Open Access Article
Open Access ArticleLigand-free iron-catalyzed benzylic C (sp3)–H amination of methylarenes with N-fluorobenzenesulfonimide†
Fengyu Bao *,
Yuanbo Cao,
Wenbo Liu and
Junhao Zhu
*,
Yuanbo Cao,
Wenbo Liu and
Junhao Zhu
College of Science, Henan Agricultural University, Zhengzhou 450002, P. R. China. E-mail: baofengyu@henau.edu.cn
First published on 4th September 2019
Abstract
Direct conversion of cheap methylarenes to benzylic amines, which are essential structural units of important drugs, is of great significance. However, the known methodologies suffer from the requirement of noble metal catalysts, heavy metal residues or strong oxidants. Herein, the first biocompatible iron-catalyzed benzylic C (sp3)–H amination of methylarenes with N-fluorobenzenesulfonimide is described. The reactions of methylarenes bearing electron-donating groups and electron-withdrawing groups ran smoothly under ligand and additional oxidant free conditions. Both toluene derivatives and 8-methylquinoline can be aminated by the same iron catalyst.
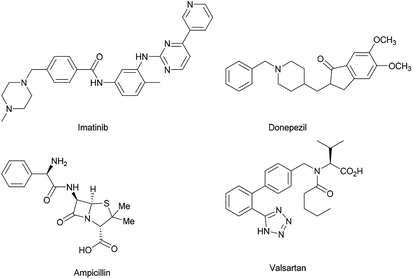
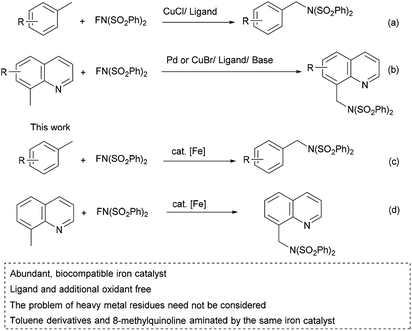
Benzylic amines are essential structural units of many important drugs, such as imatinib, donepezil, ampicillin, and valsartan (Fig. 1).1 Direct conversion of benzylic C (sp3)–H bonds to C (sp3)–N bonds to synthesize benzylic amines is of great importance. Intramolecular and intermolecular benzylic amination2–4 of aliphatic C–H bonds can be realized by Rh, Ru, Ir, Pd, Ag, Cu, Mn or Fe-catalyzed nitrene transfer reactions.2,5 In the case of amination of 8-methylquinolines, Pd, Ir, Ru or Rh can be used as catalyst.6a–e This strategy provides a powerful and direct method for the installation of benzylic amines. However, explosive azides or hypervalent iodine reagents need be used to generate nitrenes. The use of hypervalent iodine reagents may result in the generation of stoichiometric amount of environmentally unfriendly iodobenzenes. One alternative way to the benzylic amination is cross-dehydrogenative coupling (CDC) reactions catalyzed by metals or under metal free conditions.7 The strategy suffers from the additional oxidants which are essential to activate benzylic C (sp3)–H. Among the oxidants, potentially explosive peroxides are usually used. Pandey has reported visible-light-catalyzed benzylic amination via CDC procedures under metal and external oxidant free conditions,7e but the substrates could not be totally consumed. Radical addition allows benzylic amination,8 but peroxides need be used in the processes. N-Fluorobenzenesulfonimide (NFSI) is a kind of internal oxidant,9 thus no external oxidant is needed when it is used as amination reagent. Therefore, NFSI is a promising amination reagent. Zhang, Zhang, Liu and co-workers have reported copper-catalyzed benzylic amination by NFSI (Scheme 1a) in the presence of ligand.10a Remote benzylic amination with NFSI catalyzed by palladium or prompted by hypervalent iodine reagent was also investigated.10b–d Álvarez, Muñiz and co-workers have described Pd-catalyzed amination of 8-methylquinolines with NFSI (Scheme 1b).6f Zheng and co-workers have reported Cu-catalyzed amination of 8-methylquinolines in the presence of ligand and base (Scheme 1b).6g Toluene and xylene are among the cheapest, the most abundant chemical raw materials, but important materials for the production of industrially important chemicals. Their benzylic C–H bonds functionalization11 (including amination) to value-added products under environment friendly and economic conditions are highly desirable. 8-Methylquinolines are idea substrates for the synthesis of quinolin-8-ylmethanamines, which are building blocks in medicinal chemistry.12 The amination of toluene derivatives and 8-quinolines is significant. However, the known methodologies suffer from noble and or heavy metal catalysts. Their catalytic reactions' applications in the pharmaceutical industry may be limited, for the problem of heavy metal residues must be considered. Iron is abundant, nontoxic, biocompatible, environment-friendly,2d thus it is idea catalyst for the benzylic amination reactions. However, there is no report on iron-catalyzed benzylic amination of methylarenes under additional oxidants and nitrenes free conditions. There is no catalyst which can aminate both toluene derivatives and 8-methylquinoline. Herein, we'd like to report the first biocompatible iron-catalyzed benzylic C (sp3)–H amination of methylarenes (including toluene derivatives and 8-methylquinoline) with NFSI under ligand and additional oxidant free conditions (Scheme 1c and d). Our work provides a direct method for preparation of benzylic amines from toluene derivatives and 8-methylquinoline without the problem of heavy metal residues. The other advantage is to avoid the use of a large excess amount of methylarenes. Stoichiometric amount of toluene derivatives and 8-methylquinoline were used in this work. The examples of functionalization of stoichiometric amount of methylarenes are still limited.11a
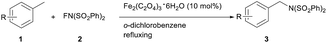
Initially, toluene was chosen as substrate with iron catalyst to optimize the reaction conditions (Table 1). Benzylic C (sp3)–H of toluene was selectively aminated to produce benzylic amine 3a in the presence of C (sp2)–H bonds. Zero valent diiron nonacarbonyl showed catalytic activity, and 3a was obtained in 27% yield (entry 1). The use of Cp2FePF6 as catalyst could slightly increase the yield (from 27% to 28%, entry 2), while the other ferrocenium salt Cp2FeBF4 clearly improved the yield (from 27% to 36%, entry 3).With nano Fe3O4 as catalyst, the yield became lower (entry 4). Notably, anionic iron also showed catalytic activity (entry 5). Iron(III) salts were investigated. Iron(III) nitrate nonahydrate, iron(III) acetylacetonate (Fe(acac)3) and iron(III) p-toluenesulfonate hexahydrate (Fe(OTs)3·6H2O) were found to be able to catalyze the amination, but they had little effects on the improvement of the yields (entry 7–9). We were pleased to find that iron(III) oxalate hexahydrate (Fe2(C2O4)3·6H2O) gave 3a in 59% yield (entry 10). Iron(II) oxalate was tested. 3a was afforded in lower yield (entry 11). The results indicate that the catalytic activity of iron(III) salt is higher than of iron(II) salt. The decrease of the amount of NFSI lowered the yield (entry 12). However, the increase of the amount of NFSI almost could not improve the yield (entry 13). The decrease of the amount of the catalyst Fe2(C2O4)3·6H2O resulted in lower yield (entry 14). With chlorobenzene as solvent, the conversion of C (sp3)–H to C (sp3)–N was limited. 3a was obtained only in 28% yield (entry 15). With 1,2-dichloroethane (DCE), acetonitrile, 1,4-dioxane or N,N-dimethylformamide (DMF) as solvent, 3a could not be detected by thin layer chromatography (TLC). When the reaction was carried out at 120 °C, 3a could not be detected by TLC.
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2 (1.5 equiv., 0.75 mmol), catalyst (10 mol%) in 10 mL 1, 2-dichlorobenzene under refluxing condition in air.b Isolated yield.c 1.0 equiv. NFSI.d 2.0 equiv. NFSI.e 5 mol% Fe2(C2O4)3·6H2O.f Chlorobenzene as solvent. | ||
| 1 | Fe2(CO)9 | 27 |
| 2 | Cp2FePF6 | 28 |
| 3 | Cp2FeBF4 | 36 |
| 4 | Fe3O4 (nano) | 24 |
| 5 | Na3FeF6 | 25 |
| 6 | Fe2TiO5 | 25 |
| 7 | Fe(NO3)3·9H2O | 29 |
| 8 | Fe(acac)3 | 22 |
| 9 | Fe(OTs)3·6H2O | 31 |
| 10 | Fe2(C2O4)3·6H2O | 59 |
| 11 | FeC2O4 | 54 |
| 12c | Fe2(C2O4)3·6H2O | 37 |
| 13d | Fe2(C2O4)3·6H2O | 60 |
| 14e | Fe2(C2O4)3·6H2O | 46 |
| 15f | Fe2(C2O4)3·6H2O | 28 |
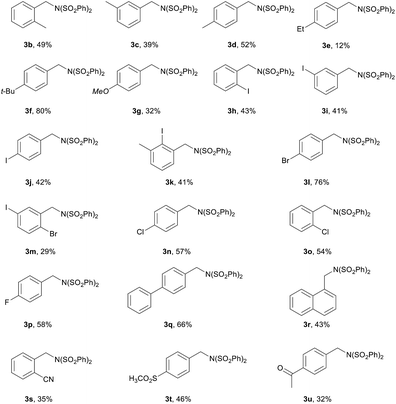
With the optimized reaction conditions (Table 1, entry 10), we began to investigate the amination of toluene derivatives (Table 2). The amination of o-xylene, m-xylene, p-xylene could be catalyzed by 10 mol% Fe2(C2O4)3·6H2O, and the corresponding monoamination products (3b–d) were obtained under the reaction conditions. The amination of both electron-deficient and electron-rich toluene derivatives underwent smoothly to produce the corresponding benzylic amines. Toluene bearing electron-donating substituent tert-butyl at the para position gave 3f in 80% yield. Notably, primary benzylic C (sp3)–H was selectively aminated in the presence of secondary benzylic C (sp3)–H, and 3e was afforded. Toluene substrates bearing iodo, bromo, chloro and fluoro substituents were good candidates for the amination, the corresponding products (3h–l and 3n–p) were obtained in satisfactory yields (from 41% to 76%). Iodo substituent at the ortho, meta or para position of the benzene ring has little effect on the benzylic amination. It is noticed that reactive iodo and bromo substituents remain in the amination products, thus further transformation can be considered. Toluene bearing phenyl substituent at the para position was aminated efficiently to produce 3q in 66% yield. 1-Methyl naphthalene was also suitable substrate. Its amination gave 3r in 43% yield. Electron-withdrawing substituents, such as cyano, sulfonyl, carbonyl, are tolerant, and the corresponding products were obtained (3s–u). 3t was afforded in 46% yield.
| a Reaction conditions: 1 (0.5 mmol), 2 (1.5 equiv., 0.75 mmol), Fe2(C2O4)3·6H2O (10 mol%) in 20 mL 1, 2-dichlorobenzene under refluxing condition in air. |
|---|
 |
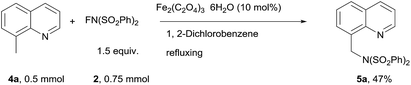
The amination of 8-methylquinoline was then studied (Scheme 2). 8-Methylquinoline was successfully aminated, 5a was obtained in 47% yield.
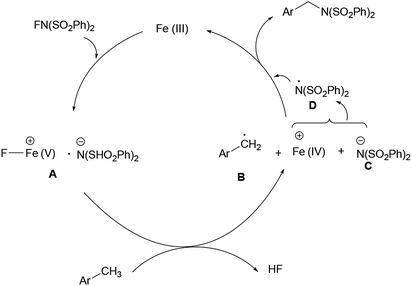
A possible path of the amination involves cationic iron species. As is known, NFSI is a kind of electrophilic oxidant.6f,13 In the amination of 8-methylquinolines with NFSI catalyzed by Pd(II), Álvarez and Muñiz proposed a mechanism which involved Pd(II) to a cationic Pd(IV) path.6f As for the interaction of iron(II) catalyst and NFSI, Fu proposed a cationic iron species.14 On the other hand, iron(III) can be oxidated to iron(V).15 Therefore, it is reasonable to assume that Fe(III)-catalyzed amination of methylarenes proceeds via cationic iron(V) path (Scheme 3). The interaction of iron(III) catalyst with NFSI produces cationic iron complex A, which is an oxidant to activate benzylic C (sp3)–H to produce benzylic type radical B.16 The following reaction of cationic Fe(IV) and anionic C generates radical D along with the regeneration of Fe(III). The coupling of radical B and D gives the amination product.
In summary, we have developed the first biocompatible iron-catalyzed benzylic C (sp3)–H amination of methylarenes with NFSI under ligand and additional oxidant free conditions. The amount of methylarenes is stoichiometric. Both electron-deficient and electron-rich methylarenes are suitable substrates. Electron-withdrawing substituents and electron-donating substituents are tolerant. Both toluene derivatives and 8-methylquinoline can be aminated by the same abundant iron catalyst.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- (a) J. P. Clark, K. Feng, A. Sookezain and M. C. White, Nat. Chem., 2018, 10, 583 CrossRef CAS PubMed; (b) Z.-l. Li and C. Cai, ChemistrySelect, 2017, 2, 8076 CrossRef CAS.
- For reviews on benzylic amination, see: (a) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef CAS PubMed; (b) Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247 CrossRef CAS PubMed; (c) J. M. Alderson, J. R. Corbin and J. M. Schomaker, Acc. Chem. Res., 2017, 50, 2147 CrossRef CAS PubMed; (d) P. Wang and L. Deng, Chin. J. Chem., 2018, 36, 1222 CrossRef CAS; (e) M. D. Käkäs, Chem. Soc. Rev., 2018, 47, 5786 RSC.
- For selected intramolecular benzylic amination, see: (a) S. M. Paradine, J. R. Griffin, J. Zhao, A. L. Petronico, S. M. Miller and M. C. White, Nat. Chem., 2015, 7, 987 CrossRef CAS PubMed; (b) M. Huang, T. Yang, J. D. Paretsky, J. F. Berry and J. M. Schomaker, J. Am. Chem. Soc., 2017, 139, 17376 CrossRef CAS PubMed; (c) E. A. Wappes, K. M. Nakafuku and D. A. Nagib, J. Am. Chem. Soc., 2017, 139, 10204 CrossRef CAS PubMed; (d) P. Dydio, H. M. Key, H. Hayashi, D. S. Clark and J. F. Hartwig, J. Am. Chem. Soc., 2017, 139, 1750 CrossRef CAS PubMed; (e) P. Becker, T. Duhamel, C. J. Stein, M. Reiher and K. Muñiz, Angew. Chem., Int. Ed., 2017, 56, 8004 CrossRef CAS PubMed; (f) T. Duhamel, C. J. Stein, C. Martínez, M. Reiher and K. Muñiz, ACS Catal., 2018, 8, 3918 CrossRef CAS; (g) P. Becker, T. Duhamel, C. Martínez and K. Muñiz, Angew. Chem., Int. Ed., 2018, 57, 5166 CrossRef CAS PubMed.
- For selected intermolecular benzylic amination, see: (a) C. K. Prier, R. K. Zhang, A. R. Buller, S. Brinkmann-Chen and F. H. Arnold, Nat. Chem., 2017, 9, 629 CrossRef CAS PubMed; (b) A. Bakhoda, Q. Jiang, J. A. Bertke, T. R. Cundari and T. H. Warren, Angew. Chem., Int. Ed., 2017, 56, 6426 CrossRef CAS PubMed; (c) J. Bergès, B. García and K. Muñiz, Angew. Chem., Int. Ed., 2018, 57, 15891 CrossRef PubMed; (d) N. D. Chiappini, J. B. C. Mack and J. D. Bois, Angew. Chem., Int. Ed., 2018, 57, 4956 CrossRef CAS PubMed; (e) L. Wang, D. W. Agnew, X. Yu, J. S. Figueroa and S. M. Cohen, Angew. Chem., Int. Ed., 2018, 57, 511 CrossRef CAS PubMedFor some examples of silver or copper-catalyzed amination see: (f) L.-Y. Xie, S. Peng, L.-L. Jiang, X. Peng, W. Xia, X. Yu, X.-X. Wang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 6, 167 RSC; (g) L.-Y. Xie, S. Peng, F. Liu, Y.-F. Liu, M. Sun, Z.-L. Tang, S. Jiang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 7193 CrossRef CAS.
- For iron-catalyzed benzylic amination via nitrene transfer, see: (a) E. R. King, E. T. Hennessy and T. A. Betley, J. Am. Chem. Soc., 2011, 133, 4917 CrossRef CAS PubMed; (b) Y. Liu, X. Guan, E. L.-M. Wong, P. Liu, J.-S. Huang and C.-M. Che, J. Am. Chem. Soc., 2013, 135, 7194 CrossRef CAS PubMed; (c) E. T. Hennessy and T. A. Betley, Science, 2013, 340, 591 CrossRef CAS PubMed; (d) D. A. Iovan and T. A. Betley, J. Am. Chem. Soc., 2016, 138, 1983 CrossRef CAS PubMed; (e) N. C. Thacker, Z. Lin, T. Zhang, J. C. Gilhula, C. W. Abney and W. Lin, J. Am. Chem. Soc., 2016, 138, 3501 CrossRef CAS PubMed; (f) B. Bagh, D. L. J. Broere, V. Sinha, P. F. Kuijpers, N. P. v. Leest, B. d. Bruin, S. Demeshko, M. A. Siegler and J. I. v. d. Vlugt, J. Am. Chem. Soc., 2017, 139, 5117 CrossRef CAS PubMed; (g) I. T. Alt, C. Guttroff and B. Plietker, Angew. Chem., Int. Ed., 2017, 56, 10582 CrossRef CAS PubMed; (h) K.-P. Shing, Y. Liu, B. Cao, X.-Y. Chang, T. You and C.-M. Che, Angew. Chem., Int. Ed., 2018, 57, 11947 CrossRef CAS PubMed.
- For amination of 8-methylquinolines, see: (a) H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048 CrossRef CAS PubMed; (b) T. Kang, Y. Kim, D. Lee, Z. Wang and S. Chang, J. Am. Chem. Soc., 2014, 136, 4141 CrossRef CAS PubMed; (c) B. Liu, B. Li and B. Wang, Chem. Commun., 2015, 51, 16334 RSC; (d) N. Wang, R. Li, L. Li, S. Xu, H. Song and B. Wang, J. Org. Chem., 2014, 79, 5379 CrossRef CAS PubMed; (e) X. Huang and J. You, Chem. Lett., 2015, 44, 1685 CrossRef CAS; (f) Á. Iglesias, R. Álvarez, Á. R. d. Lera and K. Muñiz, Angew. Chem., Int. Ed., 2012, 51, 2225 CrossRef PubMed; (g) X. Zhang, R. Wu, W. Liu, D.-W. Qian, J. Yang, P. Jiang and Q.-Z. Zheng, Org. Biomol. Chem., 2016, 14, 4789 RSC; (h) H. Wang, G. Tang and X. Li, Angew. Chem., Int. Ed., 2015, 54, 13049 CrossRef CAS PubMed; (i) Y.-Q. Zhu, J.-L. He, Y.-X. Niu, T.-F. Han and K. Zhu, ChemistrySelect, 2019, 4, 576 CrossRef CAS.
- For selected benzylic amination via CDC procedures, see: (a) D. A. Powell and H. Fan, J. Org. Chem., 2010, 75, 2726 CrossRef CAS PubMed; (b) H. J. Kim, J. Kim, S. H. Cho and S. Chang, J. Am. Chem. Soc., 2011, 133, 16382 CrossRef CAS PubMed; (c) Q. Xue, J. Xie, H. Li, Y. Cheng and C. Zhu, Chem. Commun., 2013, 49, 3700 RSC; (d) W. Liu, C. Liu, Y. Zhang, Y. Sun, A. Abdukadera, B. Wang, H. Li, X. Ma and Z. Zhang, Org. Biomol. Chem., 2015, 13, 7154 RSC; (e) G. Pandey and R. Laha, Angew. Chem., Int. Ed., 2015, 54, 14875 CrossRef CAS PubMed; (f) Z.-l. Li, L.-k. Lin and C. Cai, Org. Biomol. Chem., 2017, 15, 1317 RSC; (g) J. Xiao, P. Li, Y. Zhang, D. Xie, Z. Peng, D. An and W. Dong, Tetrahedron, 2018, 74, 4558 CrossRef CASFor Iron-catalysts, see: (h) Q. Xia, W. Chen and H. Qiu, J. Org. Chem., 2011, 76, 7577 CrossRef CAS PubMed; (i) D. Chen, F. Pan, J. Gao and J. Yang, Synlett, 2013, 24, 2085 CrossRef CAS; (j) N. C. Thacker, P. Ji, Z. Lin, A. Urban and W. Lin, Faraday Discuss., 2017, 201, 303 RSC; (k) C.-J. Yue, X.-D. Hu, L.-P. Gu and B.-L. Liu, Monatsh. Chem., 2018, 149, 1161 CrossRef CAS.
- A. Samzadeh-Kermani, New J. Chem., 2018, 42, 4766 RSC.
- C. Zhu, H. Zeng, F. Chen, Z. Yang, Y. Cai and H. Jiang, Angew. Chem., Int. Ed., 2018, 57, 17215 CrossRef CAS PubMed.
- (a) Z. Ni, Q. Zhang, T. Xiong, Y. Zheng, Y. Li, H. Zhang, J. Zhang and Q. Liu, Angew. Chem., Int. Ed., 2012, 51, 1244 CrossRef CAS PubMedFor remote benzylic C-H amination, see: (b) T. Xiong, Y. Li, Y. Lv and Q. Zhang, Chem. Commun., 2010, 46, 6831 RSC; (c) Y. Zheng, T. Xiong, Y. Lv, J. Zhang and Q. Zhang, Org. Biomol. Chem., 2013, 11, 7923 RSC; (d) Y. Yang, Y. Yu, Y. Wang, Q. Zhang and D. Li, Tetrahedron, 2018, 74, 1085 CrossRef CAS.
- For reviews of the reactions of methylarenes, see: (a) R. Vanjari and K. N. Singh, Chem. Soc. Rev., 2015, 44, 8062 RSC; (b) J.-B. Feng and X.-F. Wu, Appl. Organomet. Chem., 2014, 29, 63 CrossRef; (c) J.-P. Lumb, Angew. Chem., Int. Ed., 2017, 56, 9276 CrossRef CAS PubMed.
- (a) N. C. Warshakoon, J. Sheville, R. T. Bhatt, W. Ji, J. L. Mendez-Andino, K. M. Meyers, N. Kim, J. A. Wos, C. Mitchell, J. L. Paris, B. B. Pinney, O. Reizes and X. E. Hu, Bioorg. Med. Chem. Lett., 2006, 16, 5207 CrossRef CAS; (b) P. S. Kharkar, M. N. Deodhar and V. M. Kulkarni, Med. Chem. Res., 2009, 18, 421 CrossRef CAS.
- K. M. Engle, T.-S. Mei, X. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2011, 50, 1478 CrossRef CAS.
- S. Y. Lee, S. Neufeind and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 8899 CrossRef CAS PubMed.
- (a) F. Minisci and F. Fontana, Tetrahedron Lett., 1994, 35, 1427 CrossRef CAS; (b) F. Minisci, F. Fontana, S. Araneo and F. Recupero, Tetrahedron Lett., 1994, 35, 3759 CrossRef CAS; (c) C. Bardin, D. H. R. Barton, B. Hu, R. Rojas-Wahl and D. K. Taylor, Tetrahedron Lett., 1994, 35, 5805 CrossRef CAS; (d) D. H. R. Barton, B. Hu, T. Li and J. MacKinnon, Tetrahedron Lett., 1996, 37, 8329 CrossRef CAS.
- D. Mazzarella, G. E. M. Crisenza and P. Melchiorre, J. Am. Chem. Soc., 2018, 140, 8439 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05294a |
| This journal is © The Royal Society of Chemistry 2019 |