Open Access Article
Open Access ArticleControllable synthesis of CsPbI3 nanorods with tunable photoluminescence emission
Zhikai Wenab,
Wei Zhaiab,
Chang Liuab,
Jing Lin *ab,
Chao Yu
*ab,
Chao Yu *ab,
Yang Huang
*ab,
Yang Huang *ab,
Jun Zhang
*ab,
Jun Zhang ab and
Chengchun Tang
ab and
Chengchun Tang ab
ab
aSchool of Materials Science and Engineering, Hebei University of Technology, Tianjin 300130, P. R. China. E-mail: linjing@hebut.edu.cn; yuchao20130426@126.com; huangyang@hebut.edu.cn
bHebei Key Laboratory of Boron Nitride Micro and Nano Materials, Hebei University of Technology, Tianjin 300130, P. R. China
First published on 12th August 2019
Abstract
So far the controllable synthesis of one-dimensional (1D) CsPbI3 nanocrystals still remains a challenge due to the fast reaction kinetics of the iodine system as compared with other halide perovskites. Here we report the direct synthesis of high-quality 1D CsPbI3 nanorods by a facile solvothermal method. The as-prepared CsPbI3 nanorods show high purity and uniform morphology with ultrafine diameters down to ∼5 nm. By simply changing the solvothermal reaction conditions, fine-tuning of the sizes of the CsPbI3 nanorods can be well achieved, which leads to the successful modulation of their photoluminescence (PL) emission. The solvothermal reaction offers relatively low crystal growth rate, which is of great importance for the size control of the CsPbI3 nanocrystals. PL quantum yields (PLQYs) and lifetime results indicate that the obtained nanorods maintain a good surface state over long reaction time. Our work not only provides a reliable means for the synthesis of 1D iodine-related perovskites, but also expands the study of size-related PL properties on perovskites nanocrystals.
Introduction
In recent years, all-inorganic perovskite CsPbX3 (X = Br, Cl, I) nanocrystals with high absorption coefficient,1 high photoelectric conversion efficiency,2 adjustable band gap,3 high photoluminescence (PL) quantum yield (QY)4 and better air stability5 have shown high application value in the fields of semiconductor devices such as solar cells, light-emitting diodes, and photodetectors.6–9 It is well known that perovskite nanocrystals have strong quantum confinement effects, and their PL characteristics are influenced by their morphology and size.10–13 Among them, iodide perovskite nanocrystals have attracted great attention due to the unique narrow band gap and tunable efficient red emission.14,15 More noteworthy is the one-dimensional (1D) CsPbI3 nanocrystals with a particular crystallographic direction. Different from other structures and compositions of inorganic perovskite nanocrystals, 1D CsPbI3 nanocrystals have fewer grain boundaries, limits carrier transport area and shortens the carrier transport distance, which provides conditions for the generation of high-efficiency optoelectronic devices.16,17So far, many efforts have been developed for preparation of 1D CsPbI3 nanocrystals. For example, Fang et al. synthesized CsPbI3 nanorods with diameter of ∼45 nm using a polar solvent controlled ionization method.18 Zhang et al. used a solution-phase method to synthesize CsPbI3 nanowires with tens to hundreds of diameters.19 Recently, Chen et al. reported a two-step injection method for directly synthesizing CsPbI3 nanowires with high stability.20 In addition, the indirect synthesis of small-scale 1D CsPbI3 nanocrystals (anion exchange,21 chemical cutting22) has also received great attention. However, the development of simple and efficient direct synthesis of 1D CsPbI3 nanocrystals with high purity and high quality is still challenging. In addition, the size control of the 1D CsPbI3 nanocrystals can better meet the needs of semiconductor devices of different specifications.
Here, we use a simple solvothermal method to directly synthesize 1D CsPbI3 nanorods with ultrafine diameter. The CsPbI3 nanorods have high purity and narrow emission peak with a full width at half maxima (FWHM) of 29 nm. The strong quantum confinement effect allows the nanorods to achieve tunable size-dependent PL emission. Finally the PLQYs, PL lifetimes and stability are further studied.
Experimental
Preparation of PbI2 precursor
2 mmol of PbI2 and 20 mL of octadecene (ODE) were loaded into a 50 mL three-necked flask containing with 3 mL of oleic acid (OA) and 3 mL of oleylamine (OLA). The mixed solution was heated in an oil bath at 120 °C and kept under stirring until the PbI2 salt was completely dissolved.Preparation of Cs-oleate precursor
0.2 mmol of Cs2CO3, 18 mL of ODE and 2.5 mL of OA were added into a 50 mL three-necked flask. The solution was heated in an oil bath at 125 °C and stirred constantly until all Cs2CO3 had reacted with OA. Then the solution was cooled down to room temperature.Solvothermal synthesis of CsPbI3 nanorods
In a typical experiment, 26 mL of PbI2 precursor and 2 mL of Cs-oleate precursor were loaded into a Teflon lined autoclave (100 mL) at room temperature. The mixed solution was stirred for 3 min and then reacted at 100–180 °C for different times (1, 10, 40, 60, 90 min). After reaction, the crude solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the residual reactants. The substrate was dispersed in 10 mL of n-hexane and centrifuged at 5000 rpm for 5 min to remove large particles.
000 rpm for 10 min to remove the residual reactants. The substrate was dispersed in 10 mL of n-hexane and centrifuged at 5000 rpm for 5 min to remove large particles.
Characterization
The crystallinity and morphology were measured by X-ray diffraction (XRD, BRUKER D8 DISCOVER) and transmission electron microscopy (TEM, Philips Tecnai F20 microscope). UV-Vis absorption spectra were collected using a Japan HITACHI U-3900H spectrometer. PLQY and lifetime measurements were performed by Fluorolog-3 Horiba Jobin-Yvon spectrofluorimeter equipped with an integrating sphere. The actual contents of metal loading were analyzed by inductively coupled plasma emission spectroscopy (ICP-AES, Optima 8300).Results and discussion
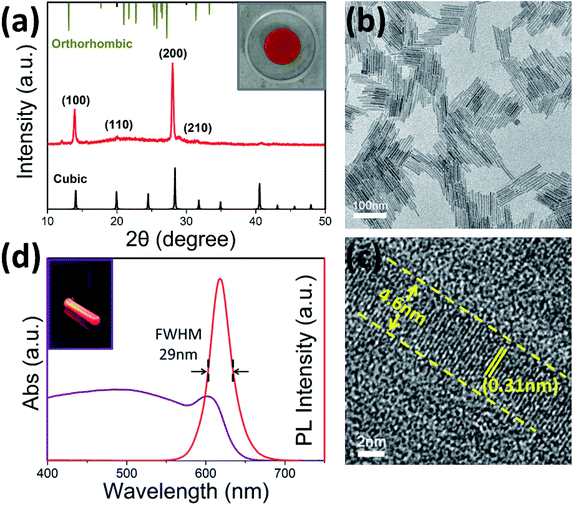
The XRD pattern indicates that the prepared CsPbI3 nanorods are crystallized in cubic phase (Fig. 1a). The main peaks of the samples are located at 14.3°, 20.3°, 28.8° and 32.2°, corresponding to the (100), (110), (200) and (201) planes of cubic CsPbI3, respectively.23,24 It is well known that CsPbI3 is stable existence in the form of a orthorhombic phase at ambient temperature.25 The presence of orthorhombic phase was not observed in the XRD pattern of our sample, demonstrating the high purity of the sample. Typical TEM image of the sample is shown in Fig. 1b. The product consists of numerous 1D rod-shaped nanoparticles. The nanorods have uniform morphology with average length of ∼80 nm. Interestingly, all of the nanorods tend to arrange as regular arrays. In addition, no other impurities such as nanoparticles can be observed, illustrating the high purity and regular morphology of the product. High-resolution TEM (HRTEM) image of an individual nanorod (Fig. 1c) demonstrates that the nanorod has an ultrafine diameter of ∼4.6 nm. The crystal structure of the sample can also been verified by HRTEM observation. The planes are perpendicular to the long axis of the nanorods and show an interplanar spacing of 0.31 nm, which can be attributed to the (200) plane of cubic CsPbI3. So the nanorods should grow along [100] direction. Due to the small size and uniform morphology, the nanorods still have good dispersibility in hexane, which is undoubtedly advantageous for solution reprocessing and the measurement of optical properties of the samples.The UV-Vis absorption and PL emission spectra of the CsPbI3 nanorods are shown in Fig. 1d. The PL spectrum displays an emission peak centered at ∼618 nm, which is consistent with the phenomenon that the sample emits visible bright red light in the light box irradiated by 365 nm ultraviolet light (shown in inset of Fig. 1d). As compared with bulk CsPbI3 which often displays red emission at 710 nm,26 here the PL emission with a blue shift of ∼92 nm can be attributed to the strong quantum confinement effect in the ultrafine nanorods. In fact, the nanorods have diameters far below the Bohr radius (∼6.5 nm) of CsPbI3.27 It is also noteworthy that the emission peak has a narrow full width at half maxima (FWHM) of only 29 nm, further confirming the high uniformity of the nanorods. Normally the synthesis of 1D CsPbI3 nanocrystals may require higher temperatures with the aim of ensure the uniformity of sample size (Zhang synthesized CsPbI3 nanowires at temperatures higher than 180 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19), whereas our synthesis process can be carried out at a relatively low temperature, which should be attributed to the high pressure environment in the autoclave. The UV-Vis absorption peak is located at ∼601 nm, and the nanorod has a typical Stokes shift of 13 nm.
19), whereas our synthesis process can be carried out at a relatively low temperature, which should be attributed to the high pressure environment in the autoclave. The UV-Vis absorption peak is located at ∼601 nm, and the nanorod has a typical Stokes shift of 13 nm.
The fine-tune on the morphology of the CsPbI3 nanorods can be well achieved by simply changing the solvothermal reaction times (0–90 min). TEM images (Fig. 2a–d) indicate that the sizes of the samples change regularly. The sample obtained after 1 min's reaction (1 min after the reaction temperature rises to 160 °C) consists of nanorods with high aspect ratios and flexible feature (Fig. 2a). When the reaction time is extended to 90 min, the shape of the product gradually changes into straight and short nanorods (Fig. 2d). Statistical diagrams of the length and diameter distributions based on the statistics of more than 100 nanorods in each sample are shown in Fig. 2e–h. With an increase of reaction time, the average length of the sample decreases from ∼264 to ∼62 nm, while the diameter changes slightly from ∼4.1 to ∼5.1 nm. This indicates that the sizes of the CsPbI3 nanorods can be fine controlled by changing the solvothermal reaction times. As compared with the obviously decrease in nanorod lengths, the slightly change in their diameter may be attributed to the 1D growth direction. In addition, all of the samples show well monodispersity with no obvious impurities. Even if the reaction is extended to 90 min, the sample maintains regular 1D morphology. The results indicate that the solvothermal conditions can effectively control the reaction rate between Cs-oleate and PbI2 precursors. The sample in the reaction can be kept in a relatively stable state, and the generation of impurities can be suppressed.
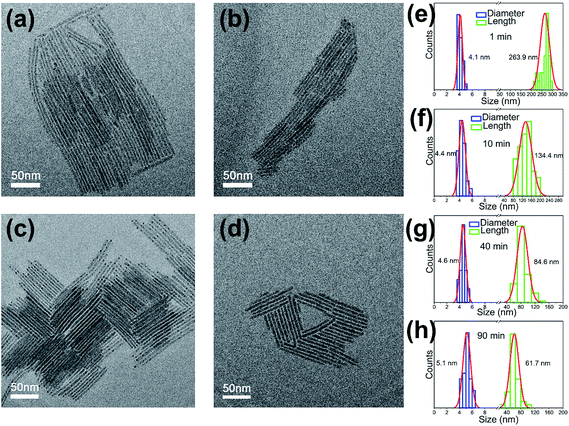 | ||
| Fig. 2 TEM images of CsPbI3 nanorods prepared at 1 min (a), 10 min (b), 40 min (c) and 90 min (d). (e–h) The corresponding diameter and length distribution histograms. | ||
It is noteworthy that iodine system always has faster reaction kinetics as compared with other halide pervoskites. However, the fast kinetics always result in larger diameters (tens or hundreds of nanometers). Therefore, the controllable synthesis of 1D CsPbI3 nanocrystals still remains challenges. With the aim of controllable synthesis of CsPbI3 nanocrystals, a relatively mild growth environment which can effectly suppress the reaction kinetics of iodine system is highly desirable. Our results have shown that ultrafine 1D CsPbI3 nanocrystals with diameters of less than 10 nm can be successfully obtained by the solvothermal method and the sizes can be well tuned by adjusting the reaction times. The solvothermal reaction in the sealed environment offers relatively low crystal growth rate, which is of great importance for the size control. It has also been experimentally proved that changes in external pressure will lead to changes in the band gap of the synthesized perovskite.28 It is believed that the high pressure environment also plays an important role in the synthesis of CsPbI3 nanocrystals. In addition, the role of the ligands cannot be ignored. In the reaction process, oleic acid and oleylamine as surface ligands can change the kinetic pathway, promote the formation of anisotropic nanostructures, and stabilize the final colloidal nanostructure.29 Here, we used ICP to understand the amount of organic ligands in the sample. In detail, the CsPbI3 nanorods with organic surfactants on surfaces were separated from n-hexane dispersion by ultracentrifugation and filtration and then weighted. Then the CsPbI3 nanorods with organic surfactants on surfaces were redissolved in distilled water and the concentration of Pb+ was measured by ICP-AES. Finally we could calculate the amount of organic surfactants in the samples. The mass ratio of CsPbI3 nanocrystals to surface ligands is calculated to be ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. We believe that a relatively high amount of ligands is beneficial to inhibit the rapid growth of CsPbI3 nanocrystals. Moreover, the proper ratio of oleic acid and oleylamine leads to the generation of 1D structure.
6. We believe that a relatively high amount of ligands is beneficial to inhibit the rapid growth of CsPbI3 nanocrystals. Moreover, the proper ratio of oleic acid and oleylamine leads to the generation of 1D structure.
The as-prepared CsPbI3 nanorods display distinct size-dependent PL properties. The PL emission peaks of samples prepared with an increasing reaction times show a significant red shift from 609 to 627 nm (Fig. 3a). The nanorods have diameters relative to the exciton Bohr diameters of CsPbI3. Therefore, the tunable sizes of the nanorods is responsible for the red shift of PL emission due to the quantum confinement effect. Correspondingly, the absorption peaks of the nanorods also change from 585 nm to 619 nm (Fig. 3b). The PLQY is an important performance parameter for the luminescent materials. Fig. 3c shows the measured PLQYs of the samples prepared at different reaction times. The PLQYs of nanorods are 40%, 37%, 46%, 41%, and 15%, respectively. The PL decay curves of the as-prepared nanorods are shown in Fig. 3d. The average lifetimes of the different samples are 18, 16, 17, 20, and 32 ns, respectively. The lifetimes can be analyzed by double-exponential fitting method, as shown in Table 1. All of the average lifetimes contain short lifetime components (τ1) and long lifetime components (τ2). The long lifetimes are associated with non-radiative recombination, while the short lifetime components represent radiation recombination.30 In our work, the sample obtained with short reaction time has a higher ratio of short lifetime component (A1), indicating the fast radiation recombination rate and therefore has higher PLQYs. The luminescent properties of perovskite nanocrystal are also affected by the binding between it and the ligand.31 Only when the reaction is extended to 90 min, the PLQY and lifetime of the nanorods show significant changes, which means that the obtained nanorods are able to maintain these properties stably over a relatively long reaction time in a solvothermal environment, thereby ensuring stable luminescence.
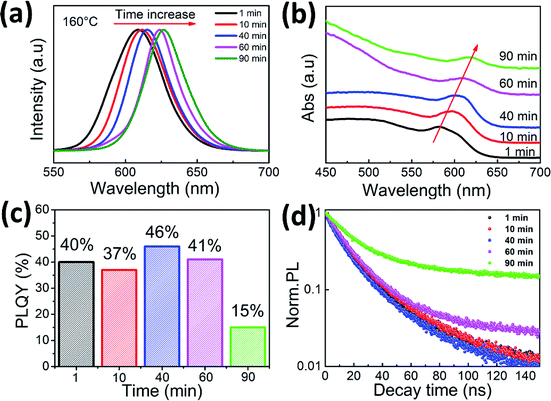 | ||
| Fig. 3 PL emission spectra (a), UV-Vis absorption spectra (b), PLQYs (c) and time-resolved PL decay curves (d) of CsPbI3 nanorods prepared at different times. | ||
| CsPbI3 sample | τ1 (ns) | A1 (%) | τ2 (ns) | A2 (%) | τavg (ns) |
|---|---|---|---|---|---|
| 1 min | 13.6 | 60.98 | 48 | 39.02 | 18 |
| 10 min | 10.1 | 58.39 | 40.2 | 41.61 | 16 |
| 40 min | 13.6 | 55.05 | 44 | 44.95 | 17 |
| 60 min | 14.5 | 69.09 | 56.3 | 30.91 | 20 |
| 90 min | 17.4 | 44.96 | 108 | 55.04 | 32 |
The size-dependent PL emission can also be achieved by controlling of the solvothermal reaction temperatures. Fig. 4a–c show typical TEM images of different samples prepared at temperatures from 100 °C to 180 °C, respectively. It should be noted that at lower temperature conditions (100 °C), both nanowires and nanosheets are present in the product, wherein the nanosheets have lateral sizes of 50–100 nm. As the temperature increased, the samples show higher purity with rodlike morphology (Fig. 4b and c), which means that in a solvothermal reaction, a higher temperature is advantageous for the 1D growth of the nanocrystals. In addition, some black spots were observed in our samples because the perovskite nanocrystals were not stable in the TEM test environment, especially after prolonged exposure. The structure of the perovskite is easily destroyed by the electron beam, which results in the production of a large number of lead points. Such phenomena have also been reported in the synthesis of other perovskites.32 The PL properties of the products also change accordingly. As shown in Fig. 4d, the samples have tunable PL emission with a red shift from 613 to 624 nm. As compared with the red shift caused by the change of reaction time (∼28 nm), here the narrower shift is due to the relative smaller size-change resulted by the change of reaction temperatures. Therefore, we believe that the changing of solvothermal reaction time without affecting the purity of the product is a more effective means of regulating the PL properties of the CsPbI3 nanorods.
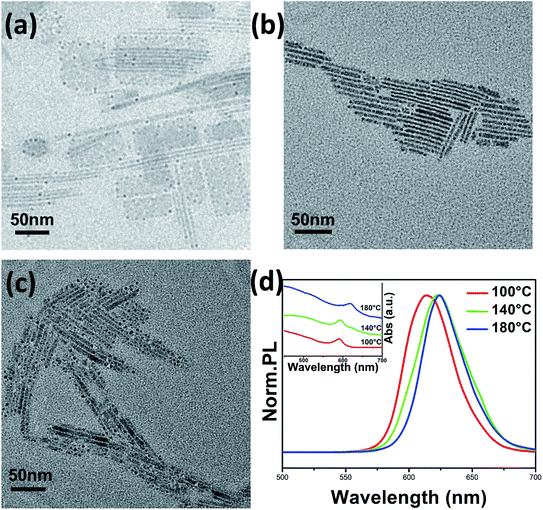 | ||
| Fig. 4 TEM images of CsPbI3 nanocrystals prepared at 100 °C (a), 140 °C (b), and 180 °C (c) for 1 h. (d) PL emission spectra and UV-Vis absorption spectra of the samples. | ||
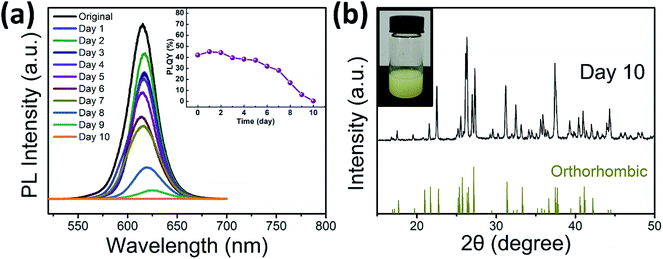
Finally, the stability of the as-prepared CsPbI3 nanorods has also been evaluated. The PL emission spectra of the sample (prepared at 160 °C for 40 min) after stored in air for several days are shown in Fig. 5a. The PL intensity decreases slowly within the first 5 days, and the measured PLQY (shown inset of Fig. 5a) does not even have obvious attenuation. However, after 5 days, the decay rate of the PL intensity is obviously faster, and PLQY is also attenuated from 38% to 1%. In addition, a red shift of the emission peak (from 515 to 525 nm) can be observed, which may be due to particle size changes caused by agglomeration of the nanorods. XRD result (Fig. 5b) indicates that the cubic CsPbI3 was converted to orthorhombic CsPbI3 after 10 days of storage. The color of the sample also changes from red to pale yellow (inset of Fig. 5b). In addition to the normal temperature phase instability of cubic CsPbI3, moisture in the air may also accelerate its structural changes.20 Therefore, inert gas protection and proper packaging are necessary.
Conclusions
In summary, monodisperse and uniform-sized CsPbI3 nanorods were synthesized by a solvothermal method. The controlled size adjustment can be achieved by changing the reaction time and temperatures, which provides a simple and feasible solution for the regulation on the morphology of perovskite nanocrystals, especially in the 1D direction. The narrow emission and adjustable PL properties indicate that the CsPbI3 nanorods have good application potential in the field of luminescence. Our work not only expands the morphological study of perovskite nanocrystals, but also expands the study of its size-related PL properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51572068, 51772075, 21806029), the Natural Science Foundation of Hebei Province (E2019202086, E2019202347), the Hundred Talents Program of Hebei Province (E2014100011), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: IRT17R33).Notes and references
- J. De Roo, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Kovalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS PubMed.
- H. Kim, J. S. Han, J. Choi, S. Y. Kim and H. W. Jang, Small Methods, 2018, 2, 1700310 CrossRef.
- H. Zhou, S. Yuan, X. Wang, T. Xu, X. Wang, H. Li, W. Zheng, P. Fan, Y. Li and L. Sun, ACS Nano, 2016, 11, 1189–1195 CrossRef PubMed.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase and T. Minemoto, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS.
- J. S. Han, Q. Van Le, J. Choi, K. Hong, C. W. Moon, T. L. Kim, H. Kim, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2018, 28, 1705783 CrossRef.
- B. Li, Y. Zhang, L. Fu, T. Yu, S. Zhou, L. Zhang and L. Yin, Nat. Commun., 2018, 9, 1076 CrossRef PubMed.
- Y. Tian, C. Zhou, M. Worku, X. Wang, Y. Ling, H. Gao, Y. Zhou, Y. Miao, J. Guan and B. Ma, Adv. Mater., 2018, 30, 1707093 CrossRef PubMed.
- W. Zhai, J. Lin, C. Li, S. Hu, Y. Huang, C. Yu, Z. Wen, Z. Liu, Y. Fang and C. Tang, Nanoscale, 2018, 10, 21451–21458 RSC.
- Y. Wang, M. Yasar, Z. Luo, S. Zhou, Y. Yu, H. Li, R. Yang, X. Wang, A. Pan and L. Gan, Small, 2018, 14, 1803010 CrossRef PubMed.
- H. He, B. Tang and Y. Ma, Nanotechnology, 2018, 29, 55601 CrossRef PubMed.
- W. Zhai, J. Lin, Q. Li, K. Zheng, Y. Huang, Y. Yao, X. He, L. Li, C. Yu and C. Liu, Chem. Mater., 2018, 30, 3714–3721 CrossRef CAS.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS PubMed.
- Y. Wu, X. Li and H. Zeng, ACS Energy Lett., 2019, 4, 673–681 CrossRef CAS.
- L. Martinez-Sarti, S. H. Jo, Y. H. Kim, M. Sessolo, F. Palazon, T. W. Lee and H. J. Bolink, Nanoscale, 2019, 11, 12793–12797 RSC.
- A. Dutta, S. K. Dutta, S. Das Adhikari and N. Pradhan, Angew. Chem., Int. Ed., 2018, 57, 9083–9087 CrossRef CAS PubMed.
- W. Tian, H. Zhou and L. Li, Small, 2017, 13, 1702107 CrossRef PubMed.
- T. Yang, Y. Zheng, Z. Du, W. Liu, Z. Yang, F. Gao, L. Wang, K. C. Chou, X. Hou and W. Yang, ACS Nano, 2018, 12, 1611–1617 CrossRef CAS PubMed.
- F. Fang, W. Chen, Y. Li, H. Liu, M. Mei, R. Zhang, J. Hao, M. Mikita, W. Cao and R. Pan, Adv. Funct. Mater., 2018, 28, 1706000 CrossRef.
- D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. Yang, J. Am. Chem. Soc., 2015, 137, 9230–9233 CrossRef CAS PubMed.
- Z. Chen, L. Dong, H. Tang, Y. Yu, L. Ye and J. Zang, CrystEngComm, 2019, 21, 1389–1396 RSC.
- D. Zhang, Y. Yang, Y. Bekenstein, Y. Yu, N. A. Gibson, A. B. Wong, S. W. Eaton, N. Kornienko, Q. Kong and M. Lai, J. Am. Chem. Soc., 2016, 138, 7236–7239 CrossRef CAS PubMed.
- Y. Tong, M. Fu, E. Bladt, H. Huang, A. F. Richter, K. Wang, P. Müller-Buschbaum, S. Bals, P. Tamarat and B. Lounis, Angew. Chem., 2018, 130, 16326–16330 CrossRef.
- G. E. Eperon, G. M. Paterno, R. J. Sutton, A. Zampetti, A. A. Haghighirad, F. Cacialli and H. J. Snaith, J. Mater. Chem. A, 2015, 3, 19688–19695 RSC.
- P. Luo, W. Xia, S. Zhou, L. Sun, J. Cheng, C. Xu and Y. Lu, J. Phys. Chem. Lett., 2016, 7, 3603–3608 CrossRef CAS PubMed.
- Y. Zhou and Y. Zhao, Energy Environ. Sci., 2019, 12, 1495–1511 RSC.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019–9038 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- Y. Cao, G. Qi, C. Liu, L. Wang, Z. Ma, K. Wang, F. Du, G. Xiao and B. Zou, J. Phys. Chem. C, 2018, 122, 9332–9338 CrossRef CAS.
- A. Pan, B. He, X. Fan, Z. Liu, J. J. Urban, A. P. Alivisatos, L. He and Y. Liu, ACS Nano, 2016, 10, 7943–7954 CrossRef CAS PubMed.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435–2445 CrossRef CAS.
- D. Yang, X. Li, W. Zhou, S. Zhang, C. Meng, Y. Wu, Y. Wang and H. Zeng, Adv. Mater., 2019, 1900767 CrossRef PubMed.
- Q. A. Akkerman, S. G. Motti, A. R. Srimath Kandada, E. Mosconi, V. D'Innocenzo, G. Bertoni, S. Marras, B. A. Kamino, L. Miranda and F. De Angelis, J. Am. Chem. Soc., 2016, 138, 1010–1016 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |