Open Access Article
Open Access ArticlePhotoluminescence and afterglow behavior of Ce3+ activated Li2Sr0.9Mg0.1SiO4 phosphor
Yingjun Xiao ,
Dongyun Zhang
,
Dongyun Zhang * and
Chengkang Chang
* and
Chengkang Chang *
*
School of Materials Science and Engineering, Shanghai Institute of Technology, 100 Haiquan Road, Shanghai 200235, China. E-mail: dyz@sit.edu.cn; ckchang@sit.edu.cn
First published on 30th August 2019
Abstract
A blue emitting phosphor Li2Sr0.9Mg0.1SiO4:Ce3+, with long persistence, was synthesized via a high-temperature solid phase method. According to the X-ray diffraction analysis result, the introduction of Mg2+ and Ce3+ ions has no influence on the structure of the host material. Typical 5d-2F5/2 and 5d-2F7/2 transitions of Ce3+ ions were detected by PL spectra, which corresponded to the CIE chromaticity coordinates of x = 0.1584, y = 0.0338. An optimal doping concentration of Ce3+ was determined as of 0.4 at%. Furthermore, the Li2Sr0.9Mg0.1SiO4:Ce3+ phosphor showed a typical triple-exponential afterglow behavior when the UV source was switched off. The highest lifetime of the electrons within the material reached a value of 73.9 s. Thermal stimulated luminescence study indicated that the afterglow of Li2Sr0.9Mg0.1SiO4:Ce3+ was due to the recombination of the electrons with holes released from the traps generated by the doping of Ce3+ ions in the Li2Sr0.9Mg0.1SiO4 host. The afterglow mechanism of Li2Sr0.9Mg0.1SiO4:Ce3+ is illustrated and discussed in detail on the basis of the experimental results.
1. Introduction
Long-lasting phosphors (LLPs) are a kind of energy storage materials with the decay time extended to seconds, minutes, or even hours after the removal of ultraviolet (UV) and visible light at room temperature.1–4 These materials have been widely studied by many researchers because of their excellent properties, such as long persistence time, stable crystal structure, high physical and chemical stabilities.5–8 The most commonly used long lasting phosphors (LLPs) are CaAl2O4:Eu2+, Nd3+ (blue), SrAl2O4:Eu2+, Dy3+ (green) and Y2O2S:Eu3+, Ti4+, Mg2+ (red).9,10 It is generally agreed that long luminescence lifetimes can be achieved by adjusting the host crystal bandgap and the energy level by introducing rare-earth ions. However, it is difficult to generate an appropriate trap depth in these host materials; hence, the progress in this field is quite slow. Therefore, extensive studies still need to be carried out in order to achieve the best performance of the long-lasting phosphorescent materials.Recently, a lithium silicate system has been regarded as a suitable host with high chemical stability, and it can produce a suitable crystal environment imposing on the emission centers. Thus, such system has been widely investigated and silicate phosphors with various colors have been well recorded in documents.11–17 We also reported some LLPs in the silicate system, where Li2SrSiO4 and Li2Ca0.6Sr0.4SiO4 were suggested as excellent hosts for LLPs.18,19
It is also recommended that the phosphorescence of Ce3+ in most of the hosts is caused by the 5d-2F5/2 and 5d-2F7/2 energy-level transitions.20–23 However, there has been no detailed report on the long lasting phosphorescence phenomenon of Ce3+ in lithium silicate phosphors. To understand the afterglow behavior of the Ce3+ doped lithium silicate phosphors, it is important to find a suitable host crystal with a certain bandgap that matches with the energy level of the Ce3+ ions. In this study, a series of blue emitting LLPs Li2Sr0.9Mg0.1SiO4:x% Ce3+ (x = 0.1, 0.2, 0.3, 0.4, and 0.5), was investigated to carry out the survey. The results demonstrated that it was the Ce3+ ions incorporated into the substrate that caused the afterglow. Thermal stimulated luminescence study indicated that the afterglow of Li2Sr0.9Mg0.1SiO4:Ce3+ was due to the recombination of the electrons with holes released from the traps generated by the doping of Ce3+ ions in the Li2Sr0.9Mg0.1SiO4 host and we discussed the long-lasting phosphorescent phenomenon in detail by means of a completely trapping model.
2. Experimental procedures
2.1 Sample synthesis
The Li2Sr0.9Mg0.1SiO4:Ce3+ phosphor was prepared by the traditional high-temperature solid phase reaction, using the raw materials, SrCO3, Li2CO3, MgO, SiO2, and CeO2, each with a purity of 99.99%. The Ce3+ doping level ranged from 0.1%, 0.2%, 0.3%, 0.4% to 0.5%. The raw materials were mixed in stoichiometric ratios, ground for 1 h and finally sintered at 850 °C under a weak reductive atmosphere (5% H2 + 95% N2). The samples were kept at this temperature for 10 h and then cooled to room temperature while maintaining the flow of the reducing gas till the furnace reached room temperature.2.2 Characterization
X-ray diffraction was performed using a TD-3500 X-ray diffractometer with Cu Kα irradiation to collect the crystal information of the as-prepared phosphors. A Hitachi F7000 fluorescence spectrophotometer was used to detect the excitation and emission spectra of the samples. The afterglow behavior and the trap energy level determinations were measured using an FJ427-A1 thermally stimulated spectrometer.3. Results and discussion
3.1 Phase identification
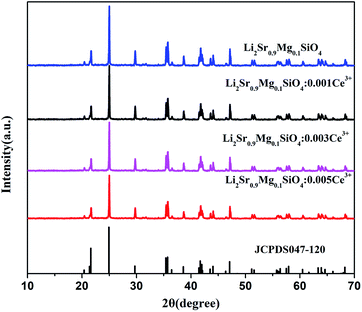
The phase and structure of the samples were determined using X-ray powder diffraction. Fig. 1 indicates the composition of Li2Sr0.9Mg0.1SiO4 phosphors doped with different concentrations of Ce3+ (0.1%, 0.3% and 0.5%), which are compared to the standard file from the JCPDS card no. 047-120. It is possible to suggest that the peaks of the samples correspond to single hexagonal phase.11 Despite the increase in the Ce3+ dopant concentration (up to 0.5%), no other product was observed. The possible reason is that the radius of Ce3+ ions (r = 1.196 Å) being very close to that of Sr2+ (1.31 Å), the Ce3+ ions are expected to occupy the Sr2+ sites in the Li2Sr0.9Mg0.1SiO4 host lattice. It can be concluded from the results that the doped Ce3+ ions as well as Mg2+ ions, have been successfully incorporated into the lattice of the silicate compound, and the co-doping of Ce3+ and Mg2+ ions did not disturb the host crystal lattice.3.2 Photoluminescence properties
Fig. 2 portrays the photoluminescence behavior of the Li2Sr0.9Mg0.1SiO4:0.004Ce3+ phosphor. A typical photoluminescence excitation and emission spectra of 0.4 at% Ce3+ activated Li2Sr0.9Mg0.1SiO4 phosphor is shown in Fig. 2a. From the PLE spectrum, a broad band is observed in the wavelength region of 250–350 nm, centering at about 277 nm and located at 253 nm, 276 nm and 314 nm respectively, which originates from the 5d → 4f transition of the Ce3+ ions.24,25 As we can see, the emission spectra of the phosphor consist of two bands, peaking at 391 and 416 nm in the wavelength region of 350–550 nm under the excitation of 277 nm. Such emissions are typical for that of Ce3+ ascribed to the 5d-2F5/2 and 5d-2F7/2 transitions. Fig. 2b shows the corresponding CIE 1931 chromaticity diagram of the Li2Sr0.9Mg0.1SiO4:0.004Ce3+ phosphor. Point with chromaticity coordination (x = 0.1584, y = 0.0338) is located in the region of the blue color, indicating that the color of LLL in Li2Sr0.9Mg0.1SiO4:0.004Ce3+ is blue. In our study, the high quantum efficiency of the PL emission yields of up to 60% at 300 K were obtained. The value of the quantum efficiency in this case is higher than the Eu2+ ions as activator in the Li2SrSiO4 matrix in ref. 26, which means that the Ce3+ as an activator in the Li2Sr0.9Mg0.1SiO4 matrix is superior and valuable. | ||
| Fig. 2 PLE and PL (λex = 277 nm) spectra (a), and CIE 1931 chromaticity coordinates of the Li2Sr0.9Mg0.1SiO4:0.004Ce3+phosphor (b). | ||
The effects of doping concentration on the PL properties were further investigated. When being excited by 277 nm, the emission spectra of Li2Sr0.9Mg0.1SiO4:Ce3+ phosphors with different concentrations of Ce3+ are depicted in Fig. 3. It can be observed that the emission intensity of Li2Sr0.9Mg0.1SiO4:Ce3+ increases initially with an increase in the Ce3+ concentration, and then decreases with a maximum intensity reaching at a doping concentration of 0.4 at%. This phenomenon results from the concentration quenching, which was studied by Chen.27 These results demonstrate that the optimal doping concentration of Ce3+ in Li2Sr0.9Mg0.1SiO4 experimentally determined to be 0.4 at%.
3.3 Long afterglow properties
As mentioned above, the Li2Sr0.9Mg0.1SiO4:0.004Ce3+ phosphors show a blue emission when excited under an ultraviolet source of 277 nm and exhibit long-lasting phosphorescence. The room-temperature decay curves of the long afterglow phosphorescence of Li2Sr0.9Mg0.1SiO4:0.004Ce3+ are depicted in Fig. 4. The decay characteristics of the Li2Sr0.9Mg0.1SiO4:0.004Ce3+ phosphor can be roughly divided into two processes, the rapid-decay process and the slow-decay process. The decay curves of the afterglow of the phosphors can be evaluated by the curve fitting technique. In this study, a triple-exponential equation, which has been used widely, can fit the experimental decay curves very well:28
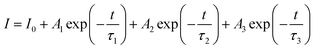 | (1) |
3.4 Thermoluminescence performance
In order to further investigate the nature of the trap in Li2Sr0.9Mg0.1SiO4:0.004Ce3+, thermoluminescence studies were carried out. The thermoluminescence curves indicating the integrated emission intensity is depicted in Fig. 5. As we all know, a proper energy level of the traps is essential for good afterglow properties and the depth of traps and the trap density in the phosphors can be dealt with the equation:31,32
 | (2) |
 | (3) |
 | (4) |
The calculated trap depth and density of Li2Sr0.9Mg0.1SiO4:0.004Ce3+ phosphors are shown in Fig. 5. The trap level and trap concentrations are two useful indicators to evaluate the long duration phosphorescence of different phosphors. Having an electron trap of suitable energy level is necessary to create the afterglow behavior. The asymptotes can be found practically in all experiments, where the values of I, S and E are between 0.7 and 2.5, between 105 s−1 and 1013 s−1, and between 0.1 and 1.6 eV, respectively. In this case, the corresponding values were in these ranges. It is well known that the trap created by the lattice defects play a very important role on the afterglow properties of the phosphors. According to Kuang's report, the trap depths should lie between 0.4 and 0.6 eV if the materials are to show excellent persistent phosphorescence performance.35 The calculated trap concentration was 1.14 × 1010 mol−1 and the depth of the trap energy level of Li2Sr0.9Mg0.1SiO4:0.004Ce3+ was 0.46 eV, which were very close to the values reported. We observed that the trap depth of 0.46 eV for 0.4 at% Ce3+ doped Li2Sr0.9Mg0.1SiO4 is suitable for excellent afterglow at room temperature.
3.5 Possible mechanism of persistence
Based on the above description and analyses, we are trying to elucidate the phosphorescence mechanism of Li2Sr0.9Mg0.1SiO4:Ce3+. In Ce3+-doped phosphor, it seems reasonable that the Ce3+ (r = 1.196 Å) ions are expected to occupy the incorporated Sr2+ (r = 1.31 Å) sites of the Li2Sr0.9Mg0.1SiO4 host due to their close ionic radius. The replacement can be explained by the mechanism: . In order to maintain the charge balance, it is the only replacement way possible, as three Sr2+ ions can be replaced by two Ce3+ ions, which induce two positive defects
. In order to maintain the charge balance, it is the only replacement way possible, as three Sr2+ ions can be replaced by two Ce3+ ions, which induce two positive defects  and one negative defect
and one negative defect  in the host. In our case, on the basis of our PL spectra and TL analysis results, Ce3+ not only acts as the luminescent center in the host lattice, but also serves as the trap center.
in the host. In our case, on the basis of our PL spectra and TL analysis results, Ce3+ not only acts as the luminescent center in the host lattice, but also serves as the trap center.
The detailed mechanism of the long-lasting phosphorescence is yet to be known. The afterglow of Ce3+ doped in the Li2Sr0.9Mg0.1SiO4 phosphor and the cause of such phenomenon was assumed to be due to the thermo-stimulated recombination of the holes at the traps induced by irradiation, which leave the holes or the electrons in a meta-stable excited state at room temperature. Based on the above results, a simple mechanism for the LLP of Li2Sr0.9Mg0.1SiO4:Ce3+ is proposed, which is presented in Fig. 6. Upon UV-irradiation (step 1), the electrons in the valence band are excited to the conduction band, resulting in the formation of free electrons and holes at the same time in the phosphor. Then, the energy associated with the excited electrons is transferred to the 5d level of Ce3+. The subsequent jumping of electron traps to the ground level 2F5/2 and 2F7/2 states of Ce3+ and the recombination of holes give rise to the characteristic emission of Ce3+ ions (step 2 and step 5). In this process, a number of free holes and excited electrons can be captured by the  and
and  trapping centers (step 3). After the UV irradiation is removed, these electrons trapped by Ce3+ will be released from the traps and transferred via the host to the luminescence center (step 4), followed by the recombination of free electrons and holes, leading to the characteristic emission of Ce3+ ions (step 5). When the decay ratio of the carries released from the electron traps to the 5d state of the Ce3+ ions is proper, the blue-emitting LLP of Ce3+ can be obtained.
trapping centers (step 3). After the UV irradiation is removed, these electrons trapped by Ce3+ will be released from the traps and transferred via the host to the luminescence center (step 4), followed by the recombination of free electrons and holes, leading to the characteristic emission of Ce3+ ions (step 5). When the decay ratio of the carries released from the electron traps to the 5d state of the Ce3+ ions is proper, the blue-emitting LLP of Ce3+ can be obtained.
4. Conclusions
In summary, a kind of blue-emitting long afterglow phosphor Li2Sr0.9Mg0.1SiO4:Ce3+ was successfully synthesized by the traditional high-temperature solid phase reaction. The doping of Ce3+ had no influence on the Li2Sr0.9Mg0.1SiO4 lattice. Upon UV illumination, typical 5d-2F5/2 and 5d-2F7/2 transitions from Ce3+ ions were observed by PL spectra, and the optimal doping concentration for Ce3+ of 0.4 at% was determined. After the UV irradiation was removed, an afterglow was observed from the Li2Sr0.9Mg0.1SiO4:Ce3+ phosphor, which was later confirmed in terms of the triple-exponential model with a maximum lifetime value (τ3) of 73.9 s. The LLP phenomenon of the matrix was supposed to be caused by the thermo-stimulated recombination of holes and free electrons, which were released from the trapped metastable state at room temperature.Conflicts of interest
There are no conflicts to declare.References
- Y. Lin, Z. Tang and Z. Zhang, Preparation of long-afterglow Sr4Al14O25 based luminescent material and its optical properties, Mater. Lett., 2001, 51, 14–18 CrossRef CAS.
- Y. Lin, Z. Tang and Z. Zhang, Preparation of a new long afterglow blue-emitting Sr2MgSi2O7-based photoluminescent phosphor, J. Mater. Sci. Lett., 2001, 20, 1505–1506 CrossRef CAS.
- X. Wang, Z. Zhang and Z. Tang, Characterization and properties of a red and orange Y2O2S-based long afterglow phosphor, Mater. Chem. Phys., 2003, 80, 1–5 CrossRef CAS.
- I. Ahemen and F. B. Dejene, Spectroscopic Investigation of Ce3+/Eu3+ Co-Doped Li2bazro4 Nanocrystalline Phosphors, J. Alloys Compd., 2018, 735, 2436–2445 CrossRef CAS.
- Y. Chen, X. Cheng and M. Liu, et al., Comparison study of the luminescent properties of the white-light long afterglow phosphors: CaxMgSi2O5+x: Dy3+(x= 1, 2, 3), J. Lumin., 2009, 129, 531–535 CrossRef CAS.
- Y. Jin, Y. Hu, L. Chen, X. Wang, Z. Mu, G. Ju and T. Wang, A novel orange emitting long afterglow phosphor Ca3Si2O7: Eu2+ and the enhancement by R3+ ions (R= Tm, Dy and Er), Mater. Lett., 2014, 126, 75–77 CrossRef CAS.
- J. Ding, Q. Wu and Y. Li, Self-Activated Yellow Light Emitting Phosphors of α, β-Ca3B2N4 with Long Afterglow Properties, Inorg. Chem., 2016, 55, 10990–10998 CrossRef CAS PubMed.
- T. Cui, P. Ma and Y. Sheng, et al., Preparation of CaAl2O4: Eu2+, Nd3+ and SrAl2O4: Eu2+, Dy3+ long afterglow luminescent materials using oil shale ash, Opt. Mater., 2017, 67, 84–90 CrossRef CAS.
- W. Li, Y. Liu and P. Ai, Synthesis and luminescence properties of red long-lasting phosphor Y2O2S:Eu3+, Mg2+, Ti4+ nanoparticles, Mater. Chem. Phys., 2010, 119, 52–56 CrossRef CAS.
- Y. Mei, H. Xu, J. Zhang, Z. Ci, M. Duan and S. Peng, Design and spectral control of a novel ultraviolet emitting long lasting phosphor for assisting TiO2photocatalysis: Zn2SiO4:Ga3+, Bi3+, J. Alloys Compd., 2015, 622, 908–912 CrossRef CAS.
- M. P. Saradhi and U. V. Varadaraju, Photoluminescence studies on Eu2+ activated Li2SrSiO4 a potential orange-yellow phosphor for solid-state lighting, Chem. Mater., 2006, 18, 5267–5272 CrossRef CAS.
- S. M. Levshov, I. V. Berezovskaya and N. P. Efryushina, Synthesis and luminescence properties of Eu2+ doped Li2SrSiO4, Inorg. Mater., 2011, 47, 285–289 CrossRef CAS.
- J. Liu, J. Sun and C. Shi, A new luminescent material: Li2CaSiO4:Eu2+, Mater. Lett., 2006, 60, 2830–2833 CrossRef CAS.
- S. Cheng, X. Xu, J. Han, J. Qiu and B. Zhang, Design, synthesis and characterization of a novel orange-yellow long-lasting phosphor: Li2SrSiO4:Eu2+, Dy3+, Powder Technol., 2015, 276, 129–133 CrossRef CAS.
- P. You, Effect of Tb3+doped Concentration on Properties of Li2SrSiO4:Tb3+ Phosphor, Adv. Mater. Res., 2014, 919, 2052–2056 Search PubMed.
- E. Erdoğmuş, I. Pekgözlü and E. Korkmaz, Synthesis and Photoluminescence Properties of Li2SrSiO4: Pb2+, J. Appl. Spectrosc., 2014, 81, 336–340 CrossRef.
- H. He, R. Fu and Y. Cao, et al., Ce3+→Eu2+ energy transfer mechanism in the Li2SrSiO4:Eu2+, Ce3+ phosphor, Opt. Mater., 2010, 32, 632–636 CrossRef CAS.
- X. Y. Li, D. Y. Zhang, Y. Chen and C. K. Chang, Preparation and characterization of a green emitting Li2Ca0.4Sr0.6SiO4: Tb3+ phosphor with afterglow behavior, Ceram. Int., 2017, 43, 1677–1681 CrossRef CAS.
- X. Y. Li, D. Y. Zhang and C. K. Chang, Preparation and characterization of orange -yellow emitting Li2Ca0.4Sr0.6SiO4: Eu2+, Dy3+ phosphor with afterglow behavior, J. Lumin., 2017, 183, 48–52 CrossRef CAS.
- W. R. Liu, C. H. Huang and C. P. Wu, et al., High efficiency and high color purity blue-emitting NaSrBO3: Ce3+phosphor for near UV light-emitting diodes, J. Mater. Chem., 2011, 21, 6869–6874 RSC.
- X. Y. Huang, J. Liang, X. X. Li and A. He, Manipulating Upconversion Emission of Cubic Bagdf5:Ce3+/Er3+/Yb3+ Nanocrystals through Controlling Ce3+ Doping, J. Alloys Compd., 2017, 721, 374–382 CrossRef CAS.
- D. Pasiński, E. Zych and J. Sokolnicki, Ce3+ to Mn2+ Energy Transfer in Sr3Y2Ge3O12: Ce3+, Mn2+ Garnet Phosphor, J. Alloys Compd., 2015, 653, 636–642 CrossRef.
- R. A. Talewar, S. Mahamuda, A. Vyas, A. S. Rao and S. V. Moharil, Enhancement of 1.54 mm Emission in Ce3+/Er3+ Codoped Ca4si2o7f2 Phosphor, J. Alloys Compd., 2019, 775, 810–817 CrossRef CAS.
- D. W. Cooke, B. L. Bennett and R. E. Muenchausen, Intrinsic ultraviolet luminescence from Lu2O3, Lu2SiO5 and Lu2SiO5: Ce3+, J. Lumin., 2004, 106(2), 125–132 CrossRef CAS.
- H. Suzuki, T. A. Tombrello, C. L. Melcher and J. S. Schweitzer, Light Emission Mechanism of Lu2(SiO4)O: Ce, IEEE Trans. Nucl. Sci., 1993, 40, 380–388 CAS.
- D. Wen, J. Shi and M. Wu, et al., Studies of terbium bridge: saturation phenomenon, significance of sensitizer and mechanisms of energy transfer, and luminescence quenching, ACS Appl. Mater. Interfaces, 2014, 6(13), 10792–10801 CrossRef CAS PubMed.
- J. Chen, C. Guo and Z. Yang, et al., Li2SrSiO4: Ce3+, Pr3+ Phosphor with Blue, Red, and Near-Infrared Emissions Used for Plant Growth LED, J. Am. Ceram. Soc., 2016, 99, 218–225 CrossRef CAS.
- T. Katsumata, T. Nabae and K. Sasajima, et al., Effects of Composition on the Long Phosphorescent SrAl2O4: Eu2+, Dy3+ Phosphor Crystals, J. Electrochem. Soc., 1997, 144, L243–L245 CrossRef CAS.
- G. Che, C. Liu and X. Li, et al., Luminescence properties of a new Mn2+ activated red long-afterglow phosphor, J. Phys. Chem. Solids, 2008, 69, 2091–2095 CrossRef CAS.
- C. Liu, G. Che and Z. Xu, et al., Luminescence properties of a Tb3+ activated long-afterglow phosphor, J. Alloys Compd., 2009, 474, 250–253 CrossRef CAS.
- E. W. Forsythe, D. C. Morton, C. W. Tang and Y. L. Gao, Trap states of tris-8-(hydroxyquinoline) aluminum and naphthyl-substituted benzidine derivative using thermally stimulated luminescence, Appl. Phys. Lett., 1998, 73, 1457–1459 CrossRef CAS.
- M. Shi, D. Zhang and C. Chang, Dy3+: Ca2SnO4, a new yellow phosphor with afterglow behavior, J. Alloys Compd., 2015, 639, 168–172 CrossRef CAS.
- Z. Q. Liang, J. S. Zhang, J. S. Sun, X. P. Li, L. H. Cheng, H. Y. Zhong, S. B. Fu, Y. Tian and B. J. Chen, Enhancement of green long-lasting phosphorescence in CaSnO3: Tb3+ by addition of alkali ions, Physica B, 2013, 412, 36–40 CrossRef CAS.
- R. Chen, Glow curves with general order kinetics, J. Electrochem. Soc., 1969, 116, 1254–1260 CrossRef.
- J. Y. Kuang and Y. L. Liu, Luminescence properties of a Pb2+ activated long-afterglow phosphor, J. Electrochem. Soc., 2006, 153, G245–G247 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |