Open Access Article
Open Access ArticleProteomic analysis of the earthworm Eisenia fetida exposed to oxytetracycline in soil
Huabing Zhao*a,
Sanyuan Shi a,
Hong Zhaob,
Jin Guoc,
Zhen Yangc,
Hongsheng Gaoc and
Fuping Lua
a,
Hong Zhaob,
Jin Guoc,
Zhen Yangc,
Hongsheng Gaoc and
Fuping Lua
aKey Laboratory of Industrial Fermentation Microbiology, Ministry of Education, Tianjin Key Laboratory of Industrial Microbiology, National Engineering Laboratory for Industrial Enzymes, College of Biotechnology, Tianjin University of Science & Technology, Tianjin 300457, China. E-mail: zhaohuabing@tust.edu.cn; Fax: +86-22-60602298; Tel: +86-22-60601958
bAnimals, Plants and Food Testing Center of Tianjin Customs District, Tianjin 300461, China
cTianjin Key Laboratory for Prevention and Control of Occupational and Environmental Hazard, Logistics University of Chinese People's Armed Police Forces, Tianjin 300162, China
First published on 16th December 2019
Abstract
Increasing attention has been paid to the toxicity and hazards of antibiotics on non-target organisms in soil ecosystems because redundant antibiotics in the excretion of treated animals are being brought into the soil by way of manure and sewage irrigation. In order to understand the toxic mechanisms of antibiotics in soil ecosystems, the earthworm Eisenia fetida was exposed to 500 mg kg−1 of oxytetracycline (OTC) as a typical antibiotic for 7, 14 and 21 days. The total proteins of E. fetida in each treatment were separated by two-dimensional gel electrophoresis and differential expressed proteins were identified by MALDI-TOF/TOF-MS. A total of 30 proteins were successfully identified and divided into four categories based on the function. It was surprisingly found that more than 50% of identified proteins belong to the actin family, and all of them were down-regulated more than 2.0-fold. In the meantime, the fibrinolytic enzymes, an important protease with plasminogen activator activity, were suppressed in the last two weeks. The validations in the mRNA level were performed using RT-PCR. However, due to the incomplete genome sequence of E. fetida, we failed to identify more proteins response to OTC stress. This study may provide a new insight into the discovery of novel biomarkers for continuous-poured and low-toxicity pollutants.
1 Introduction
As food additives or veterinary and human medicines, most of antibiotics are poorly retained in the stomach and intestine and cannot totally be absorbed by animals and human beings. According to some literature articles,1–3 about 40–90% of the antibiotics in their bodies could be excreted in faeces and urine in the forms of parent or metabolites. According to an investigation made by Lin,4 the concentration of tetracyclines in some agricultural soils from suburbs of Beijing and Tianjin was up to 119–307 mg kg−1 due to irrigation with domestic wastewater and application with pig feces and sewage sludge.5 Excessive antibiotics in the environment may potentially and adversely affect non-target organisms.6,7 Thus, more and more concerns have been paid to the toxicity and hazards of antibiotics in soil ecosystems with their wide application in agriculture, including disease therapy, health maintain, growth rate enhancement and production improvement of livestocks. Particularly, continually accumulated antimicrobials and their byproducts probably bring about the resistant bacteria which are discharged, fertilized or irrigated into waters, soils or other environmental media and allowed to enter the food chain.8 Although we can partially know the adverse effects of these pharmacologically active substances in soil ecosystems, their toxicity and hazards at the level of proteomics are still vague.In soil ecosystems, earthworms are identified as the key species of invertebrates and can indicate soil health and feedback contamination of terrestrial ecosystems.9 Increasingly, the earthworm species Eisenia fetida is suggested as the model organism for sensitive diagnosis of soil contamination, because they keep constantly exposed to the contaminated soil,10 which has been regarded as the standardization of toxicological experiments for industrial chemicals by the International Standard Organization (ISO).
Oxytetracycline (OTC) is a kind of widely used tetracycline antibiotics. Bao11 found that the concentration of OTC in soil and aquaculture industry sediments was up to 200 mg kg−1 and 285 mg kg−1, respectively. A few studies have disclosed toxic effects of OTC on the structure and function of soil microbial communities, the activity of enzymes and the growth of plants.12,13 Hu et al.14 investigated the distribution of antibiotic OTC resistance gene in the environment. However, few research reports are available on toxic effects of OTC on terrestrial organisms, especially on earthworm species at the level of proteomics.
In this study, the earthworm species Eisenia fetida was adopted as the model organism for examining the toxicity and hazards of OTC as a typical antibiotic at the level of proteomics. In particular, changes in the total protein expression profiles of the earthworm species under the stress of OTC would be analyzed comparatively. The results from this present study can undoubtedly help in understanding about toxicological mechanisms of antibiotics in soil ecosystems.
2 Materials and methods
2.1 Test animals and chemicals
The earthworms E. fetida, weighing between 300 and 600 mg, were purchased from the Earthworm Breeder Company in Tianjin, China. After transported to a laboratory without any physical stress, the worms were bred at ambient temperature of 20 ± 2 °C, using the peat moss as the bedding material. Worms were fed with commercial cattle manure once a week and the humidity of the soil was maintained around 70% by spraying purified water every 3 days. Adult worms with the weight of 400 ± 50 mg and fully developed clitellum were used for all the experiments. Oxytetracycline hydrochloride with more than 95% purity was purchased from Sigma-Aldrich and all other reagents were of analytical or molecular biology grade with the purity of 95–99%.2.2 Tested soils
Clean soils were collected from the surface layer (0–20 cm in depth) of a natural forest park in Tianjin, China, then air-dried and sieved with 2 mm mesh. The physical chemical properties and baseline concentration of OTC in the tested soil were listed in Table 1. Use the sterilized soil.2.3 Protein extraction
Total proteins were extracted based on the trichloroacetic acid-acetone (TCA-A) method previously described by Wang15 with a little modification. Briefly, fresh whole E. fetida samples (2 g) collected from three replications were pulverized to a fine powder with liquid nitrogen and a mortar and pestle. The powdered homogenate was placed in a 50 mL centrifuge tube and the protein was precipitated overnight with 8–10 times volume of ice-cold TCA-A solution containing 20% TCA (w/v) and 0.1% DTT (w/v). After centrifugation at 4 °C and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 40 min, the precipitate above was washed twice with 30 mL pre-chilled acetone solution containing 0.1% DTT (w/v) and once with 80% washing solution above. The resulting pellets were vacuum freeze dried until the remaining acetone was all removed, and then redissolved in lysis buffer [7 M urea, 2 M thiourea, 4% CHAPS (w/v), 65 mM DTT, and 0.5% (w/v) Bio-lyte (pH 4–6 and pH 5–7, Bio-rad, USA)]. The undissolved protein particulars in the lysis buffer were sonicated with the ultrasonic cell disruptor (JY92-II Ultrasonic Crasher, Ningbo Scientz Biotechnology Co., Ltd, China) at 4 °C and 300 W for 40 apm (actions per minute) and then centrifuged at 4 °C and 13
000 × g for 40 min, the precipitate above was washed twice with 30 mL pre-chilled acetone solution containing 0.1% DTT (w/v) and once with 80% washing solution above. The resulting pellets were vacuum freeze dried until the remaining acetone was all removed, and then redissolved in lysis buffer [7 M urea, 2 M thiourea, 4% CHAPS (w/v), 65 mM DTT, and 0.5% (w/v) Bio-lyte (pH 4–6 and pH 5–7, Bio-rad, USA)]. The undissolved protein particulars in the lysis buffer were sonicated with the ultrasonic cell disruptor (JY92-II Ultrasonic Crasher, Ningbo Scientz Biotechnology Co., Ltd, China) at 4 °C and 300 W for 40 apm (actions per minute) and then centrifuged at 4 °C and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 × g for 40 min. The supernatant was collected and the protein concentration was measured according to the Bradford's method using Coomassie Brilliant Blue G-250 (Bio-Rad, USA) and BSA as a standard.16 And then the protein samples were stored in 1.5 mL EP tubes in 100 μL aliquots at −80 °C.
500 × g for 40 min. The supernatant was collected and the protein concentration was measured according to the Bradford's method using Coomassie Brilliant Blue G-250 (Bio-Rad, USA) and BSA as a standard.16 And then the protein samples were stored in 1.5 mL EP tubes in 100 μL aliquots at −80 °C.
2.4 Two-dimensional electrophoresis (2-DE)
Isoelectric focusing (IEF) was carried out using immobilized pH gradient (IPG) gel strips (17 cm, pH 4–7; Bio-Rad, USA). And the dry gel strips were passively rehydrated for 8 h with 300 μL rehydration buffer containing about 2 mg of protein and focused on a PROTEAN IEF Cell (Bio-Rad, USA) at 20 °C. The condition performed was set at 50 V for 8 h to active rehydration and 200 V for 1 h slow (the build up mode of voltage), 1000 V for 1 h slow, 8000 V for 2 h linear then 8000 V for 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 V h (voltage × hours). Prior to the second dimension separation, the focused strips were equilibrated for 15 min in 6 mL equilibration solution (6 M urea, 2% SDS (w/v), 0.375 M pH 8.8 Tris–HCl and 20% glycerol (v/v)) with 2% (w/v) DTT. This is followed by a further 15 min equilibration using the same solution containing 2.5% (w/v) iodoacetamide (IAA) instead of DTT.
000 V h (voltage × hours). Prior to the second dimension separation, the focused strips were equilibrated for 15 min in 6 mL equilibration solution (6 M urea, 2% SDS (w/v), 0.375 M pH 8.8 Tris–HCl and 20% glycerol (v/v)) with 2% (w/v) DTT. This is followed by a further 15 min equilibration using the same solution containing 2.5% (w/v) iodoacetamide (IAA) instead of DTT.
The separation in the second dimension was performed with a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) containing 30% acrylamide (w/v), 1.5 M pH 8.8 Tris–HCl, 10% SDS (w/v), 10% ammonium persulphate (w/v) on a PROTEAN II xi Cell system (Bio-Rad, USA). Gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 and then destained.
2.5 Image scan and analysis
The gels incompletely destained by destaining solution containing 50% methanol (w/w) and 10% glacial acetic acid (w/w) were then washed with boiled deionized water until the background was clear. Stained gels were scanned by ScanMaker i800 (Microtek, Shanghai, China). Automated protein spots detection and matching were carried out using PDQuest 8.0.1 software (Bio-Rad, USA) with subsequent manual editing and modifying. Three well-resolved gels of each sample were chosen as the replicate groups. By the software, the ‘Analysis sets’ of each treatment groups were quantitatively and qualitatively processed. The spots included in the Boolean's intersection of exhibiting 1.50-fold increase or 0.67-fold decrease between the controlled groups and treated groups and statistically different at a level of p < 0.05 based on one-way ANOVA analysis were considered to be differentially expressed proteins. And the statistical significance was determined by one-way analysis of variance (ANOVA) using SPSS 20.0.2.6 In-gel digestion
Protein spots were excised with a diameter of 1–2 mm from the 2-D gels and transferred to a 500 μL Safe-lock tube (Eppendorf, Germany). The cut gels granules were washed twice for 10 min with 200 μL of Milli-Q water and then destained for 20 min in a mixture of 25 mM ammonium bicarbonate (NH4HCO3) and 50% acetonitrile (CH3CN) (w/w) until the gels were clear. The destaining solution should be blotted up before the dehydration of the gels by 100% acetonitrile (w/w). The protein spots were digested overnight with 5 ng μL−1 trypsin (Promega, USA) dissolved in 25 mM NH4HCO3 at 37 °C and then centrifuged. The supernatant was collected for the next step.2.7 Identification by mass spectrometry
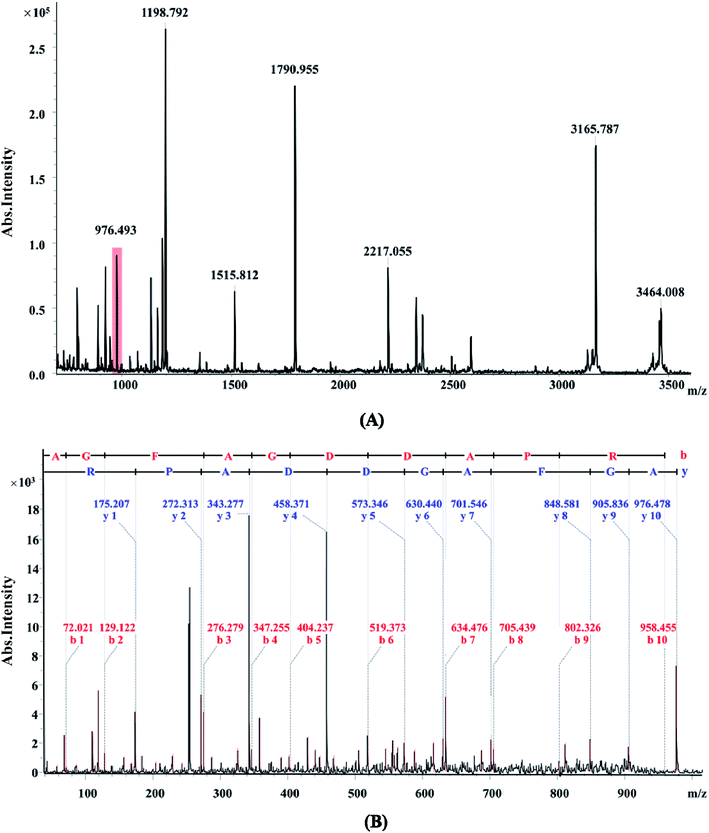
Firstly, 1 μL of peptide mixtures from the 2-DE gel spots were spotted on the AnchorChip target (Bruker Daltonics, Bremen, Germany) and then the air-dried sample was covered with 1 μL of α-cyano-4-hydroxycinnamic acid (HCCA) in 70% CH3CN (v/v) and 0.1% trifluoroacetic acid (TFA) (v/v) as a matrix. Analysis of mass spectra was carried out on the instrument Autoflex MALDI-TOF/TOF purchased from Bruker Daltonics (Bremen, Germany). Data acquisition was performed by Flex Control software 3.0 using PeptideCalibStandard II (Bruker Daltonics) as an external calibration the mass spectra were evaluated using Flex Analysis software. The protein matching using the peptide mass data were performed in the NCBInr database with the MASCOT search engine with the mass accuracy of 100 ppm and at most two peptide cleavage sites. The reliable identification results were selected considering high MASCOT score, maximum sequences coverage, and consistency of molecular weight (MW) and isoelectric point (pI) given by the database and the electropherogram. Function of the identified proteins was determined using the UniProt Knowledgebase (UniProtKB), which is a comprehensive resource for protein sequence and annotation data.2.8 Quantitative real-time PCR
Total RNA was extracted from 50 mg of six whole earthworm tissues using TRIzol® Reagent (Ambion®, Invitrogen, USA) and the extraction was repeated three times for each group (three biological replicates). RNA purity and integrity were checked by electrophoresis on 2% agarose gels (w/v) stained with ethidium bromide and by ensuring the absorbance ratios (OD260/OD280) were between 1.8 and 2.0. First strand cDNA was synthesized using GeneAmp® PCR System 9700 (Applied Biosystems, USA) with the TransScript® First-Strand cDNA Synthesis SuperMix (TransGen, China). Primer pairs of the E. fetida actin gene were designed using Gene Tool 1.0 Lite software according to the sequence of Lumbricus terrestris actin gene which has a high sequence similarity (95%) to that of E. fetida, and the β-actin gene, which was submitted by Brulle et al.,17 was selected as a reference gene (Table 2). Quantitative PCR reactions were performed in a StepOne Plus Real-Time PCR System (Applied Biosystems, USA) in triplicate wells following the instructions (1 cycle at 95 °C for 30 s and 40 amplification cycles at 95 °C for 5 s, 53 °C for β-actin/57 °C for actin 30 s, and 72 °C for 20 s). The mRNA expression level of actin gene and the E. fetida β-actin mRNA, selected as an internal reference gene, was determined in parallel for each group. The differential expression changes results were analyzed using the 2−ΔΔCT method of Livak and Schmittgen.183 Results and discussion
3.1 Identification of OTC respective proteins
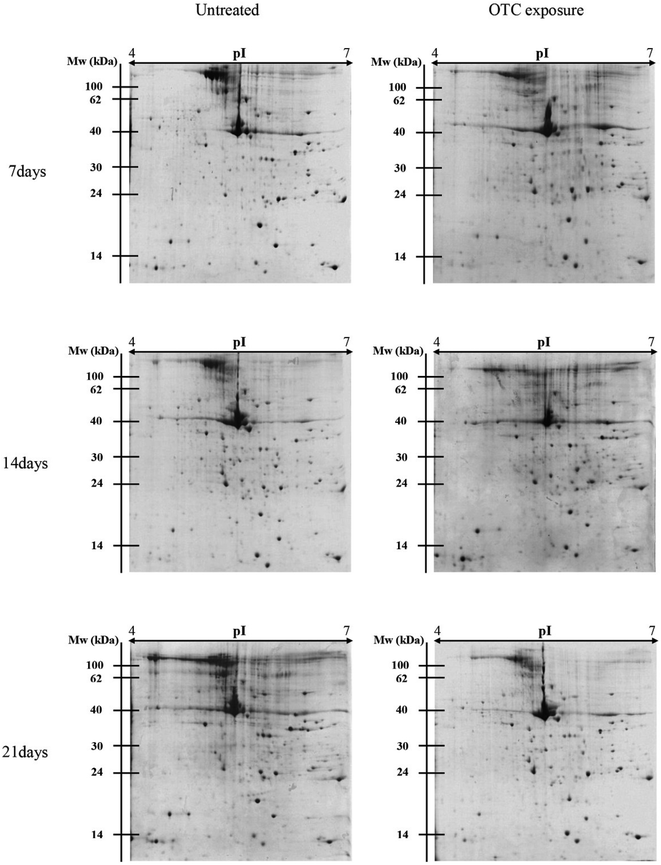
There were different protein profiles of the earthworm E. fetida between the control group and OTC-treated group at 7, 14 and 21 days (Fig. 1). On average, about 1200 protein spots could be detected on each 2-DE gel of the three replications. All of the protein spots from the earthworm E. fetida present a wide distribution on the gel between the isoelectric point range from 4 to 7 and an obvious molecular mass between 14 kDa and 100 kDa. The experimental MW and pI of the identified proteins were probably inconsistent with the theoretical values in the consequence of the post-translational modifications (PTMs) of the proteins. Analysis of the gels with PDQuest 8.0.1 software expounded the significant (p < 0.05) changes of protein spots between OTC-treated groups and control groups at least during one time point. Total 107 spots were significantly (p < 0.05 and more than 1.50-fold variation) regulated and subsequently 31 proteins were determined with MALDI-TOF/TOF-MS and MASCOT Database (Table 3). The identification results of spot 5555 are depicted in Fig. 2 as a representative. The function information of some identified proteins was evaluated by UniProtKB resource and their NCBI database accession numbers.| Spot no.a | NCBI GI. no. | Protein name (organism) | Theor. MW/pIb | Exp. MW/pIc | MASCOT scored | SCe (%) |
|---|---|---|---|---|---|---|
| a The spot numbers are acquired from the PDQuest 8.0.1 software during analysis of 2-D gels.b The theoretical MW and pI are offered by the MASCOT search engine.c The experimental molecular mass (kDa) and pI are estimated from 2-DE gels according to their relative position.d Proteins identified are listed with the MASCOT score as a criteria for MS or MS/MS identification.e SC is short for sequence coverage. | ||||||
| Actin family | ||||||
| 6453 | gi|3319951 | Actin (Helobdella triserialis) | 41.703/5.38 | 34.62/6.0 | 95 | 29 |
| 6306 | gi|3319951 | Actin (Helobdella triserialis) | 41.703/5.38 | 30.24/5.8 | 98 | 36 |
| 3344 | gi|397881222 | Actin (Lineidae sp. TWL-2008) | 42.132/5.30 | 25.14/5.0 | 121 | 39 |
| 4113 | gi|1707573 | Actin (Lumbricus terrestris) | 42.161/5.30 | 19.65/5.3 | 155 | 38 |
| 6003 | gi|3046400 | Actin 1 (Schmidtea polychroa) | 8.257/5.48 | 12.95/5.8 | 81 | 79 |
| 5363 | gi|358332531 | Actin beta/gamma 1 (Clonorchis sinensis) | 35.711/5.04 | 28.33/5.4 | 144 | 57 |
| 3642 | gi|405964580 | Actin, cytoplasmic (Crassostrea gigas) | 42.010/5.30 | 48.12/4.9 | 163 | 55 |
| 4014 | gi|3452277 | Beta actin (Pseudopleuronectes americanus) | 14.537/5.28 | 13.26/5.4 | 84 | 60 |
| 9002 | gi|3452277 | Beta actin (Pseudopleuronectes americanus) | 14.537/5.28 | 13.81/6.6 | 79 | 43 |
| 5260 | gi|197320840 | Beta-actin (Gallus gallus) | 10.298/6.17 | 24.00/5.5 | 107 | 79 |
| 5555 | gi|2829750 | RecName: Full = actin (Lumbricus rubellus) | 41.582/5.46 | 45.50/5.6 | 159 | 40 |
| 5749 | gi|2829750 | RecName: Full = actin (Lumbricus rubellus) | 41.582/5.46 | 60.68/5.7 | 189 | 54 |
| 4117 | gi|2492669 | RecName: Full = actin, cytoskeletal 3; AltName: Full = LPC3 (Lytechinus pictus) | 19.652/5.78 | 16.88/5.3 | 107 | 34 |
| 6313 | gi|1703136 | RecName: Full = actin, cytoskeletal; AltName: Full = M; flags: precursor (Heliocidaris erythrogramma) | 42.063/5.30 | 32.36/5.9 | 134 | 42 |
| 4403 | gi|1703137 | RecName: Full = actin, cytoskeletal; AltName: Full = M; flags: precursor (Heliocidaris erythrogramma) | 42.077/5.30 | 37.40/5.2 | 97 | 37 |
| 5024 | gi|27883553 | Alpha actin (Ictalurus punctatus) | 16.770/5.28 | 14.10/5.7 | 92 | 60 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Metabolism | ||||||
| 8401 | gi|16660643 | Fibrinolytic enzyme (Eisenia fetida) | 20.221/4.59 | 34.10/6.2 | 80 | 31 |
| 6203 | gi|110341195 | Fibrinolytic protease 0 (Eisenia fetida) | 23.588/5.31 | 24.79/5.8 | 88 | 40 |
| 9323 | gi|220924493 | GTP-binding protein EngA (Methylobacterium nodulans ORS 2060) | 48.460/8.66 | 30.29/6.8 | 106 | 18 |
| 1107 | gi|61657939 | Myosin heavy chain, skeletal muscle, adult (Gallus gallus) | 224.010/5.63 | 17.66/4.7 | 101 | 12 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Hypothetical and predicted proteins | ||||||
| 4301 | gi|268531882 | Hypothetical protein CBG02838 (Caenorhabditis briggsae) | 17.058/4.54 | 30.37/5.2 | 81 | 34 |
| 5558 | gi|436835559 | Hypothetical protein FAES_2173 (Fibrella aestuarina BUZ 2) | 7.091/6.07 | 38.70/5.7 | 93 | 50 |
| 5750 | gi|332017101 | Hypothetical protein G5I_14087 (Acromyrmex echinatior) | 150.374/8.67 | 64.18/5.5 | 92 | 22 |
| 3434 | gi|395827452 | Predicted: ATP-dependent zinc metalloprotease YME1L1-like (Otolemur garnettii) | 80.347/8.98 | 36.21/5.0 | 96 | 27 |
| 5364 | gi|530606293 | Predicted: dnaJ homolog subfamily C member 25-like (Chrysemys picta bellii) | 42.272/9.10 | 25.98/5.6 | 84 | 33 |
| 6112 | gi|507644426 | Predicted: dolichyl-phosphate beta-glucosyltransferase isoform X2 (Octodon degus) | 33.609/9.34 | 20.55/5.8 | 84 | 33 |
| 3346 | gi|514704523 | Predicted: interleukin-17B isoform X3 (Anas platyrhynchos) | 21.123/9.35 | 29.33/4.8 | 90 | 50 |
| 2666 | gi|488526628 | Predicted: low quality protein: vinculin (Dasypus novemcinctus) | 104.217/5.38 | 54.93/4.7 | 123 | 22 |
| 6550 | gi|488510571 | Predicted: protein phosphatase 1B isoform 2 (Dasypus novemcinctus) | 26.106/5.11 | 38.88/5.9 | 83 | 32 |
| 7006 | gi|498969699 | Predicted: restin homolog isoform X7 (Ceratitis capitata) | 202.861/4.99 | 14.16/6.1 | 94 | 19 |
| 5191 | gi|512836262 | Predicted: torsin-1A-interacting protein 2 isoform X9 (Heterocephalus glaber) | 15.666/6.88 | 16.75/5.4 | 80 | 35 |
3.2 Changes of protein expression under OTC stress
To avoid the errors caused by the experiment operation and biological variances, the identified proteins would be considered if there were more than 1.50 times regulation (up-regulation or down-regulation) of protein expression at one or more time point between the control groups and the OTC-stressed groups at a significant level (p < 0.05). Totally, 107 spots satisfied the condition and were cut down from all the gels to be analyzed by MALDI-TOF/TOF-MS. By the use of Biotools 3.0 (Bruker, Daltonics) and MASCOT search engine with NCBI database, 5 spots were identified by matching the sequence of amino acids with 31–54% sequence coverage from the earthworm species. Due to the genome of earthworms was not fully sequenced, so most of proteins were identified from other organisms. The identified proteins could be divided into three categories according to their function, among them, surprisingly, more than 50% of identified proteins belong to the actin family which suggested the OTC exposure increased the cytoskeleton degradation in vivo.After a 7 day exposure to 500 mg kg−1 of OTC, 23 proteins were up-regulated while 7 proteins were down-regulated. However, the situation reversed when there were 22 and 20 proteins down-regulated after a 14 and 21 day OTC exposure, respectively, which might suggest a stress-adaptation-compensation mode if we investigate as long as enough. There were both 7 proteins up-regulated at the last two time points. The cytoskeletal protein actin (spot 4117) was the most up-regulated (3.46-fold) after a 7 day exposure, while the myosin heavy chain protein was the most down-regulated (−11.99-fold) after a 14 day exposure.
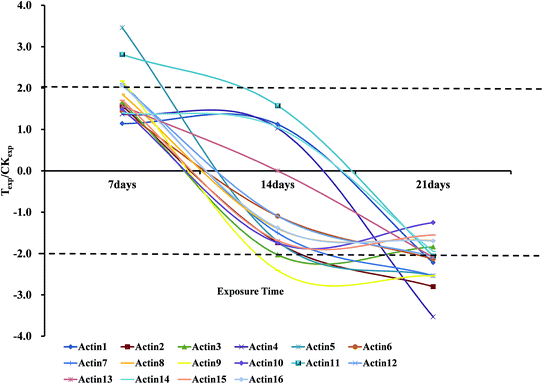
We also found that the regulation of some spots from different position on the gel (with different MW and pI) showed the same changing trends. The combined results of 2-DE and MALDI-TOF-MS showed that 16 spots were identified as the actin protein (or actin-like fragments) in Table 4, although 3 of them were annotated as earthworm actin proteins, both gene and protein sequences of the other actin proteins had a quite high similarity of more than 95%. Most of these actin proteins were nearly intact proteins, but a few of them seemed to be the fragments of actin. All of the actin proteins were up-regulated and 11 of them increased more than 1.50-fold compared with the normal group after 7 days exposure to OTC, and among them about 50% increased more than 2.00-fold of normal group. However, on the 14th day, the expressions of the 16 actin proteins consistently decreased. Moreover, 11 out of 16 actin proteins showed the obviously down-regulation. At the last exposure time point of 21 days, expression of almost all the actin proteins were down-regulated relative to the control group shown in Fig. 3, and the most down-regulation was 3.53-fold (Spot 4113).
| Spot no. | Exposure time (day) | ||
|---|---|---|---|
| 7 | 14 | 21 | |
| a 1.5 fold < the protein expression changes < 2.0 fold.b 2.0 fold < the protein expression changes < 3.0 fold.c The protein expression changes > 3.0 fold. | |||
| 5555 | 1.14 | 1.12 | −2.22b |
| 5363 | 1.57a | −1.74a | −2.80b |
| 5260 | 1.65a | −2.03b | −1.84a |
| 4113 | 1.37 | 1.03 | −3.53c |
| 4117 | 3.46c | −1.71a | −2.54b |
| 5024 | 1.45 | −1.09 | −2.13b |
| 4014 | 2.11b | −1.50a | −2.54b |
| 6003 | 1.85a | −1.40 | −1.69a |
| 6453 | 2.17b | −2.41b | −2.54b |
| 6306 | 1.49 | −1.74a | −1.25 |
| 3642 | 2.81b | 1.57a | −2.06b |
| 4403 | 2.06b | −1.09 | −2.07b |
| 5749 | 1.57a | 0.00 | −2.09b |
| 9002 | 1.40 | 1.05 | −1.95a |
| 6313 | 1.70a | −1.69a | −1.55a |
| 3344 | 2.07b | −1.38 | −1.70a |
3.3 mRNA level expression changes of actin genes
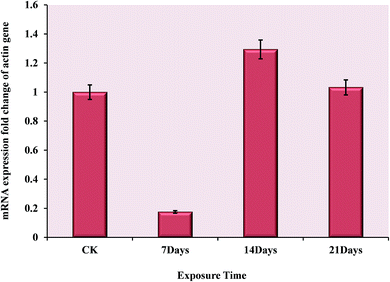
To investigate the changes in mRNA level during the OTC stress and validate the novel interesting changes that so many actin proteins all down-regulated, quantitative PCR of three biological replicates was performed. The sequence of forward and reverse primers are listed in Table 2. The result indicated that the mRNA–protein expression correlation was relatively inconsistent at the same time point. Comparing with the normal group, the actin gene was very significantly down-regulated at 7 days, but it turned to be up-regulated after exposure to OTC for another week. Finally, at 21 days, its expression returned to the normal level shown in Fig. 4. There may be spatial–temporal differences in transcription and translation of the actin genes caused by complicated biological processes, such as transcriptional splicing, post-transcriptional splicing, translational modifications and translational regulation.4 Discussion
Many studies have focused on physiological mechanisms including cellular structure, DNA damage and enzyme activity variation of earthworms under the stress of pollutants.4,19–22 In recent years, Wang et al.10 investigated effects of cadmium (Cd) on earthworms at the level of proteomics. However, the effect of antibiotics on earthworms has not been reported at the level of proteomics. Thus, protein expression profiles of the earthworm E. fetida exposed to OTC was analyzed as a demonstration by the two-dimensional gel electrophoresis. Totally, 107 protein spots with a significant (p < 0.05) variation (1.50-fold) between the control and the OTC-stressed groups during at least one time point were identified using the MALDI-TOF/TOF-MS. It indicated that almost of the proteins were up-regulated after a 7 day exposure and then down-regulated after a longer exposure.Actin is a natural and abundant protein. It is responsible for a wide range of cellular activities including cytoplasmic streaming, cell transport, and cell division. It comprises more than 12–15% of the total proteins in motile cells or cells which exhibit cytoplasmic streaming, and accounts for about 30% of muscle cell proteins. Even in nonmotile cells such as red blood cells, actin comprises about 1–2% of the cytoskeletal proteins.23 Actin is present in all eukaryotic cells, and most of the organisms have several genes encoding this protein.24
Oxidative stress routinely caused by cellular processes, xenobiotic intoxication or exposure to metals was an imbalance between reactive oxygen species (ROS) production and the antioxidant defence systems.25 The cytoskeletal protein composed of actin protein was reported as a target for oxidative stress. Proteomic study of four model contaminants (Aroclor 1254, Cu2+, tributyltin and As3+) stress on the clam Chamaelea gallina found that two actin proteins of different species with high homology were down-regulated, and one of them was identified as a actin-like fragment.24 Coincidentally, Cu2+ exposure of the mussels caused a severe perturbation and rearrangement in Mytilus galloprovincialis haemocyte actin, and the phalloidin staining showed that cell morphology was altered including the disappearance of filamentous actin and the disorganization of actin cortical meshwork, due to the carbonylation and glutathionylation of the actin in order to response to the oxidization-altered state.26,27 The similar results were also achieved when the E. fetida was exposed to 80 mg kg−1 Cd that the intermediate filament protein, which consist of actin monomer, significantly down-regulated at 21 days exposure.10 As well, the genes expression results showed that the intermediate filament protein gene was down-regulated when exposed to B(a)P, Cd and PCP.28
During exposed to OTC, it could be observed that the outer layer of the earthworms came to be soft and festered after 14 and/or 21 days exposure, and the activity of worms in the stressed group were obviously weakened compared with the normal group. Maybe, the perturbation of water29 and calcium ion30 homeostasis in the worms caused by the oxidative damage, through the xenobiotics interacting with the membrane receptors trigger a signaling pathway, then the activated inositol-triphosphate (IP3) released from the pathway subsequently triggers the release of the intracellular storaged calcium ions. As a result, the increase in cytoplasmic free Ca2+ concentration guides reorganisation of cytoskeletal proteins, mainly actin and tubulin. High free Ca2+ concentrations activate some specific protein-kinases phosphorylating cytoskeletal proteins, and calmodulin-dependent cytoskeletal phosphodiesterases and phosphatases, which could hydrolyze the actin filaments and then impair cell morphology and motility.31
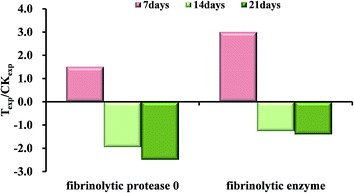
Fibrinolytic enzyme (Spot 8401) and fibrinolytic protease 0 (Spot 6203) were found to be significantly (p < 0.05 and fold change > 1.50) up-regulated at 7 days, but they both turned to be down-regulated during the following two-week exposure to OTC shown in Fig. 5. Earthworm fibrinolytic enzymes (EFEs) also referred to as lumbrokinases (LK), are a group of alkaline serine proteases with both strong plasminogen activator activity and direct fibrin-degrading activity.32–34 The changes were possibly caused by the prevention mechanism against the stress activated by xenobiotics just like high-concentration OTC, or the oxidative injury caused by the toxicity of the antibiotics. In previous study, earthworm fibrinolytic enzymes could decrease the pulmonary fibrosis induced by bleomycin in rat.35 Maybe, we speculated that the high-concentration OTC stress, to some extent, led to the formation of blood clot or the tissue fibrosis in the early days. As a result, the expressions of fibrinolytic proteases were induced for hydrolysis to repair and remodel the damaged tissue or cytoskeletal structure discussed above.
As a result of the validation test, at all the exposure time points, the contrary expression changes were shown in mRNA level (Fig. 4) comparing with protein level (Fig. 3). Presumably, the toxicity of OTC was not so strong as heavy metal, polycyclic aromatic hydrocarbon (PAHs), polychlorinated biphenyl (PCBs) or other organic pollutants for earthworm, which was harmed only by contacting or eating the contaminated soil. In contact with the high concentration OTC, the outer layer of the earthworm was damaged on the mechanism discussed above. These may cause the oxidative damage in the outer layer cells during the first seven days, which suppressed the expression of actin genes. Subsequently, the body tissue ‘sensed’ the damage, such as degradation of cytoskeletal proteins, and enhanced expression of the actin genes for repair at the phase of 14th day. Finally, the long-term stress may cause an irreversible damage on gene, so 21 days later, the mRNA expression started to decrease with respect to 14th day.
Previous studies of our group21,36 showed that tetracycline antibiotics could cause the breakage of earthworm Eisenia fetida DNA by a comet assay, and with an positive dose-response relationship, the changes in antioxidant enzymes (CAT and SOD) activities and DNA damage were observed in earthworms exposed to 300 mg kg−1 of chlortetracycline and tetracycline. But enzymes activity was confirmed as an unreliable, susceptible and poorly repeatable indicator. In this study, it's the first time to investigate the expression changes of earthworms on the antibiotics (oxytetracycline) stress in the proteome level using the 2-DE and MALDI-TOF/TOF-MS technology. The results suggested that unlike the heavy metal, PAHs and PCBs with a relatively high values of LC50/EC50, toxicity of OTC with a concentration in field and soil environment, as a widely used veterinary, couldn't make a significant adverse effect on the earthworms' important physiological and biochemical process in short or medium term exposure. However, we found that 500 mg kg−1 of OTC caused the significant changes of cytoskeletal proteins which were discovered due to the sensibility of the proteomics. The injury of cellular structure and the alteration of membrane permeability may directly expose the earthworms to the contaminated surroundings without sound membrane systems protection. In total, all the previous and current studies results indicated that the cytoskeletal and membrane proteins of some invertebrate, which were characterized by their soft bodies and living in physically contact with environment, like earthworm and molluscs, were refered to as the first mainly protective barrier to toxicants. So they should be the most sensitive as both targets and biomarkers. The future studies should focus on the mechanism and pathway of the cell structure damage caused by the pollutants with low dose and low toxicity. In addition, various contaminants may be existed simultaneously in realistic environments, the proteomic analysis of combined effects of pollutants mixture should be equally investigated.
5 Conclusions
This study investigated the toxicity and hazards of earthworms exposed to soil containing OTC, and changes in the total protein expression profiles of the earthworm species under the stress of OTC. A total of 30 proteins were successfully identified and divided into four categories based on the function. More than 50% of identified proteins response to OTC stress belong to the actin family. All of the actin proteins were significantly up-regulated at 7 days, but down-regulated during the following two-week and the fibrinolytic enzyme and fibrinolytic protease were induced as the same trend, which might suggest a stress-adaptation-compensation mode. Meanwhile, it could be observed that the outer layer of the earthworms came to be soft and festered after 14 and/or 21 days exposure. This study may provide an new insight into the prevention mechanism against the stress activated by xenobiotics just like high-concentration OTC, or the oxidative injury caused by the toxicity of the antibiotics.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (No. 21477163 and No. 21037002).References
- B. N. N. S. Halling-Sørensen, P. F. Lanzky, F. Ingerslev, H. C. Holten Lüzhøft and S. E. Jørgensen, Occurrence, fate and effects of pharmaceutical substances in the environment- A review, Chemosphere, 1998, 36, 357–393, DOI:10.1016/s0045-6535(97)00354-8.
- P. K. Jjemba, The potential impact of veterinary and human therapeutic agents in manure and biosolids on plants grown on arable land:a review, Agric., Ecosyst. Environ., 2002, 93, 267–278, DOI:10.1016/s0167-8809(01)00350-4.
- I. Phillips, M. Casewell, T. Cox, B. De Groot, C. Friis, R. Jones, C. Nightingale, R. Preston and J. Waddell, Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data, J. Antimicrob. Chemother., 2004, 53(1), 28–52, DOI:10.1093/jac/dkg483.
- D. Lin, Q. Zhou, Y. Xu, C. Chen and Y. Li, Physiological and molecular responses of the earthworm (Eisenia fetida) to soil chlortetracycline contamination, Environ. Pollut., 2012, 171, 46–51, DOI:10.1016/j.envpol.2012.07.020.
- X. Xie, Q. Zhou, Q. Bao, Z. He and Y. Bao, Genotoxicity of tetracycline as an emerging pollutant on root meristem cells of wheat (Triticum aestivum L.), Environ. Toxicol., 2011, 26(4), 417–423, DOI:10.1002/tox.20567.
- Y. G. Zhu, Soil science in the understanding of the security of food systems for health, Asia Pac. J. Clin. Nutr., 2009, 18(4), 516–519 Search PubMed.
- M. Isidori, M. Lavorgna, A. Nardelli, L. Pascarella and A. Parrella, Toxic and genotoxic evaluation of six antibiotics on non-target organisms, Sci. Total Environ., 2005, 346(1–3), 87–98, DOI:10.1016/j.scitotenv.2004.11.017.
- K. Kummerer, Significance of antibiotics in the environment, J. Antimicrob. Chemother., 2003, 52(1), 5–7, DOI:10.1093/jac/dkg293.
- E. J. LaCourse, M. Hernandez-Viadel, J. R. Jefferies, C. Svendsen, D. J. Spurgeon, J. Barrett, A. J. Morgan, P. Kille and P. M. Brophy, Glutathione transferase (GST) as a candidate molecular-based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus, Environ. Pollut., 2009, 157(8–9), 2459–2469, DOI:10.1016/j.envpol.2009.03.015.
- X. Wang, L. Chang, Z. Sun, Y. Zhang and L. Yao, Analysis of earthworm Eisenia fetida proteomes during cadmium exposure: an ecotoxicoproteomics approach, Proteomics, 2010, 10(24), 4476–4490, DOI:10.1002/pmic.201000209.
- Y. Y. Bao, Environmental Behavior and Eco-toxicity of Tetracycline Antibiotics in Soils. Postdoctoral Research Report, Nankai University, Tianjin, China, 2008 Search PubMed.
- W. D. Kong, Y. G. Zhu, B. J. Fu, P. Marschner and J. Z. He, The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community, Environ. Pollut., 2006, 143, 129–137, DOI:10.1016/j.envpol.2005.11.003.
- F. Liu, G. G. Ying, R. Tao, J. L. Zhao, J. F. Yang and L. F. Zhao, Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities, Environ. Pollut., 2009, 157(5), 1636–1642, DOI:10.1016/j.envpol.2008.12.021.
- J. Hu, J. Shi, H. Chang, D. Li, M. Yang and Y. Kamagata, Phenotyping and Genotyping of Antibiotic-Resistant Escherichia coli Isolated from a Natural River Basin, Environ. Sci. Technol., 2008, 42, 3415–3420, DOI:10.1021/es7026746.
- X. Wang, L. Chang, G. Wang, Z. Sun, H. Ma, Q. Sun and J. Li, Protein extraction from the earthworm Eisenia fetida for 2-DE, Proteomics, 2010, 10(5), 1095–1099, DOI:10.1002/pmic.200900488.
- M. M. Bradford, A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem., 1976, 72, 248–254, DOI:10.1016/0003-2697(76)90527-3.
- F. Brulle, G. Mitta, C. Cocquerelle, D. Vieau, S. Lemiere, A. Lepretre and F. vandenBulcke, Cloning and real-time PCR testing of 14 potential biomarkers in Eisenia fetida following cadmium exposure, Environ. Sci. Technol., 2006, 40(8), 2844–2850, DOI:10.1021/es052299x.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods, 2001, 25(4), 402–408, DOI:10.1006/meth.2001.
- J. Rombke, K. A. Krogh, T. Moser, A. Scheffczyk and M. Liebig, Effects of the veterinary pharmaceutical ivermectin on soil invertebrates in laboratory tests, Arch. Environ. Contam. Toxicol., 2010, 58(2), 332–340, DOI:10.1007/s00244-009-9414-8.
- B. Wen, R. Huang, P. Wang, Y. Zhou, X. Q. Shan and S. Zhang, Effect of complexation on the accumulation and elimination kinetics of cadmium and ciprofloxacin in the earthworm Eisenia fetida, Environ. Sci. Technol., 2011, 45(10), 4339–4345, DOI:10.1021/es104034g.
- L. Dong, J. Gao, X. Xie and Q. Zhou, DNA damage and biochemical toxicity of antibiotics in soil on the earthworm Eisenia fetida, Chemosphere, 2012, 89(1), 44–51, DOI:10.1016/j.chemosphere.2012.04.010.
- A. J. Baguer, J. Jensen and P. H. Krogh, Effects of the antibiotics oxytetracycline and tylosin on soil fauna, Chemosphere, 2000, 40, 751–757, DOI:10.1016/s0045-6535(99)00449-x.
- M. Kekic and C. G. dos Remedios, Electrophoretic monitoring of pollutants: Effect of cations and organic compounds on protein interactions monitored by native gel electrophoresis, Electrophoresis, 1999, 20(10), 2053–2058, DOI:10.1002/(sici)1522-2683(19990701)20:10<2053::aid-elps2053>3.0.co.
- M. J. Rodríguez-Ortega, B. E. Grøsvik, A. Rodríguez-Ariza, A. Goksøyr and J. López-Barea, Changes in protein expression profiles in bivalve molluscs (Chamaelea gallina) exposed to four model environmental pollutants, Proteomics, 2003, 3(8), 1535–1543, DOI:10.1002/pmic.200300491.
- I. Dalle-Donne, R. Rossi, A. Milzani, P. Di Simplicio and R. Colombo, The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself, Free Radicals Biol. Med., 2001, 31(12), 1624–1632 CrossRef CAS PubMed.
- B. McDonagh, R. Tyther and D. Sheehan, Carbonylation and glutathionylation of proteins in the blue mussel Mytilus edulis detected by proteomic analysis and Western blotting: Actin as a target for oxidative stress, Aquat. Toxicol., 2005, 73(3), 315–326, DOI:10.1016/j.aquatox.2005.03.020.
- B. McDonagh and D. Sheehan, Effects of oxidative stress on protein thiols and disulphides in Mytilus edulis revealed by proteomics: actin and protein disulphide isomerase are redox targets, Mar. Environ. Res., 2008, 66(1), 193–195, DOI:10.1016/j.marenvres.2008.02.069.
- S. O. Agbo, J. Lemmetyinen, M. Keinanen, S. Keski-Saari, J. Akkanen, M. T. Leppanen, Z. Wang, H. Wang, D. A. Price and J. V. Kukkonen, Response of Lumbriculus variegatus transcriptome and metabolites to model chemical contaminants. Comparative biochemistry and physiology, CBP, Part C: Toxicol. Pharmacol., 2013, 157(2), 183–191, DOI:10.1016/j.cbpc.2012.11.005.
- Y. Noda, S. Horikawa, Y. Katayama and S. Sasaki, Water channel aquaporin-2 directly binds to actin, Biochem. Biophys. Res. Commun., 2004, 322(3), 740–745, DOI:10.1016/j.bbrc.2004.07.195.
- A. Gómez-Mendikute, A. Etxeberria, I. Olabarrieta and M. P. Cajaraville, Oxygen radicals production and actin filament disruption in bivalve haemocytes treated with benzo(a)pyrene, Mar. Environ. Res., 2002, 54(3–5), 431–436, DOI:10.1016/s0141-1136(02)00177-0.
- F. Cima and L. Ballarin, Tributyltin induces cytoskeletal alterations in the colonial ascidian Botryllus schlosseri phagocytes via interaction with calmodulin, Aquat. Toxicol., 2000, 48(4), 419–429, DOI:10.1016/s0166-445x(99)00064-8.
- I. H. Cho, E. S. Choi, H. G. Lim and H. H. Lee, Purification and characterization of six fibrinolytic serine-proteases from earthworm Lumbricus rubellus, J. Biochem. Mol. Biol., 2004, 37(2), 199–205, DOI:10.5483/Bmbrep.2004.37.2.199.
- F. Wang, C. Wang, M. Li, L. Gui, J. Zhang and W. Chang, Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida, Biotechnol. Lett., 2003, 25(13), 1105–1109, DOI:10.1023/a:1024196232252.
- Q. Q. Bi, J. X. Chu, Y. L. Feng, Z. Q. Jiang, B. Q. Han and W. S. Liu, Purification and Characterization of a New Serine Protease with Fibrinolytic Activity from the Marine Invertebrate, Urechis unicinctus, Appl. Biochem. Biotechnol., 2013, 170(3), 525–540, DOI:10.1007/s12010-013-0168-4.
- Y. Xue, H. P. Dai, A. Cui, S. J. Niu and B. S. Pang, Effects of aerosolized earthworm fibrinolytic enzyme on bleomycin induced pulmonary fibrosis in rats, Zhonghua yi xue za zhi, 2012, 92(48), 3429–3433, DOI:10.3760/cma.j.issn.0376-2491.2012.48.013.
- H. Z. Wang, Y. Luo, W. Q. Xu, Q. X. Zhou, B. H. Tang and Y. Y. Wang, Ecotoxic Effects of Tetracycline and Chlortetracycline on Aquatic Organisms, J. Agro-Environ. Sci., 2008, 27(4), 1536–1539 CAS.
| This journal is © The Royal Society of Chemistry 2019 |