Open Access Article
Open Access ArticleRetracted Article: Upregulation of miR-26b alleviates morphine tolerance by inhibiting BDNF via Wnt/β-catenin pathway in rats
Xing Liu†
a,
Jiefeng Geng†b,
Huilian Bua,
Junqi Maa and
Yanqiu Ai *a
*a
aDepartment of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, No. 1, East Jianshe Rd, Zhengzhou, 450052, China. E-mail: peimqq@163.com; Tel: +86-371-67966114
bDepartment of Neurosurgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
First published on 11th December 2019
Abstract
Background: Morphine is a commonly used analgesic drug. However, long-term use of morphine will cause tolerance which limits its clinical application in pain treatment. MicroRNAs (miRNAs) have been reported to be involved in the morphine tolerance, but the underlying mechanism is still poorly understood. Methods: Tail flick test was used to measure the maximum possible effect (MPE). Quantitative real-time PCR was employed to detect miR-26b, BDNF, and Wnt5a expression in rat dorsal root ganglia (DRG). Luciferase report assay was introduced to verify the binding relationship between miR-26b and Wnt5a. BDNF, Wnt5a and β-catenin protein level were tested by western blotting. Results: MiR-26b was down-regulated during the development of morphine tolerance while BDNF was upregulated. Overexpression of miR-26b or BDNF inhibition alleviated morphine tolerance. Wnt5a was directly targeted and inhibited by miR-26b via binding to the 3′-UTR of Wnt5a. The Wnt/β-catenin pathway was active in morphine tolerant rats. Moreover, overexpression of Wnt5a could partially enhance miR-26 mimic-mediated morphine tolerance, while a Wnt5a inhibitor could attenuate the tolerance. Conclusion: The present study demonstrated that miR-26b overexpression alleviated morphine tolerance by inhibiting BDNF via the Wnt/β-catenin pathway in rats, highlighting a promising target for the treatment of morphine tolerance.
Introduction
Morphine, as a member of the opiate family, is a pain medication which may relieve acute pain and chronic pain. It acts directly on the central nervous system (CNS) to decrease the pain feeling. However, long-term use or repeat-use could lead to morphine tolerance,1 which is becoming the most critical challenge. In clinic, morphine tolerance is defined as a gradual loss of drug potency of efficacy and reduced duration of action.2 There will be more morphine needed to maintain the same analgesic effect in morphine-tolerant patients that could result in more side effects. Still now, the molecular origin of morphine tolerance is poorly understood. But many researches have suggested that various factors including opioid receptor,3 glutamate receptor,4 G-protein,5 protein kinase C, and microRNAs6 may be involved in the tolerance.MicroRNAs (miRNAs) are small, non-coding single-stranded RNAs, about 22 nucleotides in length which may inhibit translation initiation and protein synthesis at the post-transcriptional level and mediate messenger RNA (mRNA) degradation by binding to the 3′-untranslated region (3′-UTR). Recent studies demonstrate that the miRNA/mRNA axis plays important role in the pathophysiology of the nervous system7 and morphine tolerance. MiRNA microarray analysis revealed that miR-26b, miR-218a, miR-10a-5p, miR-338, miR-219a-5p, let-7a-2, miR-196a, miR-873, miR-665 and miR-365 were downregulated in the rat spinal cord after induction of morphine tolerance.8
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family which is synthesized in dorsal root ganglia (DRG) neurons. BDNF acts on certain neurons of the central nervous system and peripheral nervous system to support the survival of existing neurons and encourage the growth and differentiation of neurons and synapses.9 When there is peripheral inflammation and nerve injury,10 BDNF expression are dramatically changed in DRG. In morphine tolerance, BDNF expression is increased due to the demethylation regulation of the promoter.11 However, whether there is a relationship of BDNF and miR-26b in the morphine tolerance is still unknown.
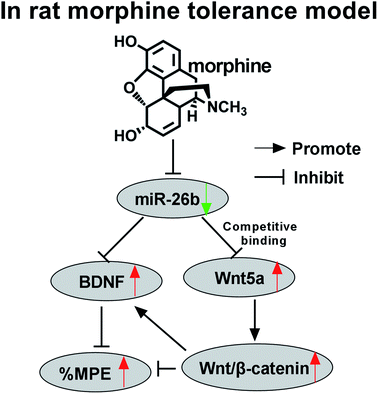
In present study, rat morphine tolerance model was induced by repeated intrathecal injection of morphine. And we evidenced that miR-26b was involved in the morphine tolerance modulation. Overexpression of miR-26b led to decreased expression of the direct target Wnt5a, β-catenin, and BDNF and an attenuation of morphine tolerance. As a direct target of miR-26b, Wnt5a inhibition also alleviated morphine tolerance. So miR-26b might be a promising target in treatment of morphine tolerance.
Materials and methods
Animals and treatment
Female and male Sprague-Dawley (SD) rats (250–300 g, n = 60 in total) were purchased from the First Affiliated Hospital of Zhengzhou University. All animal experiments were performed with the approval of the Ethics Committee for Animal Experimentation of the First Affiliated Hospital of Zhengzhou University and conducted in accordance with the National Institutes of Health Guidelines. The rats were housed in the animal facility and great efforts were made to minimize the number of animals used and their suffering. All rats were randomly divided into ten groups (n = 6 each group, Table 1).| Group (n = 6 each group) | Treatment regimens | Effects |
|---|---|---|
| NC | Rats received no treatment | As blank control |
| Sal | Saline was injected into cord spinal of the rats via intrathecal infection at the dosage of 10 μl twice a day for 7 days | As negative control for Mor group |
| Mor | Morphine was injected into cord spinal of the rats via intrathecal infection at the dosage of 10 μg twice a day for 7 days | Compared with NC, miR-26b was downregulated and BDNF was upregulated in the dorsal root ganglia of morphine-tolerant rats. (5 test animals were effected) |
| Mor + LV-miR-NC | Rats received an injection of morphine and 10 × 106 PFU miR-NC-expressing lentivirus vectors | As negative control for Mor + LV-miR-26b group |
| Mor + LV-miR-26b | Rats received an injection of morphine and 10 × 106 PFU miR-26b-expressing lentivirus | Compared with Mor + LV-miR-NC, miR-26b overexpression reversed the morphine tolerance effect. (5 test animals were effected) |
| Mor + sh-NC | Rats received an injection of morphine and 10 × 106 PFU sh-NC-expressing lentivirus vectors | As negative control for Mor + sh-BDNF group |
| Mor + sh-BDNF | Rats received an injection of morphine and 10 × 106 PFU sh-BDNF-expressing lentivirus | Compared with Mor + sh-NC, sh-BDNF reversed the morphine tolerance effect. (6 test animals were effected) |
| Mor + LV-miR-26b + LV-vector | Rats received an injection of morphine and miR-26b-expressing lentivirus and negative lentivirus vectors | As negative control for Mor + LV-miR-26b + LV-Wnt5a group |
| Mor + LV-miR-26b + LV-Wnt5a | Rats received an injection of morphine and miR-26b-expressing lentivirus and Wnt5a-overexpressing lentivirus | Compared with Mor + LV-miR-26b + LV-vector, overexpressed Wnt5a could partially destroy the morphine tolerance (4 test animals were effected) |
| IWR-1-endo− (Mor) | Rats received an injection of morphine | As negative control for IWR-1-endo + group |
| IWR-1-endo+ | Rats received an injection of morphine and IWR-1-endo | Compared with NC, IWR-1-endo + treatment inhibited Wnt/β-catenin signal pathway and reversed the morphine tolerance effect. (5 test animals were effected) |
Morphine was used to construct the rat morphine tolerance model. Morphine was injected into cord spinal of the rats via intrathecal infection at the dosage of 10 μg twice a day for 7 days.12 As control, nothing or 10 μl saline were injected.
IWR-1-endo was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and 20 ng was injected into the rat spinal cord every two days followed by the morphine injection.
Behavioral test
The morphine antinociceptive tolerance was assessed by the tail flick test as previously described.13 In brief, the tail flick test was performed before and 30 min after NC, saline and morphine injection. 3 min interval was performed between the three trails and the tail position would be changed in each trail. The mean latency for 3 trails was defined as the tail flick latency. Antinociception was calculated by this following formulation: maximum possible effect (MPE)% = (test latency − predrug latency)/(cutoff time − predrug latency) × 100%. To prevent the damage to the tissues, the cutoff time was 10 s. Antinociceptive responses were presented as mean ± SEM.12Transient transfection
Lentivirus vectors expressing miR-26b (LV-miR-26b), shRNA against BDNF (sh-BDNF), Wnt5a overexpression vector (LV-Wnt5a) and the corresponding negative controls (LV-miR-NC, sh-NC and LV-vector) were purchased from GenePharma Co., Ltd. (Shanghai, China). 10 μl of lentivirus (108 PFU ml−1) was intrathecally injected in vivo.Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from DRG cells using TRIzol (Invitrogen) and RNA concentration was detected by NanoDropND-1000 spectrophotometer. The TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and qScript cDNA Synthesis Kit (QuantaBio, Beverly, MA, USA) were applied to reverse-transcribe into cDNA. MiR-26b, BDNF and Wnt5a expression was determined using SYBR® Green (Promega, Madison, WI, USA) according to the manufacturer's protocol. U6 small nuclear RNA (snRNA) was used as the reference gene for miR-26b. 18s rRNA was used as a reference gene for BNDF and Wnt5a. qRT-PCR was carried out in the iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The relative expression of miR-26b, BDNF and Wnt5a were calculated by the 2−ΔΔCt method. Primer sequences: miR-26b forward 5′-ATTAGCCCTGTCCCTCAATC-3′, reverse 5′-TAGATGGTTTATCTATGACC-3′. BDNF forward 5′-AGCTGAGCGTGTGTGACAGT-3′, reverse 5′-ACCCATGGGATTACACTTGG-3′. Wnt5a forward 5′-AGACGGGCATCAAAGAGT-3′, reverse 5′-AAGCGGTAGCCATAGTC-3′. U6 forward 5′-TGACACGCAAATTCGTGAAGCGTTC-3′, reverse 5′-CCAGTCTCAGGGTCCGAGGTATTC-3′. 18s forward 5′-GTAACCCGTTGAACCCCATT-3′, reverse 5′-CCATCCAATCGGTAGTAGCG-3′.Western blot
Cells were isolated from DRG and made to single cell suspension. Washed with PBS for 2 times, then added RIPA lysis buffer and maintained on ice for 30 min accompanied with vortexing 30 s per 10 min. Centrifuged for 15 min at 4 °C with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and transferred the supernatant to a new tube. The protein concentrations were measured using Nanodrop 2000 (Thermo Fisher Scientific, San Jose, CA, USA). Then added 10× loading buffer to the protein solution and heated on 95 °C for 5 min. Proteins (10 μg) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Bedford, MA, USA). After blocked with 5% dried skimmed milk in Tris-buffered saline (TBS), the membranes were incubated with specific primary antibodies including anti-BDNF, anti-Wnt5a and anti-β-catenin (1
000 rpm and transferred the supernatant to a new tube. The protein concentrations were measured using Nanodrop 2000 (Thermo Fisher Scientific, San Jose, CA, USA). Then added 10× loading buffer to the protein solution and heated on 95 °C for 5 min. Proteins (10 μg) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Bedford, MA, USA). After blocked with 5% dried skimmed milk in Tris-buffered saline (TBS), the membranes were incubated with specific primary antibodies including anti-BDNF, anti-Wnt5a and anti-β-catenin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 dilution, and all from Santa Cruz Biotechnology) and anti-β-actin antibody (1
100 dilution, and all from Santa Cruz Biotechnology) and anti-β-actin antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution, Santa Cruz Biotechnology) at 4 °C overnight. The PVDF membrane was washed five times with TBS with Tween-20 (TBST), followed by incubation with HRP-conjugated anti-mouse IgG secondary antibody (1
2000 dilution, Santa Cruz Biotechnology) at 4 °C overnight. The PVDF membrane was washed five times with TBS with Tween-20 (TBST), followed by incubation with HRP-conjugated anti-mouse IgG secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution, Santa Cruz Biotechnology) at room temperature for 1 h. After being washed with TBST five times, the protein signals were detected using PierceTM ECL western blotting substrate (Thermo Fisher Scientific).
2000 dilution, Santa Cruz Biotechnology) at room temperature for 1 h. After being washed with TBST five times, the protein signals were detected using PierceTM ECL western blotting substrate (Thermo Fisher Scientific).
Bioinformatics and dual-luciferase reporter assay
Analysis for miRNA molecular targets was performed using TargetScan Human 7.1 (http://www.targetscan.org/vert_71/). Wnt5a 3′-UTR wild-type luciferase reporter plasmid (Wnt5a-WT) harboring the miR-26b-binding site and the site-directed mutant of the miR-26b-binding site (Wnt5a-MUT) were bought from Hanbio (Shanghai, China). HEK293T cells (ATCC, Manassas, VA, USA) cultured in RIMP-1640 medium (Thermo Fisher Scientific) plus 10% FBS (Thermo Fisher Scientific) were cotransfected with Wnt5a-WT or Wnt5a-MUT and miR-NC mimic or miR-26b mimic using the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). Luciferase activities were detected using the Dual-luciferase Reporter Assay System (Promega) after 48 h transfection.Statistical analysis
The data were presented as mean ± standard deviation (SD) from three biological replicated experiments. The analysis of results was shown and plotted using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). All group comparisons were carried out using the Student's t-test. The p value less than 0.05 was regarded as statistically significant.Results
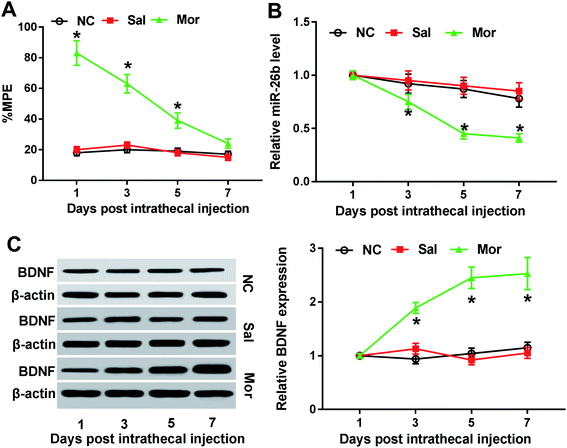
MiR-26b was downregulated and BDNF was upregulated in the dorsal root ganglia of morphine-tolerant rats.
Firstly, to construct the morphine tolerance model, we injected NC, saline and morphine to rats twice a day for 7 consecutive days. Then, as the methods described, we preformed the tail flick test to calculate the MPE after each injection. At the first three injection, the % MPEs were significantly different in Mor group compared with the NC and saline-injection group, but no difference on day 7 (Fig. 1A). Thus, morphine tolerance rat model was established successfully 7 days after morphine injection.Previous studies demonstrated that miR-26b was down-regulated in morphine tolerant rats by microRNA microarray assay.8 To validate the miR-26b expression pattern in morphine-tolerant rats, we used qRT-PCR to measure its expression level. In contrast to the control group, miR-26b expression was not changed on day 1 after morphine injection, but on day 3 it began to decline and showed significant difference on day 5 and day 7 (Fig. 1B). The trend of miR-26b alteration was related with the morphine tolerance curve in Fig. 1A, which implied that miR-26 might be involved in the morphine tolerance.
BDNF is synthesized in DRG neuron cells which could be induced by exposure to morphine.14 So as a marker of morphine stimulation, we also measured the BDNF expression in the DRG of morphine tolerant rats. The western blotting results showed that BDNF expression was gradually increased from day 3 after intrathecal injection and BDNF expression difference was shown visually by the gray scale analysis (Fig. 1C). On day 7, the BDNF increased about 3-fold compared with that on day 1 (Fig. 1C).
Overexpression of miR-26b or BDNF knockdown partially reversed the morphine tolerance effect
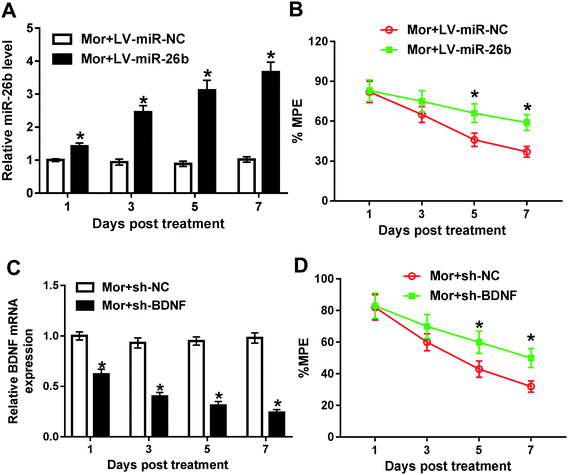
To further explore the function of miR-26 in morphine tolerance, we constructed lentivirus (LV)-miR-26b and lentivirus (LV)-miR-NC and injected the virus to the spinal cord of rat. Then, we continued to construct the morphine tolerance rat model by intermittent injection. As demonstrated by qRT-PCR, the miR-26b expression was gradually increased from day 1 in LV-miR-26b group compared with that in LV-miR-NC group (Fig. 2A). Next, we checked the % MPE via tail flick test and there were no difference on day 1 and day 3 after virus injection (Fig. 2B). However, the % MPE in LV-miR-26b group on day 5 was about 70% while the % MPE in LV-miR-NC was close to 45% (Fig. 2B). On day 7, the difference of % MPE in the two groups was even more significant (Fig. 2B). The results meant that the overexpression of miR-26b in the DRG might relieve the morphine tolerance in rats.Besides, we knocked down the BDNF expression in DRG in morphine tolerant rats. The data of qRT-PCR showed that the BDNF expression was gradually decreased after treatment (Fig. 2C). In line with miR-26b overexpression, the downregulation of BDNF expression partially reversed the morphine tolerance in rats from day 3 after treatment (Fig. 2D).
Wnt5a was a direct target of miR-26b
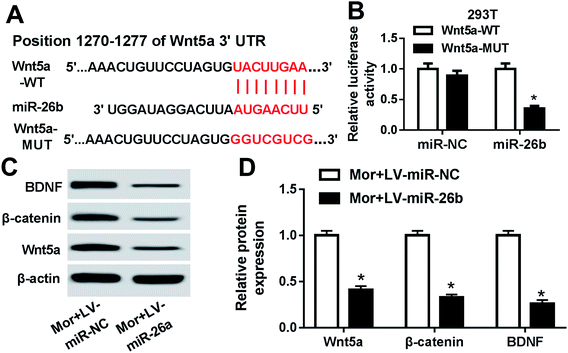
MiRNAs exert their function through inhibiting target mRNAs expression. So to identify the potential target mRNAs of miR-26b, we used bioinformatics software TargetScan to predict the candidate targets. Among all the putative target mRNAs, the position 1270–1277 of Wnt5a 3′-UTR was a putative binding site of miR-26b (Fig. 3A). We focused on Wnt5a because Wnt/β-catenin pathway could regulate the BDNF expression.15 Thus, we continued to verify whether Wnt5a is a direct target of miR-26b using a dual luciferase assay in HEK293T cells. As shown in Fig. 3B, after cotransfection with Wnt5a-WT 3′-UTR reporter vector and miR-26b mimic, the luciferase activity was significantly attenuated, however the mutant no longer elicited such effect. These findings suggested that Wnt5a was a direct target of miR-26b.Then we checked the Wnt5a, BDNF and β-catenin expression in the DRG of morphine-tolerant rats transfected with LV-miR-26b or LV-control. As shown by western blotting, all of the three proteins were prominently downregulated in the Mor + LV-miR-26b group compared with those of control (Fig. 3C and D).
Wnt5a was involved in the miR-26b-mediated morphine tolerance
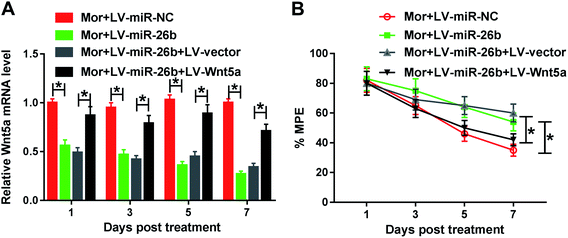
To better understand the function of miR-26b and Wnt5a, the LV-Wnt5a and LV-vector were constructed and cotransfected into the DRG of morphine tolerant rats with LV-miR-26b, respectively. As demonstrated by qRT-PCR, the Wnt5a mRNA expression was significantly decreased by the overexpression of miR-26b compared to the negative group (Fig. 4A). However, cotransfection of LV-Wnt5a dramatically reversed miR-26b-mediated repression in Wnt5a mRNA expression (Fig. 4A).Meanwhile, we evaluated the % MPE in the four groups of Mor + LV-miR-NC, Mor + LV-miR-26b, Mor + LV-miR-26b + LV-vector and Mor + LV-miR-26b + LV-Wnt5a. The % MPE in LV-miR-26b + LV-Wnt5a group was lower than that in LV-miR-26b + LV-vector group (Fig. 4B). The results suggested that miR-26b overexpression-mediated alleviation in morphine tolerance was significantly abolished by the restored Wnt5a expression in rats.
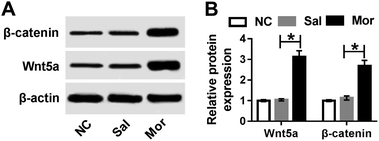
Wnt/β-catenin pathway was active in morphine tolerance
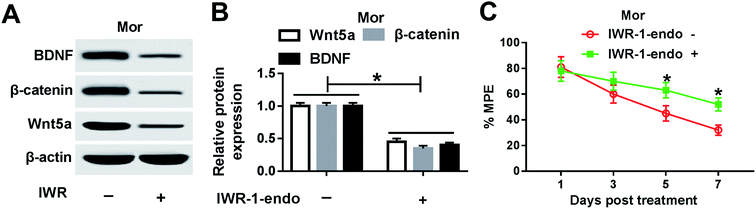
As above mentioned, the miR-26b expression was down-regulated in the morphine-tolerant rats and Wnt5a was a direct target of miR-26b. Therefore, we determined if the expression of Wnt5a was upregulated during the development of morphine tolerance. At day 7 after morphine injection, we checked the Wnt5a expression and it indeed was upregulated in the DRG of morphine-tolerant rats (Fig. 5A). Moreover, β-catenin expression was higher in Mor group than that in control groups (Fig. 5A). Gray scale analysis demonstrated that Wnt5a and β-catenin expression increased about 3 fold in morphine-tolerant rats than those in NC or saline-treated rats (Fig. 5B).To further explore the role of Wnt5a in morphine tolerance, we used IWR-1-endo, which is an inhibitor of Wnt5a. We injected 20 ng IWR-1-endo into the rat spinal cord every two days followed by the morphine injection. On day 7, the BDNF, β-catenin and Wnt5a protein level in DRG were measured by western blotting. As shown in Fig. 6A, all of the three proteins were significantly down-regulated after IWR injection. Gray scale analysis suggested that BDNF, β-catenin and Wnt5a decreased about a half compared with the negative control (Fig. 6B). Meanwhile, we checked the % MPE in morphine-injected rats, and there were no significant difference on day 1 and day 3. But on day 5, the % MPE was about 70% in IWR-1-endo treated group while it was only about 45% in the control group (Fig. 6C). On day 7, the % MPE was about 60% in IWR-1-endo treated group while it was only about 35% in control (Fig. 6C). These results indicated that IWR-1-endo, an inhibitor of Wnt5a, might partially alleviate the morphine tolerance in rats (Fig. 7).
Discussion
Inflammation, tissue damage, nerve injury, cancer and other types of disease can cause chronic pain.16 This pain stimulations may be transmitted to the spinal dorsal horn via dorsal root ganglion (DRG) or trigeminal ganglion (TG).17 In chronic pain, the microglia could be activated which is accompanied with morphological changes, cell number increase, neurotransmitter receptor expression alteration such as P2X4R, P2X7R and P2YT in the spinal cord.18 P2X4Rs may stimulate the expression and release of BDNF.19 BDNF can act on certain type of neurons in the central and peripheral nervous system to support the survival, growth and differentiation of neurons and synapses.9 When there is peripheral inflammation and nerve injury,10 BDNF expression are dramatically altered in DRG.Opioids are widely used to treat chronic pain.20 It was suggested that μ opioid receptors (MOR) are involved in the pain relief during morphine treatment.21 However, long-term use or repeat-use could lead to morphine tolerance1 which becomes the most critical challenge. There are several aspects of tolerance relevant to this issue including pharmacodynamic, pharmacokinetic, and learned tolerance, such as MOR desensitization and endocytosis, morphine analgesia and altered gene expression.22,23 In morphine tolerance, BDNF expression is increased due to the demethylation regulation of the promoter.11 In our present study, we indeed found that BDNF expression was gradually increased during the development of morphine tolerance in rat. And BDNF knockdown could alleviate the morphine tolerance in some extend.
It was firstly reported in 2007 that miRNAs are involved in the pain model. Via injecting Complete Freund's Adjuvant (CFA) into the rat masseter muscle to induce inflammatory pain, Bai et al. found that miR-124 was decreased in the early state but increased in the late state during the development of model. Besides miR-124, miR-10a, miR-29a, miR-98, miR-99a, miR-134 and miR-183 were also down-regulated.24 In addition, miR-1, miR-16 and miR-206 expression were markedly downregulated in the DRG of mouse spinal cord dorsal horn.25 Further research discovered that miR-16 played function by targeting ras-related protein (RAB23) and inhibiting p38 MAPK activation.26 MiR-134 was also downregulated under neuropathic conditions and nociceptive pains which could regulate mu-opioid type 1 receptors in dorsal root ganglion neurons.27 Overexpression of miR-143-3p which could target and inhibit the high mobility group box 1 (HMGB1) may relieved neuropathic pain development.
Besides, miRNAs are also involved in the morphine tolerance. For example, miR-365 could reverse morphine tolerance via inhibiting β-arrestin 2 expression in the spinal cord;8 miR-375 could significantly hinder morphine tolerance via inhibiting JAK2/STAT3 pathway;28 miR-93 may be involved in the tolerance by targeting Smad5;29 miR-338 could suppress the tolerance by targeting CXCR4.30 In our study, we found that miR-26b was decreased during the morphine tolerance, and miR-26b-mimic could attenuate the tolerance significantly.
Usually, microRNAs can't play the biological function solely. They may binds to 3′-UTRs of target mRNAs to inhibit translation initiation and protein synthesis at the post-transcriptional level. Considering the dramatic alteration of miR-26b expression during the morphine tolerance, we further verified that Wnt5a was a direct target of miR-26b, which is a member of Wnt superfamily. Wnt/β-catenin signaling pathway plays important role in cell proliferation, migration and homeostasis.31 Previous study demonstrated that Wnt/β-catenin signaling pathway was also involved in the morphine tolerance.32 As we found in our study, overexpression of Wnt5a could partially enhance the morphine tolerance which was alleviated by miR-26b mimic, while Wnt5a inhibitor could attenuate the tolerance.
Conclusion
In conclusion, our results demonstrate that miR-26b is involved in morphine tolerance by negatively regulating the expression of Wnt5a. Overexpression of miR-26b leads to decreased expression of the direct target Wnt5a, β-catenin, and BDNF and a significant attenuation of morphine tolerance. Meanwhile, Wnt5a inhibitor also can attenuate the morphine tolerance in rats. Although further studies are needed to demonstrate the therapeutic impacts, potential side effects of miR-26b in the modulation of morphine tolerance, miR-26b still might be a promising and novel target for the treatment of morphine tolerance.Conflicts of interest
No conflict of interest is declared by all named co-authors.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant no. 81500964 and 81601201).References
- H. McQuay, Acta Anaesthesiol. Scand., 2010, 41, 1–3 Search PubMed.
- (a) Z. J. Wang and L. X. Wang, Life Sci., 2006, 79, 1681–1691 CrossRef CAS PubMed; (b) R. N. Harden, Arch. Phys. Med. Rehabil., 2008, 89, S72–S76 CrossRef.
- L. Martini and J. L. Whistler, Curr. Opin. Neurobiol., 2007, 17, 556–564 CrossRef CAS.
- P. Sánchez-Blázquez, M. Rodríguez-Muñoz, E. Berrocoso and J. Garzón, Eur. J. Pharmacol., 2013, 716, 94–105 CrossRef PubMed.
- A. R. Gintzler and S. Chakrabarti, Mol. Neurobiol., 2000, 21, 21–33 CrossRef CAS PubMed.
- K. K. Bali and R. Kuner, Trends Mol. Med., 2014, 20, 437–448 CrossRef CAS PubMed.
- (a) O. G. Bhalala, M. Srikanth and J. A. Kessler, Nat. Rev. Neurol., 2013, 9, 328–339 CrossRef CAS PubMed; (b) F. Philipp, C. Harold and B. Christophe, Front. Mol. Neurosci., 2014, 7, 5 Search PubMed; (c) E. Sun and Y. Shi, Exp. Neurol., 2015, 268, 46–53 CrossRef CAS PubMed.
- J. Wang, W. Xu, T. Zhong, Z. Song, Y. Zou, Z. Ding, Q. Guo, X. Dong and W. Zou, Sci. Rep., 2016, 6, 38285 CrossRef CAS PubMed.
- (a) A. Acheson, J. C. Conover, J. P. Fandl, T. M. Dechiara, M. Russell, A. Thadani, S. P. Squinto, G. D. Yancopoulos and R. M. Lindsay, Nature, 1995, 374, 450–453 CrossRef CAS; (b) E. J. Huang and L. F. Reichardt, Annu. Rev. Neurosci., 2001, 24, 677–736 CrossRef CAS PubMed.
- (a) A. Merighi, C. Salio, A. Ghirri, L. Lossi, F. Ferrini, C. Betelli and R. Bardoni, Prog. Neurobiol., 2008, 85, 297–317 CrossRef CAS PubMed; (b) K. Obata and K. Noguchi, Neurosci. Res., 2006, 55, 1–10 CrossRef CAS PubMed.
- Y. C. Chao, X. Fang, X. Li, R. Guo, Y. Ning, Z. Chen, S. Rong, G. Yun, Y. Yun and W. Yun, Neurochem. Int., 2016, 97, 91–98 CrossRef CAS PubMed.
- D. E. Kellstein and D. J. Mayer, Pain, 1991, 47, 221–229 CrossRef CAS PubMed.
- C. H. Yang, H. W. Huang, K. H. Chen, Y. S. Chen, S. M. Sheenchen and C. R. Lin, Br. J. Anaesth., 2011, 107, 774–781 CrossRef CAS PubMed.
- H. Hatami, S. Oryan, S. Semnanian, B. Kazemi, M. Bandepour and A. Ahmadiani, Neuropeptides, 2007, 41, 321–328 CrossRef CAS PubMed.
- (a) Y. Wang, J. Liao, S. J. Tang, J. Shu and W. Zhang, J. Mol. Neurosci., 2017, 62, 199–208 CrossRef CAS PubMed; (b) J. W. Yang, J. Ru, W. Ma, Y. Gao, Z. Liang, J. Liu, J. H. Guo and L. Y. Li, Neuropeptides, 2015, 54, 35–46 CrossRef CAS PubMed.
- R. H. Dworkin, A. B. O'Connor, J. Kent, S. C. Mackey, S. N. Raja, B. R. Stacey, R. M. Levy, M. Backonja, R. Baron and H. Harke, Pain, 2013, 154, 2249–2261 CrossRef PubMed.
- R. Kuner, Nat. Med., 2010, 16, 1258–1266 CrossRef CAS.
- (a) M. Tsuda, K. Inoue and M. W. Salter, Trends Neurosci., 2005, 28, 101–107 CrossRef CAS PubMed; (b) J. M. Pocock and H. Kettenmann, Trends Neurosci., 2007, 30, 527–535 CrossRef CAS PubMed.
- L. Ulmann, J. P. Hatcher, J. P. Hughes, S. Chaumont, P. J. Green, F. Conquet, G. N. Buell, A. J. Reeve, I. P. Chessell and F. Rassendren, J. Neurosci., 2008, 28, 11263–11268 CrossRef CAS PubMed.
- A. Rosenblum, L. A. Marsch, H. Joseph and R. K. Portenoy, Exp. Clin. Psychopharmacol., 2008, 16, 405–416 Search PubMed.
- H. W. D. Matthes, R. Maldonado, F. Simonin, O. Valverde, S. Slowe, I. Kitchen, K. Befort, A. Dierich, M. L. Meur and P. Dollé, Nature, 1996, 383, 819 CrossRef CAS PubMed.
- G. Chang, L. Chen and J. Mao, Med. Clin. North Am., 2007, 91, 199–211 CrossRef CAS PubMed.
- H. Ueda and M. Ueda, Front. Biosci., Landmark Ed., 2009, 14, 5260–5272 CrossRef CAS PubMed.
- G. Bai, A. Rajini, W. Dong and D. Dean, Mol. Pain, 2007, 3, 15 CrossRef PubMed.
- R. Kusuda, F. Cadetti, M. I. Ravanelli, T. A. Sousa, S. Zanon, F. L. D. Lucca and G. Lucas, Mol. Pain, 2011, 7, 17 CrossRef CAS PubMed.
- W. Chen, S. Guo and S. Wang, Med. Sci. Monit., 2016, 22, 3894–3901 CrossRef CAS PubMed.
- J. Ni, Y. Gao, S. Gong, S. Guo, T. Hisamitsu and X. Jiang, Eur. J. Pain, 2013, 17, 313–323 CrossRef CAS PubMed.
- H. Li, R. Tao, J. Wang and L. Xia, J. Pain Res., 2017, 10, 1279–1287 CrossRef CAS PubMed.
- W. F. Xiao, Y. S. Li, W. Lou, T. Cai, S. Zhang, X. Y. Hu, X. W. Zhang and W. Luo, Oncotarget, 2016, 7, 52104–52114 Search PubMed.
- H. X. Mei, M. H. Zhou, X. W. Zhang, X. X. Huang, Y. L. Wang, P. F. Wang and G. H. Zhan, Biosci. Rep., 2017, 37 DOI:10.1042/bsr20160517.
- H. Clevers, Cell, 2006, 127, 469–480 CrossRef CAS PubMed.
- S. A. Dunbar, I. Karamian, L. Roberts and J. Zhang, Anesthesiology, 2006, 105, 154 CrossRef CAS PubMed.
Footnote |
| † Xing Liu and Jiefeng Geng contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |