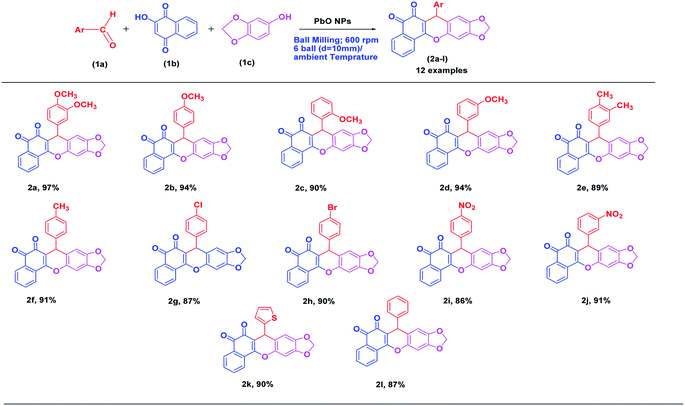
Open Access Article
Open Access ArticleMesoporous PbO nanoparticle-catalyzed synthesis of arylbenzodioxy xanthenedione scaffolds under solvent-free conditions in a ball mill†
Trimurti L.
Lambat
 *a,
Ratiram G.
Chaudhary
*a,
Ratiram G.
Chaudhary
 b,
Ahmed A.
Abdala
c,
Raghvendra Kumar
Mishra
d,
Sami
H. Mahmood
*e and
Subhash
Banerjee
*f
b,
Ahmed A.
Abdala
c,
Raghvendra Kumar
Mishra
d,
Sami
H. Mahmood
*e and
Subhash
Banerjee
*f
aDepartment of Chemistry, Manoharbhai Patel College of Arts, Commerce & Science, Deori, Gondia 441901, Maharashtra, India. E-mail: lambatges@gmail.com; Tel: +917972047470
bPost Graduate Department of Chemistry, S. K. Porwal College, Kamptee 441001, India
cChemical Engineering Programme, Texas A & M University at Qatar, POB 23784, Doha, Qatar
dIMEDA Materials, Technogetafe, Calle Eric Kandel, 2, 28906 Getafe, Madrid, Spain
eDepartment of Physics, The University of Jordan, Amman 11942, Jordan
fDepartment of Chemistry, Guru Ghasidas Vishwavidyalaya, Bilaspur, 495009, Chhattisgarh, India. E-mail: ocsb2006@gmail.com
First published on 30th September 2019
Abstract
A protocol for the efficient synthesis of arylbenzodioxy xanthenedione scaffolds was developed via a one-pot multi-component reaction of aromatic aldehydes, 2-hydroxy-1,4-naphthoquinone, and 3,4-methylenedioxy phenol using mesoporous PbO nanoparticles (NPs) as a catalyst under ball milling conditions. The synthesis protocol offers outstanding advantages, including short reaction time (60 min), excellent yields of the products (92–97%), solvent-free conditions, use of mild and reusable PbO NPs as a catalyst, simple purification of the products by recrystallization, and finally, the use of a green process of dry ball milling.
Recently, the ball milling technique has received great attention as an environmentally benign strategy in the context of green organic synthesis.1a The process of “ball milling” has been developed by adding mechanical grinding to the mixer or shaker mills. The ball milling generates a mechanochemical energy, which promotes the rupture and formation of the chemical bonds in organic transformations.1b Subsequently, detailed literature1c and books on this novel matter have been published.2a,b Several typical examples include carbon–carbon and carbon–heteroatom bond formation,2c organocatalytic reactions,2d oxidation by using solid oxidants,2e dehydrogenative coupling, asymmetric, and peptide or polymeric material synthesis, which have been reported under ball milling conditions.2e Hence, the organic reactions using ball milling activation carried out under neat reaction environments, exhibit major advantages,2f including short reaction time, lower energy consumption, quantitatively high yields and superior safety with the prospective for more improvement than the additional solvent-free conditions and clear-cut work-up.3–5
On the other hand, the organic transformations using metal and metal oxide nanoparticles6 are attracting enormous interest due to the unique and interesting properties of the NPs.7,8,9a Particularly, PbO NPs9b provide higher selectivity in some organic reactions9c and find applications in various organic reactions, like Paal–Knorr reaction,10 synthesis of diethyl carbonate,11 phthalazinediones,12 disproportionation of methyl phenyl carbonate to synthesize diphenyl carbonate,13 the capping agent in organic synthesis, and selective conversion of methanol to propylene.14 In addition, the PbO NPs are also used in many industrial materials.15,16
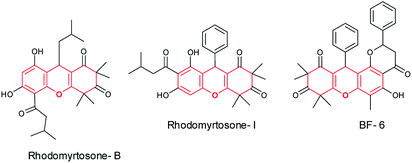
However, till date, PbO NPs have not been explored in MCRs leading to biologically important scaffolds. Among others, the xanthene scaffolds17 are one of the important heterocyclic compounds18 and are extensively used as dyes, fluorescent ingredients for visual imaging of the bio-molecules, and in optical device technology because of their valuable chemical properties.19 The xanthene molecules have conjointly been expressed for their antibacterial activity,20 photodynamic medical care, anti-inflammatory drug impact, and antiviral activity. Because of their various applications, the synthesis of these compounds has received a great deal of attention.21 Similarly, vitamin K nucleus22,23 shows a broad spectrum of biological properties, like anti-inflammatory, antiviral, antiproliferative, antifungal, antibiotic, and antipyretic.24a As a consequence, a variety of strategies24b have been demonstrated in the literature for the synthesis of xanthenes and their keto derivatives, like rhodomyrtosone-B,25a rhodomyrtosone-I,25b and BF-6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25c as well as their connected bioactive moieties. Few biologically active xanthene scaffolds are shown in (Fig. 1).
25c as well as their connected bioactive moieties. Few biologically active xanthene scaffolds are shown in (Fig. 1).
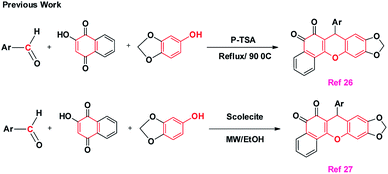
Due to the significance of these compounds, the synthesis of xanthenes and their keto derivatives using green protocols is highly desirable. Reported studies reveal that these scaffolds are synthesized by three-component condensations using p-TSA26 and scolecite27 as catalysts. However, these methods suffer from the use of toxic acidic catalysts like p-TSA, long reaction times (3 h), harsh refluxing26 or microwave reaction conditions,27 and tedious work-up procedures. The previously reported methods for the synthesis of xanthenediones are shown in Scheme 1.
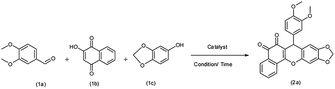
Herein, we report an economical and facile multicomponent protocol, using ball milling, for the synthesis of 7-aryl-6H-benzo[H][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione using PbO NPs as a heterogeneous catalyst (Scheme 2). The PbO NPs are non-corrosive, inexpensive, and easily accessible.
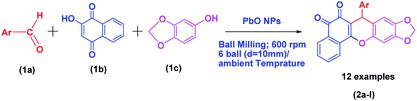 | ||
| Scheme 2 General reaction scheme of PbO NP-catalyzed synthesis of the xanthenedione scaffolds under ball milling conditions. | ||
In our protocol,28 the PbO NPs were initially prepared by mixing sodium dodecyl sulphate (2.5 mmol) and sodium hydroxide (10 mL, 0.1 N) with an aqueous methanolic solution of lead nitrate (2 mmol) under magnetic stirring at 30 °C by continuing the reaction for 2 h. Then, the obtained white polycrystalline product was filtered, washed with H2O, and dried at 120 °C, followed by calcination at 650 °C for 2 h. During this step, the white PbO NPs turned pale yellow in colour. Eventually, the synthesized PbO was then characterized by spectroscopic and analytical techniques.
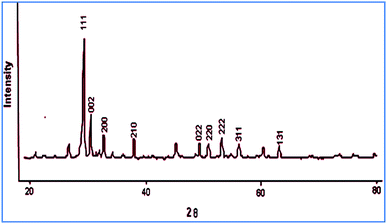
The powder X-ray diffraction (XRD) pattern revealed the crystalline nature of the PbO NPs as the diffraction peaks corresponding to (131), (311), (222), (022), (210), (200), (002), and (111) crystal planes were identified (Fig. 2). The XRD outline of the synthesized PbO NPs was further established for the formation of space group Pca21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 with a single orthorhombic structure (JCPDS card number 76-1796). The sharp diffraction peaks indicated good crystallinity, and the average particle size of the PbO NPs was estimated to be 69 nm, as calculated using the Debye–Scherer equation.
29 with a single orthorhombic structure (JCPDS card number 76-1796). The sharp diffraction peaks indicated good crystallinity, and the average particle size of the PbO NPs was estimated to be 69 nm, as calculated using the Debye–Scherer equation.
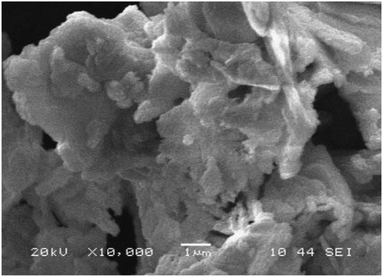
The surface morphology of the PbO NPs was analyzed by scanning electron microscopy (SEM), and the SEM image revealed the discrete and spongy appearance of the PbO NPs (Fig. 3).
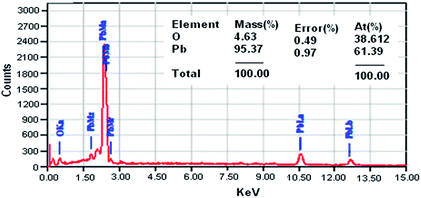
Moreover, the elemental composition obtained from energy dispersive X-ray (EDX) analysis confirmed that the material contains Pb and O elements, and no other impurity was present (Fig. 4).
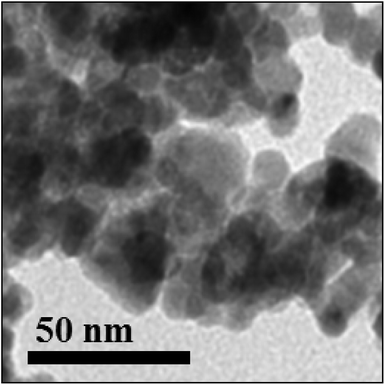
The transmission electron microscopy (TEM) image shown in Fig. 5 indicated the formation of orthorhombic crystallites of PbO with several hexagon-shaped particles. The dark spot in the TEM micrograph further confirmed the synthesis of PbO NPs, as the selected area diffraction pattern associated with such spots reveals the occurrence of the PbO NPs in total agreement with the X-ray diffraction data (Fig. 6). The average size of the PbO nanocrystals by TEM was approximated to be around 20 nm.
The Fourier transform infrared (FT-IR) spectrum (ESI, S6†) of the PbO NPs displayed peaks at 575, 641, and 848 cm−1, which corresponds to the Pb–O vibrations. Furthermore, the absorption band at ∼3315 cm−1 was due to the presence of the hydroxyl group (–OH) in the NPs.
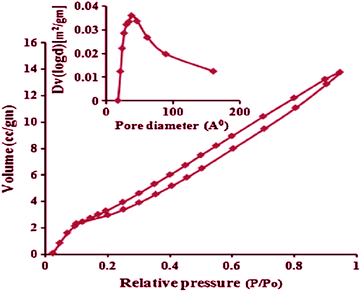
The N2 adsorption–desorption isotherms of the PbO nanoparticles shown in Fig. 7 was consistent with type IV adsorption–desorption isotherms with H1 hysteresis corresponding to the cylindrical mesoporous structure. Moreover, the surface area, pore-volume, and BJH pore diameter were found to be 32.0 m2 g−1, 0.023 cm3 g−1, and 30.9 Å, respectively.
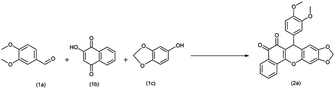
The catalytic activity of the synthesized PbO NPs was tested in a one-pot multicomponent synthesis of arylbenzodioxoloyl xanthenedione derivative under ball milling condition according to the reaction scheme 2a, with 3,4-dimethoxybenzaldehyde (166.2 mg, 1.0 mmol), 3,4-methylenedioxyphenol (138.0 mg, 1.0 mmol), and 2-hydroxy-1,4-naphthoquinone (174.0 mg, 1.0 mmol) as reactants. The reaction conditions, the ball milling parameters (speed, time, and ball to solids ratio), and the PbO nanocatalyst amount were first optimized to produce the highest yield using experimental design as shown in Table 1. The ball milling at 600 (6 balls) rpm for 60 min under solvent-free conditions was established as the optimized reaction conditions (entry 13, Table 1). Further, to understand the effect of ball milling, we also performed the model reaction in an RB flask under normal conditions without ball milling (entry 15, Table 1). However, the reaction did not initiate under these conditions, which demonstrated that ball milling provides sufficient energy to the reaction leading to the formation of the product.
| Entry | Conditions | Rotation (rpm) | Catalyst (mol%) | Time (min) | Yield (%)a |
|---|---|---|---|---|---|
| a Isolated yield; model reaction: 3,4-dimethoxybenzaldehyde (166.2 mg, 1.0 mmol), 3,4-methylenedioxyphenol (138.1 mg, 1.0 mmol), 2-hydroxy-1,4-naphthoquinone (174.1 mg, 1.0 mmol) under ball milling. b Optimized reaction conditions. c The reaction was performed under stirring condition in a RB flask. | |||||
| 1 | Ball milling | 400 | 00 | 50 | 21 |
| 2 | Ball milling | 400 | 10 | 50 | 48 |
| 3 | Ball milling | 400 | 15 | 60 | 54 |
| 4 | Ball milling | 400 | 20 | 70 | 59 |
| 5 | Ball milling | 500 | 10 | 50 | 62 |
| 6 | Ball milling | 500 | 15 | 50 | 65 |
| 7 | Ball milling | 500 | 20 | 60 | 67 |
| 8 | Ball milling | 600 | 10 | 70 | 71 |
| 9 | Ball milling | 600 | 15 | 50 | 77 |
| 10 | Ball milling | 600 | 20 | 60 | 82 |
| 11 | Ball milling | 600 | 05 | 70 | 90 |
| 12 | Ball milling | 600 | 10 | 50 | 91 |
| 13 | Ball milling b | 600 | 15 | 60 | 97 |
| 14 | Ball milling | 600 | 20 | 70 | 97 |
| 15 | No ball millingc | — | 15 | 60 | — |
Next, by utilizing the general experimental procedure (ESI† for detail experimental procedure; S2) and the aforementioned optimized conditions (Table 1), we examined the range of conditions for the synthesis of arylbenzodioxoloyl xanthenedione scaffolds. Moreover, we demonstrated that using PbO NPs as a catalyst leads to outstanding yield (92–97%) of 7-aryl-6H-benzo[h][1,3] dioxolo [4,5-b]xanthene-5.6(7H)-dione (2a–l) by the condensation of a variety of substituted aromatic aldehydes, 2-hydroxy-1,4-naphthoquinone, and 3,4-methylenedioxyphenol under the optimized conditions.
With the optimal conditions and using general experimental procedure,29 we also investigated the possible scopes of the reactants as revealed in Table 2. All these arylbenzodioxoloyl xanthenediones are well-known scaffolds and were simply recognized by the assessment of their spectroscopic information with earlier reports.26 These data are available in S4 (see ESI† for the spectroscopic data). The aromatic aldehydes comprising both electron-withdrawing (e.g., nitro group) and electron-donating (e.g., –OMe, –OH, –Cl, –Me, and –Br) groups participated proficiently in the reaction without including any electronic effects. The aromatic aldehyde with electron-donating groups (e.g., –OMe, –OH, –Cl, –Me, and –Br) increased the product yield, while in the case of aryl aldehyde having an electron-withdrawing group (e.g., –NO2), both the product yield as well as the reaction rate decreased. These findings are depicted in Table 2. All the prepared xanthenedione derivatives were purified via recrystallization from hot ethanol and extracted as solids, and thus, any column chromatography step was not required in the present protocol. The compounds were then confirmed by measuring their melting points (MP), followed by spectroscopic analysis using 1H and 13C NMR spectra provided in S5, ESI.†
Following a previously reported mechanism,26 a possible mechanism for the synthesis of arylbenzodioxoloyl xanthenedione derivative under ball milling at 600 rpm for 60 min is shown in Scheme 3. It is speculated that in the first step, the surface of the PbO NPs having free –O–H groups facilitated the carbon–carbon bond formation by activating aromatic aldehyde 1a to react with 2-hydroxy-1,4-naphthoquinone 1b leading to the intermediate B, which further undergoes dehydration, followed by the addition of 3,4-methylenedioxyphenol 1c, which upon cyclization leads to the formation of the product 2a with the recovery of the catalyst, PbO NPs.
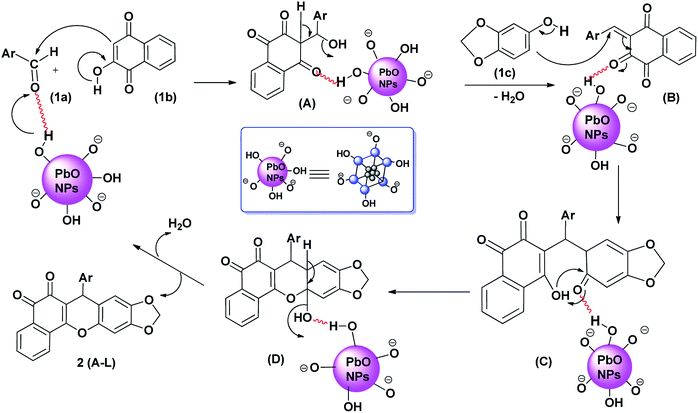 | ||
| Scheme 3 Plausible mechanism of PbO NP-catalyzed synthesis of arylbenzodioxoloyl xanthenedione (2a). | ||
Further, to signify the advantages of the current methodology, a comparative study of known methods is provided in Table 3, which clarifies the effectiveness of the PbO NP catalyst over known p-TSA and scolecites in terms of product yields, reaction times, and catalyst recyclability. A comparative study of the present method to that of the reported methods for the synthesis of arylbenzodioxoloyl xanthenediones is shown in Table 3.
Next, we investigated the reusability of the PbO nanocatalyst for the synthesis of 7-(3,4-dimethoxyphenyl)-6H-benzo[H][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione (2a) as a model reaction. After the reaction, PbO NPs were separated from the reaction mixture by centrifugation, washed consecutively with aqueous ethanol, dried, and reused for the next run. As shown in Fig. 8, the reaction yield was reduced by only 12% after eight consecutive runs. This slight decrease in the yield was observed due to the loss of PbO NPs (∼10 wt%) during the recycling process.
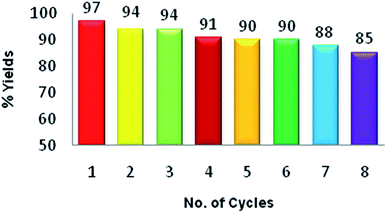 | ||
| Fig. 8 Reusability of PbO NPs for the synthesis of 7-(3,4-dimethoxyphenyl)-6H-benzo[H][1,3]dioxolo[4,5-b]xanthenes-5,6 (7H)-dione as a model reaction. | ||
The fate of the recycled PbO NPs was analyzed by performing SEM and TEM studies after the 8th run, and considerable agglomeration of NPs was observed. However, interestingly the particle size of the NPs reduced to ∼15 nm compared to fresh PbO NPs during the ball milling process (Fig. 9).
In conclusion, we demonstrated a facile and efficient method for the synthesis of 7-aryl-6H-benzo[H][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione using PbO NPs as a catalyst. The entire synthesis process was very clean and provided very high yields (86–97%) of xanthenedione derivatives (2a–l) via mild ball milling. Moreover, the present protocol has demonstrated significant development in terms of higher isolated yields, faster rate of reaction (1 h), and most importantly, it is environment-friendly. Moreover, the use of solvent-free ball milling conditions allows simple isolation and purification of the products, with no column chromatography, as well as the mild PbO NPs as a reusable catalyst made the current synthetic method more suitable and environmentally benign in nature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr T. L. Lambat thankfully acknowledges the research grant awarded from DST, New Delhi for the INSPIRE Fellowship [IF120418]. We are also grateful to Dr S. S. Deo & Dr F. S. Inam for their constant encouragement.References
- (a) N. Mukherjee, S. Ahammed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389 RSC; (b) J. Yu, G. Peng, Z. Jiang, Z. Hong and W. Su, Eur. J. Org. Chem., 2016, 32, 5340 CrossRef; (c) S. Zhao, Y. Li, C. Liu and Y. Zhao, Tetrahedron Lett., 2018, 59, 317 CrossRef CAS.
- (a) R. Thorwirth and A. Stolle, Synlett, 2011, 15, 2200 Search PubMed; (b) E. Tullberg, D. Peters and T. Frejd, J. Organomet. Chem., 2004, 689, 3778 CrossRef CAS; (c) T. Szuppa, A. Stolle, B. Ondruschka and W. Hopf, Green Chem., 2010, 12, 1288 RSC; (d) R. Thorwirth, F. Bernhardt, A. Stolle, B. Ondruschka and J. Asghari, Chem.–Eur. J., 2010, 16, 13236 CrossRef CAS PubMed; (e) T. Chatterjee, D. Saha and B. C. Ranu, Tetrahedron Lett., 2012, 53, 4142 CrossRef CAS; (f) A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC.
- B. Rodriguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213 CrossRef CAS.
- (a) R. Schimdt, R. Thorwirth, T. Szuppa, A. Stolle, B. Ondruschka and H. Hopf, Chem.–Eur. J., 2011, 17, 8129 CrossRef PubMed; (b) R. Thorwirth, A. Stolle and B. Ondruschka, Green Chem., 2010, 12, 985 RSC.
- S. Stankic, S. Suman and F. H. J. Vidic, J. Nanobiotechnol., 2016, 14, 1–20 CrossRef PubMed.
- B. Mirtamizdoust and B. Shaabani, et al. , Z. Anorg. Allg. Chem., 2012, 638, 844 CrossRef CAS.
- T. Lambat, J. Chin. Adv. Mater. Soc., 2018, 6, 134–144 CrossRef CAS.
- (a) L. S. Gadekar, S. S. Katkar, S. R. Mane, B. R. Arbad and M. K. Lande, Bull. Korean Chem. Soc., 2009, 30, 11 Search PubMed; (b) B. Gerard, J. Ryan, A. B. Beeler and J. A. Porco Jr., Tetrahedron, 2006, 62, 6405 CrossRef CAS; (c) A. Marra, A. Vecchi, C. Chiappe, B. Melai and A. Dondoni, J. Org. Chem., 2008, 73, 2458 CrossRef CAS.
- S. K. Pashaa, V. S. V. Satyanarayana, A. Sivakumar, K. Chidambaram and L. John Kennedy, Chin. Chem. Lett., 2011, 22, 891 CrossRef.
- H. An, X. Zhao, L. Guo, C. Jia, B. Yuan and Y. Wang, Appl. Catal., A, 2012, 433–434, 229 CrossRef CAS.
- R. Tayebee, B. Maleki and M. Sabeti, J. Iran. Chem. Soc., 2017, 14, 1179 CrossRef CAS.
- S. Wang, C. Li, Z. Xiao, T. Chen and G. Wang, J. Mol. Catal. A: Chem., 2016, 420, 26 CrossRef CAS.
- H. Zhang, Z. Ning, J. Shang, H. Liu, S. Han, W. Qu, Y. Jiang and Y. Guo, Microporous Mesoporous Mater., 2017, 248, 173 CrossRef CAS.
- D. Kumar, V. B. Reddy, S. Sharad, U. Dube and S. Kapur, Eur. J. Med. Chem., 2009, 44, 3805 CrossRef CAS.
- (a) T. Lambat, S. Deo, F. Inam, T. Deshmukh and A. Bhat, Karbala International Journal of Modern Science, 2016, 2, 63 CrossRef; (b) T. L. Lambat, S. S. Deo and T. B. Deshmukh, J. Chem. Pharm. Res., 2014, 6(4), 888 Search PubMed; (c) T. L. Lambat and S. S. Deo, Der Pharm. Lett., 2014, 6(3), 218 CAS; (d) T. Lambat and S. Deo, J. Chin. Adv. Mater. Soc., 2016, 5(1), 20 CrossRef; (e) T. L. Lambat and S. S. Deo, J. Chin. Adv. Mater. Soc., 2017, 5(2), 65 CrossRef CAS.
- T. R. Mandlimath, B. Umamahesh and K. I. Sathiyanarayanan, J. Mol. Catal. A: Chem., 2014, 391, 198 CrossRef CAS.
- A. Saeed, G. Shabir and S. A. Shehzadi, J. Chin. Chem. Soc., 2016, 63, 181 CrossRef CAS.
- D. Castañeda-Antonio, A. Rivera, E. Islas-Rodríguez, R. Portillo-Reyes, J. Muñoz-Rojas, F. Hernández-Aldana and D. Martínez-Carrera, ARPN J. Agric. Biol. Sci., 2015, 10, 390–393 Search PubMed.
- Ž. Lukšienė, Medicina, 2003, 29, 1137–1150 Search PubMed.
- (a) S. Banerjee and S. Santra, Tetrahedron Lett., 2009, 50, 2037 CrossRef CAS; (b) S. Banerjee, J. Das, R. Alverez and S. Santra, New J. Chem., 2010, 34, 302 RSC; (c) S. Banerjee, V. Balasanthiran, R. Koodali and G. Sereda, Org. Biomol. Chem., 2010, 8, 4316 RSC; (d) S. Banerjee, A. Horn, H. Khatri and G. Sereda, Tetrahedron Lett., 2011, 52, 1878 CrossRef CAS; (e) S. Banerjee and A. Saha, New J. Chem., 2013, 37, 4170 RSC; (f) S. Banerjee, S. Payra, A. Saha and G. Sereda, Tetrahedron Lett., 2014, 55, 5515 CrossRef CAS; (g) A. Saha, S. Payra and S. Banerjee, Green Chem., 2015, 17, 2859 RSC; (h) S. Banerjee, New J. Chem., 2015, 39, 5350 RSC.
- (a) A. Saha, S. Payra and S. Banerjee, RSC Adv., 2015, 5, 101664 RSC; (b) A. Saha, S. Payra, S. K. Verma, M. Mandal, S. Thareja and S. Banerjee, RSC Adv., 2015, 5, 100978 RSC; (c) S. Payra, A. Saha and S. Banerjee, RSC Adv., 2016, 6, 12402 RSC; (d) S. Payra, A. Saha, S. Guchhait and S. Banerjee, RSC Adv., 2016, 6, 33462 RSC; (e) S. Payra, A. Saha and S. Banerjee, RSC Adv., 2016, 6, 52495 RSC.
- (a) A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317 RSC; (b) B. Rodriguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213 CrossRef CAS.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; (b) M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS PubMed.
- (a) S. Limsuwan, E. N. Trip, T. R. Kouwen, S. Piersma, A. Hiranrat, W. Mahabusarakam, S. P. Voravuthikunchai, J. M. van Dijl and O. Kayser, Phytomedicine, 2009, 16, 645–651 CrossRef CAS PubMed; (b) A. Gervais, K. E. Lazarski and J. A. Porco, J. Org. Chem., 2015, 2, 9584–9591 CrossRef; (c) G. M. Ziarani, A. R. Badiei and M. Azizi, Sci. Iran., 2011, 18, 453–457 CrossRef CAS.
- K. Qian, Z. Fu, X. Cao, S. Li, T. Shen and Q. Song, Synth. Commun., 2017, 47, 37–43 CrossRef CAS.
- T. L. Lambat, Int. J. Appl. Biol. Pharm. Technol., 2017, 8, 11–18 CAS.
- General procedure for the preparation of PbO NPs: a combination of sodium dodecyl sulphate (720 mg, 2.5 mmol) and sodium hydroxide (10 mL, 0.1 N) in distilled water was added to a magnetically stirred lead nitrate (662 mg, 2 mmol) solution in methanol (10 mL). The reaction combination was agitated for 2 h at 30 °C. The whitish polycrystalline product was formed which was filtered, washed with distilled water (3 × 2 mL), and dried at 120 °C for 2 h. The white solid material was calcined at 650 °C for 2 h. During this process, the white PbO turned pale yellow in colour. Eventually, the formation of PbO NPs was confirmed by various analytical techniques.
- General Procedure for synthesis of xanthenedione derivatives using ball milling technique: Representative experimental procedure for the synthesis of 7-(3,4-dimethoxyphenyl)-6H-benzo[h][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione (2A): a mixture of 3,4-dimethoxybenzaldehyde (166.2 mg, 1 mmol), 2-hydroxy-1,4-naphthoquinone (138.1 mg, 1 mmol), 3,4-methylenedioxyphenol (171.1 mg, 1.0 mmol), and PbO NPs (25 mg, 15 mol%) was taken in 25 mL stainless steel beaker and ball-milling was done at 600 rpm with six balls (d = 10 mm) for 60 min. The ball-milling was carried out at inverted rotation directions, with the time interval of 10 minutes, having an interval break of 30 s. Further, the reaction mixture was extracted by carrying out elution with ethanol (5 mL), followed by solvent evaporation to furnish the crude product (2a–l). The products were purified by recrystallization in ethanol. This methodology was applicable to all the reactions listed in Table 2. The remaining catalyst was washed with ethanol (2 mL), followed by acetone (2 mL), dried up under vacuum, and reused for the next run. Analytical dada for selected products: 7-(3,4-dimethoxyphenyl)-6H-benzo[h][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione (2a): 1H NMR (DMSO-d6, 500 MHz) δ: 8.20 (d, J = 7.6 Hz, 1H), 8.10 (d, J = 7.8 Hz, 1H), 8.00 (t, J = 7.6 Hz, 1H), 7.80 (t, J = 7.7 Hz, 1H), 7.25 (s, 1H), 7.10 (s, 1H), 6.85 (s, 1H), 6.65 (d, J = 8.1 Hz, 1H), 6.80 (d, J = 8.4 Hz, 1H), 6.15 (s, 1H), 6.10 (s, 1H), 5.15 (s, 1H), 3.72 (s, 3H), 3.64 (s, 3H); 13C NMR (DMSO-d6, 125 MHz) δ: 177.9, 175.6, 160.0, 155.0, 146.5, 147.5, 146.7, 142.9, 140.6, 137.8, 135.0, 131.3, 130.2, 128.4, 124.3, 119.4, 115.0, 113.4, 111.0, 110.8, 106.8, 99.8, 98.5, 53.5, 53.5, 36.1; HRMS (ESI) calc. for C26H19O7 ([M + H]+): 443.1126. Found: 443.1133. 7-(4-methoxyphenyl)-6H-benzo[h][1,3]dioxolo[4,5-b]xanthene-5,6(7H)-dione (2b): 1H NMR (DMSO-d6, 500 MHz) δ: 8.11 (d, J = 7.6 Hz, 1H), 7.99 (dd, J = 7.5, 1.0 Hz, 1H), 7.88 (td, J = 7.6, 1.2 Hz, 1H), 7.69 (td, J = 7.5, 1.0 Hz, 1H), 7.29–7.22 (m, 2H), 7.16 (s, 1H), 6.87–6.76 (m, 3H), 6.06 (d, J = 0.9 Hz, 1H), 6.00 (d, J = 0.8 Hz, 1H), 5.04 (s, 1H), 3.69 (s, 3H); 13C NMR (DMSO-d6, 125 MHz) δ: 176.8, 176.6, 156.9, 155.9, 145.7, 143.9, 141.7, 136.3, 134.0, 130.3, 129.2, 129.2, 127.6, 127.4, 123.3, 116.0, 113.4, 112.8, 106.8, 100.7, 97.5, 54.0, 35.7; HRMS (ESI) calc. for C25H17O6 ([M + H]+): 413.1021. Found: 413.1001.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05903b |
| This journal is © The Royal Society of Chemistry 2019 |