Rixin Congc,
Heng Liub,
Feng Wang*bd,
Yanming Hub and
Xuequan Zhang*b
ab,
Huaqiang Zhangc,
Rixin Congc,
Heng Liub,
Feng Wang*bd,
Yanming Hub and
Xuequan Zhang*b
aCollege of Material Science and Engineering, Shenyang University of Chemical Technology, Shenyang 110142, China
bCAS Key Laboratory of High-Performance Synthetic Rubber and Its Composite Materials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun 130022, P. R. China. E-mail: fengwang@ciac.ac.cn; xqzhang@ciac.ac.cn
cLanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, Lanzhou, 730060, China
dSchool of Chemical Engineering, Changchun University of Technology, Changchun, Jilin, China
First published on 17th October 2019
Abstract
Copolymerization of 1,3-butadiene with four 1-substituted 1,3-diene comonomers bearing amino and alkyoxy groups by a Ziegler–Natta iron(III) catalytic system to access in-chain functionalized syndiotactic 1,2-polybutadiene is reported herein. The polar comonomer content can be easily regulated by varying the comonomer loadings or polymerization conditions, affording functionalized syndiotactic 1,2-polybutadiene with different amounts of functionalities. The incorporation of a polar comonomer showed little influence on the 1,2-content and stereoregularity of the resulting polymers, giving a 1,2-structure as high as ∼85% and an rrrr pentad of 81.0%. Significantly improved surface properties of the polymers was obtained after incorporation of polar comonomer, as revealed from the remarkably decreased water contact angles.
Introduction
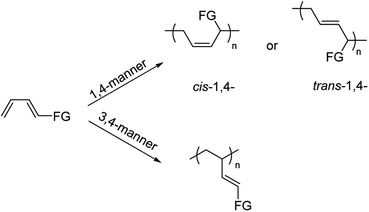
Syndiotactic 1,2-polybutadiene (sPB), a promising crystalline material that bears alternately located vinyl groups on opposite positions along the polymer main chain,1 was firstly synthesized by Natta et al.,2 and later successfully industrialized by the Japan Synthetic Rubber Co. Ltd. (JSR) by using a Co/AlR3/CS2 catalytic system.3–7 Due to its unique mechanical properties that originate from the combination of properties of plastic and rubber, sPB reveals a wide range of applications in the fields of fibres, films, moulded articles, and recently has been used as synthetic rubber reinforcing fillers.8–12 Despite this, the inherent shortfall of low surface energy makes sPB display inferior properties with respect to adhesion, toughness, paintability, miscibility and rheological properties. Improving the hydrophilicity and developing functionalized sPB has been the long-term pursuit for scientists.Similar to achieving other functionalized polyolefin materials,13–15 two strategies are generally available for accessing functionalized sPB, i.e. post functionalization of polymer products and in situ functionalization by statistical copolymerization with polar monomers. In the past few years, colossal advances have been made for the former strategy, due to the presence of highly active pendent vinyl substituents.16–22 For instance, Mecking, Ndoni, et al., presented efficient functionalization of sPB via thiol–ene reaction, through which various types of functional groups, including ester, L-cysteine, carboxylic acid were successfully introduced;23–25 Linford and Tang reported hydrosilation and hydrozirconation of 1,2-polybutadiene (1,2-PB) by Si–H and Zr–H reagents, and provided a platform for accessing diversified functional 1,2-PBs.26,27 Despite of the facility and efficiency in these reports, all of them were built on the sacrifice of pendent double bonds, which might cause irreversible damage to the inherent nature of sPB, such as crystallinity,28 molecular weights,16 and even causing cross-linking.28 On the other hand, incorporation of functional groups through coordination copolymerization strategy not only has a good control on the regio- and/or stereo-regularities, but also is able to regulate the comonomer sequence and content conveniently by varying the feed ratio. Nevertheless, up to date, no reports on this strategy were involved, perhaps due to the highly electrophilic nature of the active species, which are likely to be deactivated in the presence of heteroatom-containing polar comonomers. Moreover, directly coordination copolymerization of 1,3-butadiene with traditional polar monomers, such as methyl methacrylate, vinyl acetate, etc., is strikingly difficult due to the different mechanisms presented in diene and monoene polymerization,29 alternative comonomers can be inspired from Cui's and Mecking's recent reports, in which the preparation of in-chain functionalized cis-1,4 polydienes by using a type of new comonomers (Scheme 1).30–34 Alternatively, this unique comonomer, i.e. functional-group-substituted 1,3-butadiene, can also be polymerized in a 3,4-manner through a η3-coordinated π-allylic unit,35 and therefore serves the best candidate for accessing functionalized sPB.
 | ||
| Scheme 1 Polymerization of functional-group-substituted 1,3-butadiene (1-substiuted 1,3-diene was taken as an example). | ||
We have been interested in tapping effective Ziegler–Natta iron(III) catalytic systems to syndiotactically polymerize 1,3-butadiene,36–39 to circumvent the environmental toxicity and bad-smelling issues that encountered in the industrialized Co(II)-based systems. In the present study, we disclose the first example of functionalized sPB prepared through copolymerization strategy, detailed catalytic behaviours of the iron(III) catalytic system together with various functionalized sPB with different types and amounts of functionalities were systematically evaluated.
Experimental section
General considerations
Iron(III) acetylacetonate (Fe(acac)3) and diethyl phosphite (DEP) were purchased from J&K (Beijing, China) and diluted to 0.06 mol L−1 solution in toluene. Al(iBu)3 (1.0 mol L−1 in toluene) was a commercial product of Akzo Nobel and used as received. 1,3-Butadiene was supplied by Jinzhou Petrochemical Corporation in China and purified by passing through four columns packed with activated molecular sieves (4 Å) and KOH prior to use. Toluene and THF were refluxed over sodium/diphenylketyl under nitrogen and then distilled before use. All other commercial chemicals were used as received.1H NMR spectra were recorded on a Varian Unity-400 MHz spectrometer at 125 °C in C6D4Cl2. IR spectra was recorded on BRUKE Vertex-70 FIR spectrophotometer. The molecular weights and molecular weight distributions of the polymers were estimated by gel permeation chromatography (GPC) on a PL-GPC 220 high-temperature chromatograph at 150 °C. 1,2,4-Trichlorobenzene containing 0.05% (w/v) BHT was used as eluent at a flow rate of 1.0 mL min−1 and the values of Mn and Mw/Mn were calculated by using polystyrene calibration. The melting points were estimated on a Q100 DSC (TA Instruments) under nitrogen atmosphere. The samples were heated and cooled down at a rate of 10 °C min−1.
Synthesis and characterization of polar 1-substituted 1,3-dienes
All reactions were carried out under a nitrogen atmosphere in flame-dried glassware with magnetic stirring. A general procedure was as follows: allyltriphenylphosphonium bromide (20 g, 52.18 mmol) was reacted with potassium t-butoxide (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, dissolved in THF) in an ice bath for one hour, then 1.0 equivalent of the benzaldehyde derivative (52.18 mmol) was slowly added over 10 minutes, and the reaction was continued for 4 hours in room temperature. The reaction was quenched by addition of 150 mL of saturated aqueous ammonium chloride and then extracted with 100 mL ethyl acetate for 4 times to get crude products. The crude products were purified by silica gel chromatography (eluent: petroleum ether (or hexane)
1.2, dissolved in THF) in an ice bath for one hour, then 1.0 equivalent of the benzaldehyde derivative (52.18 mmol) was slowly added over 10 minutes, and the reaction was continued for 4 hours in room temperature. The reaction was quenched by addition of 150 mL of saturated aqueous ammonium chloride and then extracted with 100 mL ethyl acetate for 4 times to get crude products. The crude products were purified by silica gel chromatography (eluent: petroleum ether (or hexane)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 50
ethyl acetate = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the target product.
1) to give the target product.
1-(4-Methoxyl)phenyl-1,3-butadiene (a)
Yield: 7.79 g, 65%. Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E = 60
E = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.36 (d, 2H, Hph); 6.89 (d, 2H, Hph); 6.68 (m, 1H, –CH); 6.55–6.45 (m, 2H, –CH); 5.35 (d, 1H, –CH2); 5.20 (d, 1H, –CH2); 3.80 (s, 3H, –CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.29 (d, 2H, Hph); 6.92 (m, 1H, –CH); 6.89 (d, 2H, Hph); 6.41 (m, 1H, –CH); 6.20 (t, 1H, –CH); 5.29 (d, 1H, –CH2); 5.17 (d, 1H, –CH2); 3.82 (s, 3H, –CH3). 13C NMR (400 MHz, CDCl3, ppm): 159.38; 158.78; 137.48; 133.44; 132.51; 131.42; 130.33; 130.03; 129.41; 127.70; 118.97; 116.43; 114.10; 113.74; 55.23. EI-MS for C11H12O (m/z): 161.0 (M + H+).
40. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.36 (d, 2H, Hph); 6.89 (d, 2H, Hph); 6.68 (m, 1H, –CH); 6.55–6.45 (m, 2H, –CH); 5.35 (d, 1H, –CH2); 5.20 (d, 1H, –CH2); 3.80 (s, 3H, –CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.29 (d, 2H, Hph); 6.92 (m, 1H, –CH); 6.89 (d, 2H, Hph); 6.41 (m, 1H, –CH); 6.20 (t, 1H, –CH); 5.29 (d, 1H, –CH2); 5.17 (d, 1H, –CH2); 3.82 (s, 3H, –CH3). 13C NMR (400 MHz, CDCl3, ppm): 159.38; 158.78; 137.48; 133.44; 132.51; 131.42; 130.33; 130.03; 129.41; 127.70; 118.97; 116.43; 114.10; 113.74; 55.23. EI-MS for C11H12O (m/z): 161.0 (M + H+).
1-(4-N,N-Dimethylamino)phenyl-1,3-butadiene (b)
Yield: 7.93 g, 68%. Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E = 57
E = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.32 (d, 2H, Hph); 7.00 (m, 1H, –CH); 6.73–6.60 (m, 2H, Hph); 6.54–6.45 (m, 2H, –CH); 5.34 (d, 1H, –CH2); 5.18 (d, 1H, –CH2); 2.97 (s, 6H, –N(CH3)2); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.27 (d, 2H, Hph); 6.65 (m, 1H, –CH); 6.73–6.60 (m, 2H, Hph); 6.38 (m, 1H, –CH); 6.13 (t, 1H, –CH); 5.25 (d, 1H, –CH2); 5.06 (d, 1H, –CH2); 2.97 (s, 6H, –N(CH3)2). 13C NMR (400 MHz, CDCl3, ppm): 149.97; 149.45; 137.78; 133.79; 130.54; 130.03; 127.64; 127.44; 125.74; 125.48; 117.77; 114.87; 112.29; 111.99; 40.30. EI-MS for C12H15N (m/z): 174.1 (M + H+).
43. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.32 (d, 2H, Hph); 7.00 (m, 1H, –CH); 6.73–6.60 (m, 2H, Hph); 6.54–6.45 (m, 2H, –CH); 5.34 (d, 1H, –CH2); 5.18 (d, 1H, –CH2); 2.97 (s, 6H, –N(CH3)2); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.27 (d, 2H, Hph); 6.65 (m, 1H, –CH); 6.73–6.60 (m, 2H, Hph); 6.38 (m, 1H, –CH); 6.13 (t, 1H, –CH); 5.25 (d, 1H, –CH2); 5.06 (d, 1H, –CH2); 2.97 (s, 6H, –N(CH3)2). 13C NMR (400 MHz, CDCl3, ppm): 149.97; 149.45; 137.78; 133.79; 130.54; 130.03; 127.64; 127.44; 125.74; 125.48; 117.77; 114.87; 112.29; 111.99; 40.30. EI-MS for C12H15N (m/z): 174.1 (M + H+).
1-(4-Propoxyl)phenyl-1,3-butadiene (c)
Yield: 4.47 g, 46%. Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E = 83
E = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.34 (d, 2H, Hph); 6.89 (d, 2H, Hph); 6.68 (m, 1H, –CH); 6.53 (t, 1H, –CH); 6.49 (t, 1H, –CH); 5.37 (d, 1H, –CH2); 5.21 (d, 1H, –CH2); 3.92 (s, 2H, –O–CH2–); 1.75 (m, 2H, –CH2–); 1.05 (t, 3H, –CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.29 (d, 2H, Hph); 6.92 (m, 1H, –CH); 6.89 (d, 2H, Hph); 6.41 (d, 1H, –CH); 6.21 (t, 1H, –CH); 5.30 (d, 1H, –CH2); 5.12 (d, 1H, –CH2); 3.95 (s, 2H, –O–CH2–); 1.75 (m, 2H, –CH2–); 1.05 (t, 3H, –CH3). 13C NMR (400 MHz, CDCl3, ppm): 158.76; 158.15; 137.29; 133.23; 132.35; 130.11; 129.90; 129.69; 129.07; 127.46; 127.36; 118.67; 116.11; 114.48; 114.12; 69.17; 22.47; 10.40. EI-MS for C13H16O (m/z): 188.1 (M+).
17. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.34 (d, 2H, Hph); 6.89 (d, 2H, Hph); 6.68 (m, 1H, –CH); 6.53 (t, 1H, –CH); 6.49 (t, 1H, –CH); 5.37 (d, 1H, –CH2); 5.21 (d, 1H, –CH2); 3.92 (s, 2H, –O–CH2–); 1.75 (m, 2H, –CH2–); 1.05 (t, 3H, –CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.29 (d, 2H, Hph); 6.92 (m, 1H, –CH); 6.89 (d, 2H, Hph); 6.41 (d, 1H, –CH); 6.21 (t, 1H, –CH); 5.30 (d, 1H, –CH2); 5.12 (d, 1H, –CH2); 3.95 (s, 2H, –O–CH2–); 1.75 (m, 2H, –CH2–); 1.05 (t, 3H, –CH3). 13C NMR (400 MHz, CDCl3, ppm): 158.76; 158.15; 137.29; 133.23; 132.35; 130.11; 129.90; 129.69; 129.07; 127.46; 127.36; 118.67; 116.11; 114.48; 114.12; 69.17; 22.47; 10.40. EI-MS for C13H16O (m/z): 188.1 (M+).
1-(2-Methoxyl)phenyl-1,3-butadiene (d)
Yield: 3.68 g, 44%. Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E = 80
E = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.49 (d, 1H, –CH); 7.33–6.74 (m, 4H, Hph); 6.61–6.29 (m, 2H, –CH); 5.38–5.29 (m, 1H, –CH2); 5.19–5.13 (m, 1H, –CH2); 3.86 (d, 3H, –O–CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 6.95 (d, 1H, –CH); 7.33–6.74 (m, 4H, Hph); 6.61–6.29 (m, 2H, –CH); 5.38–5.29 (m, 1H, –CH2); 5.19–5.13 (m, 1H, –CH2); 3.84 (d, 3H, –O–CH3). 13C NMR (400 MHz, CDCl3, ppm): 156.97; 156.68; 137.84; 133.44; 130.48; 130.03; 128.47; 127.55; 126.36; 126.10; 120.57; 119.89; 118.76; 116.79; 110.75; 110.28; 55.25. EI-MS for C11H12O (m/z): 161.0 (M + H+).
20. For E isomer: 1H NMR (400 MHz, CDCl3, ppm): 7.49 (d, 1H, –CH); 7.33–6.74 (m, 4H, Hph); 6.61–6.29 (m, 2H, –CH); 5.38–5.29 (m, 1H, –CH2); 5.19–5.13 (m, 1H, –CH2); 3.86 (d, 3H, –O–CH3); for Z isomer: 1H NMR (400 MHz, CDCl3, ppm): 6.95 (d, 1H, –CH); 7.33–6.74 (m, 4H, Hph); 6.61–6.29 (m, 2H, –CH); 5.38–5.29 (m, 1H, –CH2); 5.19–5.13 (m, 1H, –CH2); 3.84 (d, 3H, –O–CH3). 13C NMR (400 MHz, CDCl3, ppm): 156.97; 156.68; 137.84; 133.44; 130.48; 130.03; 128.47; 127.55; 126.36; 126.10; 120.57; 119.89; 118.76; 116.79; 110.75; 110.28; 55.25. EI-MS for C11H12O (m/z): 161.0 (M + H+).
Copolymerization procedure of 1,3-butadiene and 1-substituted 1,3-dienes
All the manipulations were performed under an atmosphere of dry nitrogen. 1,3-Butadiene in toluene (1.85 mol L−1) and quantitative 1-substituted 1,3-dienes were placed in an oxygen- and moisture-free ampule capped with a rubber septum. Then, Fe(acac)3, DEP, and Al(iBu)3 at designed ratios were injected sequentially. The polymerization was kept at 50 °C for 4 h, then terminated by adding 2.0 mL of acidified ethanol containing 1.0 wt% 2,6-di-tert-butyl-4-methylphenol (BHT) as stabilizer. The resulting products were repeatedly washed with ethanol and dried under vacuum at 40 °C to constant weight.Results and discussion
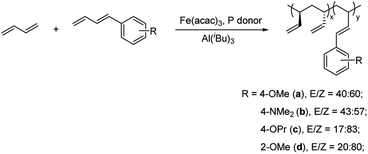
The 1-substituted 1,3-diene comonomers bearing different types of functionalities, including 1-(4-methoxyl)phenyl-1,3-butadiene (a), 1-(4-N,N-dimethylamino)phenyl-1,3-butadiene (b), 1-(4-propoxyl)phenyl-1,3-butadiene (c), 1-(2-methoxyl)phenyl-1,3-butadiene (d), were prepared by Wittig reaction between allyltriphenylphosphonium bromide and substituted benzaldehydes (Scheme 2). Further purification by flash column chromatography afforded the targeted compounds a–d in high yields (for detailed procedures and NMR spectra cf. Experimental section and ESI†). As evidenced from 1H NMR spectra, two isomers were obtained for each of the comonomer, with E/Z ratios of 40/60, 43/57, 17/83, and 20/80 for a–d, respectively.Copolymerizations of 1,3-butadiene with comonomers a–d were carried out by using syndiotactically 1,2-selective catalytic system, Fe(acac)3/Al(iBu)3/phosphorous compound. To optimize the polymerization conditions, the influences of the types and/or amounts of phosphorous donors and cocatalysts were studied with 1-(4-N,N-dimethylamino)phenyl-1,3-butadiene (b) as a comonomer. As the results shown in Table 1, in the presence of 10 mol% polar comonomer b, the polymerization proceeded smoothly, affording copolymers from mediate to high yields, and the catalytic activity and the properties of the resulting polymers were found to be highly dependent on the cocatalysts and phosphorous donors. When the polymerizations were undertaken at smaller cocatalyst/Fe ratios (Al/Fe < 15), only a small amount of copolymers were isolated, perhaps due to the insufficiency of cocatalyst to activate the active species or due to the poison of catalytic active species by dimethylamino functionality in comonomer b. These speculations can also be shed light from increment of comonomer contents in the resultant copolymers with an increasing Al/Fe ratio, in which Lewis acidic aluminum cocatalyst would bond with dimethylamino group and thus hinder it from decomposing iron(III) active centres. Moreover, polymerization at Al/Fe ratio lower than 15 gave amorphous rubber-like 1,2-PB, whereas crystalline syndiotactic sPB was obtained when higher Al/Fe is employed. This result agrees with our previous research on Fe(III)-mediated 1,3-butadiene homopolymerization.40
| Run | Pb | P/Fe | Al/Fe | Yield (%) | Mn × 10−3c | PDIc | Comon. content in polymerd (%) | Microstructure of PBd | Xce (%) | Tmf (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,2- | Trans-1,4- | ||||||||||
| a Polymerization conditions: solvent, toluene; polymerization time, 4 h; T: 50 °C; 1,3-butadiene, 1.85 mol L−1; [M]/[Fe] = 1000; comonomer b, 1.665 mmol (Bd/b = 9/1).b DEP: diethyl phosphite; DBP: dibutyl phosphite; TBP: tributyl phosphite; TPP: triphenyl phosphite.c Determined by GPC in trichlorobenzene vs. polystyrene standards.d Determined by 1H NMR analysis.36e Estimated by the formula of ΔH/ΔH0, ΔH was calculated by DSC and ΔH0 referred to standard enthalpy of 1,2-polybutadiene with 100% crystallinity, equal to 60.7 J g−1.f Determined by DSC at heat rate of 10 °C min−1. | |||||||||||
| 1 | DEP | 3 | 5 | <5% | — | — | — | — | — | — | — |
| 2 | DEP | 3 | 10 | 22 | 9.3 | 2.32 | 3.14 | 64.94 | 35.06 | — | — |
| 3 | DEP | 3 | 15 | 14 | 6.4 | 2.67 | 4.00 | 59.52 | 40.48 | — | — |
| 4 | DEP | 3 | 30 | 54 | 40.2 | 3.19 | 4.51 | 75.19 | 24.81 | 14.74 | 157 |
| 5 | DEP | 1 | 30 | 65 | 32.5 | 2.29 | 2.89 | 81.30 | 18.7 | 23.71 | 157 |
| 6 | DEP | 5 | 30 | 90 | 4.1 | 2.90 | 6.69 | 53.76 | 46.24 | — | — |
| 7 | DEP | 10 | 30 | <5% | 6.4 | 2.45 | 6.32 | 61.35 | 38.65 | — | — |
| 8 | DBP | 3 | 30 | 78 | 50.5 | 1.96 | 2.48 | 84.75 | 15.25 | 16.19 | 158 |
| 9 | TBP | 3 | 30 | <5% | 30.3 | 2.37 | 1.32 | 33.33 | 66.67 | — | — |
| 10 | TPP | 3 | 30 | 28 | 70.2 | 2.06 | 1.02 | 77.52 | 22.48 | 12.66 | 167 |
External phosphorous donors also significantly influenced the copolymerization performances. Increasing the P/Fe ratio from 1 to 10 first led to the formation of sPB and then produced amorphous 1,2-PB rubber, this is consistent with our previous conclusion that higher P/Fe molar ratio favours the formation of amorphous 1,2-polybutadiene.41 Although the best catalytic activity was observed when P/Fe = 5 in the present study, sPB can only be formed when P/Fe = 1 or 3, therefore, P/Fe = 3 was applied in the following study. Moreover, it was observed that the molecular weights of the resultant amorphous 1,2-PBs were much lower than that of crystalline sPB, which might be due to the over-coordination of DEP slowed down coordination of 1,3-butadiene monomer to the vacant site of active centre and thus reduced chain propagation rate. Different types of phosphorous donors were also investigated, and dibutyl phosphite (DBP) was found to give the highest polymer yield (78%), and simultaneously affording sPB with high 1,2-contents. Whereas tributyl phosphite (TBP) and triphenyl phosphite (TPP)-based systems showed much lower catalytic activities under identical conditions; the polymer yields were <5% and 28%, respectively.
Copolymerizations were then expanded to other 1-substituted 1,3-dienes to evaluate the influence of different functionalities on copolymerization behaviour. As the results summarized in Table 2, the presence of a small amount of polar comonomer could deactivate the active species obviously, in spite of the relative lower electrophilicity of late-transition metal iron(III) center, when comparing to early-transition or lanthanide metal counterparts. For instance, when 5% of comonomers a–d were added into the system, the isolated copolymer yields dropped significantly from 93% for 1,3-butadiene homopolymerization to 83%, 66%, 77%, and 79% for copolymerizations with a, b, c, and d, respectively. Further increasing the comonomer/Bd ratio led to gradual reduction in the polymer yields. For instance, the presence of 30% comonomer a gave the polymer yield almost half of that in the 1,3-butadiene homopolymerization (46.5% vs. 92.9%); and the presence of 20% b poisoned the active species significantly, and only oligomers, rather than copolymers, were produced eventually. The decrement of polymer yield in the copolymerizations can be explained by the formation of relatively stable intermediates through coordination of heteroatoms to iron(III) center, which retards subsequent insertion reaction. In spite of these results, to our knowledge, the synthesis of polar group-functionalized sPB via direct polymerization approach has not been reported thus far, this is the first example in this field. Regarding the effect of functionality on copolymerization performances, an order of a > c ∼ d > b was revealed from the catalytic activities. The most unreactive comonomer b was ascribed to the strongest electro-donating ability of dimethylamino group, which displayed high coordination capability with the electrophilic active species and/or the alkyl aluminum cocatalyst. Similar reasons can also be explainable for the inferior reactivity of monomers c, when comparing with a, the presence of long alkyl group generally imparts them more pronounced electron-donating ability. Nevertheless, the relative smaller reactivity of d was ascribed to steric effect, i.e., 2-substituted phenyl groups brought in a sterically hindered space, which thus prohibited itself being coordinated to the metal center.
| Run | Comon. | Comon./Bdb | Yield (%) | Mnc × 10−3 | PDIc | Comon. content in polymerd (%) | Microstructure of PBd | Xce (%) | Tmf (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1,2- | Trans-1,4- | |||||||||
| a Polymerization conditions: solvent, toluene; polymerization time, 4 h; T: 50 °C; 1,3-butadiene, 1.85 mol L−1; [M]/[Fe]/[Al]/[P] = 1000/1/30/3.b Molar ratio.c Determined by GPC in trichlorobenzene vs. polystyrene standards.d Determined by 1H NMR analysis.36e Estimated by the formula of ΔH/ΔH0, ΔH was calculated by DSC and ΔH0 referred to standard enthalpy of 1,2-polybutadiene with 100% crystallinity, equal to 60.7 J g−1.f Determined by DSC at heat rate of 10 °C min−1. | ||||||||||
| 11 | — | 0/100 | 93 | 22.8 | 2.22 | 0 | 85.47 | 14.53 | 42.75 | 165 |
| 12 | a | 2/98 | 90 | 37.3 | 1.79 | 1.63 | 84.71 | 15.29 | 35.25 | 167 |
| 13 | a | 5/95 | 83 | 38.1 | 3.93 | 3.63 | 80.65 | 19.35 | 28.19 | 157 |
| 14 | a | 10/90 | 75 | 41.0 | 3.69 | 7.40 | 80.00 | 20.00 | 12.14 | 154 |
| 15 | a | 20/80 | 61 | 45.0 | 2.97 | 8.88 | 74.92 | 25.08 | 9.41 | 147 |
| 16 | a | 30/70 | 47 | 62.7 | 2.26 | 17.18 | 72.46 | 27.54 | 6.40 | 140 |
| 17 | b | 2/98 | 82 | 75.6 | 1.78 | 0.83 | 84.03 | 15.97 | 30.97 | 158 |
| 18 | b | 5/95 | 66 | 55.2 | 5.62 | 1.84 | 80.65 | 19.35 | 24.18 | 158 |
| 19 | b | 10/90 | 54 | 40.2 | 3.19 | 4.51 | 75.19 | 24.81 | 14.74 | 157 |
| 20 | b | 20/80 | Oligomer | — | — | — | — | — | — | — |
| 21 | c | 5/95 | 77 | 30.6 | 2.26 | 1.59 | 80.65 | 19.35 | 31.83 | 163 |
| 22 | c | 10/90 | 66 | 72.2 | 2.57 | 3.03 | 78.13 | 21.87 | 30.61 | 161 |
| 23 | c | 20/80 | 47 | 67.1 | 2.41 | 5.71 | 75.76 | 24.24 | 21.37 | 159 |
| 24 | d | 5/95 | 79 | 95.6 | 2.49 | 2.12 | 81.30 | 18.70 | 30.64 | 163 |
| 25 | d | 10/90 | 66 | 176.4 | 2.08 | 4.00 | 78.74 | 21.26 | 23.94 | 156 |
| 26 | d | 20/80 | 48 | 102.9 | 2.16 | 6.29 | 77.52 | 22.48 | 13.99 | 152 |
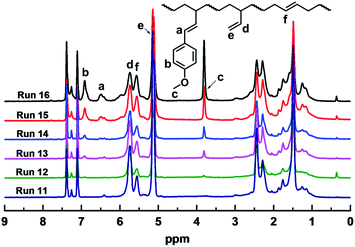
Incorporation of polar 1-substituted 1,3-diene comonomer was clearly observed for all the copolymerization studies. When comonomer a/Bd = 2/98 in the feed ratio, the resulting copolymer contained ca. 1.63% of comonomers. The increase in the comonomer feed resulted in the increment of comonomer content in the resulting polymer. Fig. 1 illustrated the 1H NMR spectra of the resultant copolymers, in which the resonance peaks at 3.81 ppm assignable to the methoxyl group gradually increased if more a was incorporated into the copolymer. Moreover, the resonance peaks of phenyl protons at 6.92 ppm, can also be clearly observed. For other comonomers b–d, a monotonous increasing of incorporation content could also be witnessed if more comonomer feed ratio was applied.
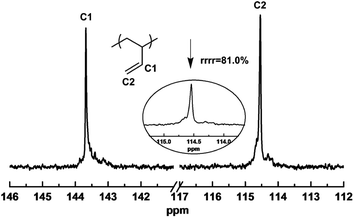
Increasing the amount of comonomer a from 2% to 30% led to slight decrement of 1,2-content in the resultant copolymers from 84.71% to 72.46%, while the rest microstructure was kept as trans-1,4- moiety. Similar results were also observed for other comonomer b, c, and d. While the increase in the comonomer feed led to a decrement in the catalytic activity (vide supra) and 1,2 selectivity, the catalytic system still exhibited syndio 1,2-selectivity, affording in-chain functionalized sPBs. As the tendency revealed from Fig. 1, the resonance peaks, that assigned to the vinyl protons, at 5.75, 5.14 ppm revealed a clear decrement. Similar conclusion can also be made for other comonomers, as revealed from Fig. S9–S11.† It is of note that, the slight decrease of 1,2-structure didn't affect the stereo-regularity of the resultant copolymers. As the representative 13C NMR shown in Fig. 2, when 10% comonomer b was incorporated into the polymer chain, the syndiotactic rrrr pentad was maintained as high as 81.0%, which is about same with that of sPB homopolymers,35 implying that the presence of polar comonomer displayed subtle influence on the stereoselectivity of the iron(III) catalytic active species.
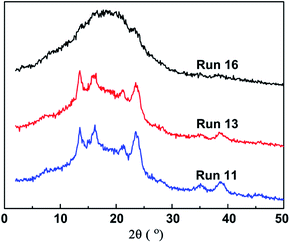
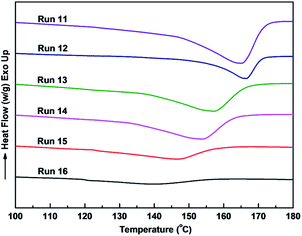
The incorporation of polar 1-substituted 1,3-diene comonomer into sPB resulted in, as expected, a decrease in the crystallinity degree Xc and melting temperature Tm of the resulting copolymers. Fig. 3 shows the WXRD spectra of the resultant copolymer, the diffraction peaks at 2θ = 13.50, 16.17, 21.22, and 23.50° ascribed to 1,2-syndiotactic PB,42 clearly weakened with an increasing incorporation amount of comonomer a. Fig. 4 illustrates the DSC analysis curves, in which a gradual decrease of Tm and gradual broadening of the melting transition were observed when more comonomer was in cooperated.
In order to clearly elucidate of the improved surface property upon incorporation of polar monomer, static water contact angles of the resultant copolymers was tested. The results demonstrated that the static water contact angle of the copolymers decreased from 115.1° (run 11) to 104.2° (run 14) with an addition of 10 mol% a. Analogous results were observed for other comonomer b–d, implying the significant increased surface energy therein (Fig. 5).
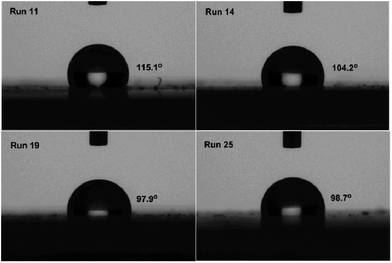 | ||
| Fig. 5 Static water contact angle measurements for the resultant copolymer products (runs 11, 14, 19, 25). | ||
Conclusions
In-chain functionalized syndiotactic 1,2-polybutadiene via copolymerization strategy is disclosed in this research. Through changing copolymerization conditions, the Ziegler–Natta catalytic system, Fe(acac)3/Al(iBu)3/P, was able to efficiently copolymerize 1,3-butadiene with 1-substituted 1,3-dienes containing amino and alkoxy groups to produce functionalized syndiotactic 1,2-polybutadiene. Copolymerization conditions, including phosphorous donors, cocatalyst, etc., imposed significant influence on the copolymerization activities, as well as the properties of resulting functionalized copolymers. Although slight decreased 1,2-contents was revealed when increasing the comonomer content, but the resultant copolymers still exhibited syndiotactic structure. The incorporation of polar comonomers greatly improved the energy surface of copolymer products.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21801236), PetroChina Company Limited (2018B-2707), Joint Funds of the National Natural Science Foundation of China (U186220053) and Jilin Provincial Science and Technology Development program (20190103122JH).Notes and references
- J. E. Mark, Handbook, Polymer Data, Oxford University Press, New York, 1999 Search PubMed.
- V. G. Natta, Makromol. Chem., 1955, 16, 213–237 CrossRef.
- H. Ashitaka, H. Ishikawa, H. Ueno and A. Nagasaka, J. Polym. Sci., Polym. Chem. Ed., 1983, 21, 1853–1860 CrossRef CAS.
- M. Ichikawa, Y. Takeuchi, A. Kogure and H. Kurita, US Pat., 3498963A, 1970.
- K. Makino, K. Komatsu, Y. Takeuchi and M. Endo, US Pat., 4182813A, 1980.
- S. Luo, US Pat., 6331594, 2001.
- S. Luo, US Pat., 6528588, 2003.
- H. Ashitaka, Y. Kusuki, S. Yamamoto, Y. Ogata and A. Nagasaka, J. Appl. Polym. Sci., 1984, 29, 2763–2776 CrossRef CAS.
- X. Hao and X. Zhang, Mater. Lett., 2007, 61, 1319–1322 CrossRef CAS.
- M. Hitrik, V. Gutkin, O. Lev and D. Mandler, Langmuir, 2011, 27, 11889–11898 CrossRef CAS.
- W. Pan, H. Chen, J. Mu, W. Li, F. Jiang, G. Weng, Y. Hu, D. Gong and X. Zhang, Polymer, 2017, 111, 20–26 CrossRef CAS.
- Y. Takahashi, X. Liang and K. Nakajima, J. Appl. Polym. Sci., 2019, 136, 47934 CrossRef.
- T. C. Chung, Prog. Polym. Sci., 2002, 27, 39–85 CrossRef CAS.
- P. Zinck, F. Bonnet, A. Mortreux and M. Visseaux, Prog. Polym. Sci., 2009, 34, 369–392 CrossRef CAS.
- N. M. G. Franssen, J. N. H. Reek and B. de Bruin, Chem. Soc. Rev., 2013, 42, 5809–5832 RSC.
- B. Marciniec, M. Lewandowski, C. Pietraszuk and Z. Foltynowicz, Polymer, 1997, 38, 5169–5172 CrossRef CAS.
- M. I. Abdullin, A. B. Glazyrin, R. N. Asfandiyarov, V. R. Akhmetova and V. N. Zaboristov, Polym. Sci., Ser. B, 2006, 48, 104–107 CrossRef.
- M. I. Abdullin, A. B. Glazyrin, R. N. Asfandiyarov and R. R. Muslukhov, Polym. Sci., Ser. B, 2009, 51, 303–308 CrossRef.
- A. B. Glazyrin, M. I. Abdullin, R. R. Muslukhov and V. A. Kraikin, Polym. Sci., Ser. A, 2011, 53, 110–115 CrossRef CAS.
- A. B. Glazyrin and M. I. Abdullin, Russ. J. Appl. Chem., 2016, 89, 1655–1661 CrossRef CAS.
- A. B. Glazyrin, M. I. Abdullin, E. R. Atnabaeva, R. M. Sultanova, V. P. Volodina and V. A. Dokichev, Polym. Bull., 2019, 76, 3643–3657 CrossRef CAS.
- F. Guo, K. Jankova, L. Schulte, M. E. Vigild and S. Ndoni, Langmuir, 2010, 26, 2008–2013 CrossRef CAS PubMed.
- B. Korthals, M. C. Morant-Miñana, M. Schmid and S. Mecking, Macromolecules, 2010, 43, 8071–8078 CrossRef CAS.
- A. Berthold, K. Sagar and S. Ndoni, Macromol. Rapid Commun., 2011, 32, 1259–1263 CrossRef CAS.
- L. Lotti, S. Coiai, F. Ciardelli, M. Galimberti and E. Passaglia, Macromol. Chem. Phys., 2009, 210, 1471–1483 CrossRef CAS.
- T. D. Wickard, E. Nelsen, N. Madaan, N. ten Brummelhuis, C. Diehl, H. Schlaad, R. C. Davis and M. R. Linford, Langmuir, 2010, 26, 1923–1928 CrossRef CAS.
- J. Zheng, F. Liu, Y. C. Lin, Z. J. Zhang, G. C. Zhang, L. Wang, Y. Liu and T. Tang, Macromolecules, 2012, 45, 1190–1197 CrossRef CAS.
- B. Korthals, M. C. Morant-Miñana, M. Schmid and S. Mecking, Macromolecules, 2010, 43, 8071–8078 CrossRef CAS.
- The growing active specieses are η3 and σ1 coordinated mode for diene and monoene monomers respectively.
- C. Yao, N. Liu, S. Long, C. Wu and D. Cui, Polym. Chem., 2016, 7, 1264–1270 RSC.
- S. Long, F. Lin, C. Yao and D. Cui, Macromol. Rapid Commun., 2017, 38, 1700227 CrossRef.
- H. Leicht, I. Göttker-Schnetmann and S. Mecking, J. Am. Chem. Soc., 2017, 139, 6823–6826 CrossRef CAS.
- H. Leicht, I. Göttker-Schnetmann and S. Mecking, Macromolecules, 2017, 50, 8464–8468 CrossRef CAS.
- H. Leicht, I. Göttker-Schnetmann and S. Mecking, ACS Macro Lett., 2016, 5, 777–780 CrossRef CAS.
- C. Yao, H. Xie and D. Cui, RSC Adv., 2015, 5, 93507–93512 RSC.
- D. Gong, W. Dong, Y. Hu, J. Bi, X. Zhang and L. Jiang, Polymer, 2009, 50, 5980–5986 CrossRef CAS.
- D. Gong, W. Pan, T. Zhu, H. Chen, Z. Zhou, F. Jiang, Y. Hu and X. Zhang, Polymer, 2016, 98, 136–142 CrossRef CAS.
- W. Pan, H. Chen, R. Sun, D. Gong, X. Jia, Y. Hu and X. Zhang, Ind. Eng. Chem. Res., 2016, 55, 7580–7586 CrossRef CAS.
- D. Gong, W. Dong, J. Hu, X. Zhang and L. Jiang, Polymer, 2009, 50, 2826–2829 CrossRef CAS.
- W. Zheng, F. Wang, J. Bi, H. Zhang, C. Zhang, Y. Hu, C. Bai and X. Zhang, J. Polym. Sci., Polym. Chem. Ed., 2015, 53, 1182–1188 CrossRef CAS.
- J. Lu, Y. Hu, X. Zhang, J. Bi, W. Dong, L. Jiang and B. Huang, J. Appl. Polym. Sci., 2006, 100, 4265–4269 CrossRef CAS.
- J. Cai, Q. Yu, X. Zhang, J. Lin and L. Jiang, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 2885–2897 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06499k |
| This journal is © The Royal Society of Chemistry 2019 |