Open Access Article
Open Access ArticleEnhanced hydrogen storage performance of graphene nanoflakes doped with Cr atoms: a DFT study
Chunqi Xianga,
Ao Lia,
Shulin Yang *a,
Zhigao Lana,
Wei Xiea,
Yiming Tanga,
Huoxi Xu*a,
Zhao Wangb and
Haoshuang Guab
*a,
Zhigao Lana,
Wei Xiea,
Yiming Tanga,
Huoxi Xu*a,
Zhao Wangb and
Haoshuang Guab
aSchool of Physics and Electronic Information, Hubei Key Laboratory for Processing and Application of Catalytic Materials, Huanggang Normal University, Huanggang 438000, P. R. China. E-mail: yangsl@hgnu.edu.cn; xuhuoxi@hgnu.edu.cn
bFaculty of Physics and Electronic Sciences, Hubei University, Wuhan 430062, P. R. China
First published on 15th August 2019
Abstract
The hydrogen storage performances of novel graphene nanoflakes doped with Cr atoms were systematically investigated using first-principles density functional theory. The calculated results showed that one Cr atom could be successfully doped into the graphene nanoflake with a binding energy of −4.402 eV. Different from the H2 molecule moving away from the pristine graphene nanoflake surface, the built Cr-doped graphene nanoflake exhibited a high affinity to the H2 molecule with a chemical adsorption energy of −0.574 eV. Moreover, the adsorptions of two to five H2 molecules on the Cr-doped graphene nanoflake were studied as well. It was found that there were a maximum of three H2 molecules stored on the graphene nanoflake doped with one Cr atom. Also, the further calculations showed that the numbers of the stored H2 molecules were effectively improved to be six (or nine) when the graphene nanoflakes were doped with two (or three) Cr atoms. This research reveals that the graphene nanoflake doped with Cr atom could be a promising material to store H2 molecules and its H2 storage performance could be effectively enhanced through modifying the number of doped Cr atoms.
1. Introduction
In recent decades, nanostructured carbon-based materials, including C60, carbon nanotubes, graphene sheets, graphene quantum dots and graphene nanoflakes, have attracted great interests all over the world due to their unique physical and chemical properties.1–4 They have been widely researched in various fields of high-performance solar cells, lithium-ion, supercapacitors, water splitting, and gas adsorptions/storages.5–9 Among all the extensively studied carbon-based nanomaterials, graphene (G) first reported in 2004 is composed of the uniform ring of six carbon atoms in a layered structure, making it exhibit an advantage of ultra-high surface area and be a promising candidate for the storage of gas molecules.10,11 However, many investigations have reported that the pure graphene-based material only interacted with the adsorbed gas molecules through weak physical adsorption due to the sp2 hybridizing of carbon atoms.12,13 Recently, researchers have taken some effective measures to solve this problem and improve the gas adsorption performance of the graphene-based substrates. For example, Chi et al. reported that the Al-doped graphene exhibited extremely higher affinities to the CO molecule and H2 molecule than the pure graphene.12 Cortés-Arriagada et al. have also reported that the Fe-doped graphene nanosheet was a more effective platform for the adsorption of the CO, CO2, SO2 and H2S compared with the untreated graphene.14 Moreover, the study by Yang and his teammates revealed that the three CO molecules could be chemically adsorbed on the modified graphene.15 Therefore, the interactions between the gas molecules and the graphene-based materials could be significantly improved through doped with metal atoms.Hydrogen (H2), a renewable and clean energy resource, has been recognized as an ideal substitution for the excessively consumed fossil fuels.16 This outstanding energy carrier is highly friendly to the environment with a wide range of sources and an extremely high energy density (143 kJ g−1).17 Some countries have devoted their efforts to studying the effective application of H2 in the typical industries of aerospace and clean-energy vehicles.18 The efficient storage of hydrogen gas is of significant importance to the safe and full use of this promising energy.19–21 According to the recent reports, one of those effective ways to store hydrogen energy is to design the solid-state material to store the gas in molecules, which has been widely reported in the modified graphene-based sheets.9,22,23 The theoretical and experimental studies of Yang et al. showed that the hydrogen storage capacity of the microporous carbon-based material could be significantly enhanced by modified with Ru due to the spillover effect.24 Fan and his workmates have also done a first-principles study on the hydrogen storage properties of the Sc-decorated graphene. They found that there were six H2 molecules in maximum adsorbed on the modified Sc atom.25 Moreover, the Ti-doped and Os-doped graphene nanoflakes were also calculated to be the potential material to achieve the high hydrogen storage due to the partially occupied 3d orbitals.26,27 Reasonably, it could be inferred that the hydrogen storage performance of the graphene-based materials could be successfully and effectively enhanced through doped with transition metal atoms.
The Cr atom, one of the typical transition metal atoms, is also reported to be selected to modify the graphene-based materials to improve their interactions with the adsorbed gas molecules.28–30 For instance, Zhang et al. have researched the strongly chemical adsorption of the formaldehyde molecule on Cr-doped graphene surface.29 The study of Villagracia and his group also showed that the Cr-doped penta-graphene could interact with the hydrogen gas molecule with the adsorption energy of −0.25 eV, exhibiting higher affinity to hydrogen gas than the pristine penta-graphene.31 However, few literatures were reported to study the interaction between the hydrogen gas and the Cr-doped graphene nanoflake, let alone systematically investigating the hydrogen storage capacity on this promising nanosheet. Furthermore, a majority of the published researches mainly focused on the hydrogen gas molecules stored on the graphene-based sheet modified with only one metal atom, the hydrogen storage performance of the sheets modified with two or more metal atoms was little reported.
In this paper, the graphene nanoflake with H atoms at the end of the C atoms located at the edge and the modified nanoflakes doped with Cr atoms were constructed to study the effects of Cr atom on the hydrogen storage performance of the graphene-based materials. The interaction between the built graphene-based sheets and the stored hydrogen molecules were studied through analysis their optimized morphologies, the density of states and electron densities. Also, the hydrogen gas molecules stored on the graphene nanoflakes doped with two or three Cr atoms were also investigated in details.
2. Calculation method
In our study, all the built modes of the storages of hydrogen gas molecules on graphene nanoflake based materials are studied through the density functional theory (DFT) method with the CASTEP code in Materials Studio. According to the previous studies, the generalized gradient approximation (GGA) parameterized with the Perdew–Burke–Ernzerhof (PBE) correction is used to investigate the full relaxation of the optimized structure.12,32 However, the pure GGA-PBE method always underestimates the adsorption energies, the long-range dispersion correction (DFT-D) proposed by the Grimme scheme is carried out to correct the effect of van der Waals interaction on all the built modes.33 The ultrasoft pseudo-potential with the cut-off energy of 300 eV is adopted in all the calculations. The quality of convergence tolerance is fine and the maximum root-mean-square, remaining stress and forces are set to be ≤1 × 10−5 eV per atom, 0.05 GPa and 0.03 eV Å−1, respectively. Spin-unrestricted calculations with k-point of 4 × 4 × 1 in the Brillouin zone are performed for all the calculated modes to study their geometry optimization structure and electronic properties. The pure graphene nanoflake (PGNF) consisting of 54 C atom and 18 H atoms was constructed through deleting the additional C atoms in the monolayer graphene and then saturating the hydrogen atoms at the end of C atoms in the built nanoflake.34–37 For the doped nanoflake, one Gr atom was directly used to replace one of the C atoms at the center of PGNF to build the Cr-doped graphene nanoflake (CrGNF). Meanwhile, the graphene nanoflakes doped with two Cr atoms (2CrGNF) or three Cr atoms (3CrGNF) were also constructed in a similar way.The average binding energy (Ēb) of Cr atoms in the PGNF is calculated with the equation of
| Ēb = [EnCrGNF − Esub − nECr]/n, | (1) |
The adsorption energies (Ead) and the average adsorption energies (Ēad) of the H2 molecules adsorbed on graphene nanoflakes doped with Cr atoms are defined as
| Ead = EnH2+CrGNF − E(n−1)H2+CrPGNF − EH2 | (2) |
| Ēad = [EnH2+CrGNF − ECrGNF – nEH2]/n, | (3) |
3. Results and discussions
The full relaxations of all the built systems in our study were calculated under the same parameters. The obtained optimized geometries (top view and side view) of the PGNF and CrGNF are displayed in Fig. 1. It can be seen that all the atoms (C and H atoms) were distributed in the same plane in PGNF. The bond lengths of C–C (at the center) and C–H (at the edge) in the nanoflake were calculated to be approximately 1.419 Å and 1.093 Å, respectively, consisting well with the results in previous literatures.40–42 In the CrGNF, the radius of the doped Cr atom (∼1.852 Å) is larger than that of C atom (∼0.913 Å), resulting in the additional stress in the nanoflake. The calculated optimized geometry of the CrGNF in Fig. 1b showed that the Cr atom moved up from the nanoflake to release this tress with the height of 1.766 Å, agreeing well with the reported results.31,43 The bond lengths of Cr atom with the near three C atoms were found to be 1.830 Å, 1.820 Å and 1.821 Å for Cr–C1, Cr–C2 and Cr–C3, respectively. Meanwhile, the further calculations confirmed that the binding energy of a Cr atom doped into the graphene nanoflake was −4.402 eV, indicating the reasonable stability of the CrGNF.44 Similar results were also found in the Mo-doped or Pd-doped graphene sheets.24,45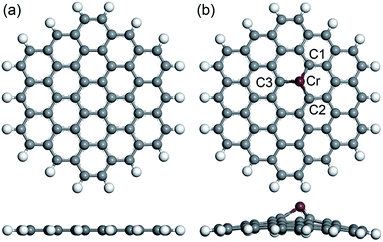 | ||
| Fig. 1 The calculated optimized geometries (top view and side view) of the built PGNF (a) and CrGNF (b). | ||
In the following research, the hydrogen molecules with different orientations were placed on the built nanoflakes to investigate the interactions between the hydrogen gas and the PGNF (or CrGNF). Two typical adsorption modes were constructed for one hydrogen molecule adsorbed on PGNF (or CrGNF): (i) the hydrogen molecule was parallel to the nanoflake with H atom above the active site (C atom for PGNF or Cr atom for CrGNF); (ii) the hydrogen molecule was perpendicular to the nanoflake with the H atom above the active site.24 Interestingly, we found that the final optimized geometries of the built adsorption modes for PGNF or CrGNF were little affected by the orientations of the placed hydrogen molecule. The optimized structures (top view and side view) of the H2 adsorbed on PGNF (mode P1H) or CrGNF (mode Cr1H) systems are shown in Fig. 2. The results presented that the hydrogen molecule placed on PGNF ran far away from the nanoflake with the long-distance (d) of 3.124 Å, indicating the weak interaction between the H2 and the PGNF, which agreed well with the reported results.46,47 While in the case of the Cr doped system, the placed H2 molecule was found to be adsorbed stably above the active Cr atom in the CrGNF. The d between the adsorbed H2 and the CrGNF was found to be 1.756 Å, much shorter than that of the pure system. Meanwhile, the bond length (l) of the H2 adsorbed on the CrGNF expanded to be 0.864 Å, which is longer than that of the H2 adsorbed on the PGNF (0.753 Å). Furthermore, the Ead of the H2 adsorbed on the CrGNF was calculated to be −0.574 eV, higher than that in the pure graphene system (−0.098 eV). The shorter d, longer l and higher Ead implied the stronger interaction between the adsorbed H2 and the graphene nanoflake doped with Cr atom, indicating that the CrGNF could be applied as a promising candidate for the storage of H2 molecules.
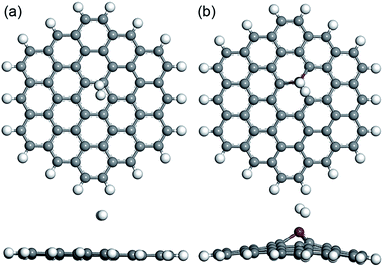 | ||
| Fig. 2 The optimized structures (top view and side view) of built modes of the H2 adsorbed on PGNF (a) and CrGNF (b). | ||
The electron densities and the partial density of states (PDOS) of the systems of one H2 molecule adsorbed on PGNF and CrGNF are further studied to better understand their gas storage performance, as displayed in Fig. 3. The results showed that the electron density of the H2 molecule in the mode P1H was mainly distributed within the molecule (seen in Fig. 3a), indicating the weak interaction between the adsorbed H2 and the substrate of graphene nanoflake. In the case of mode Gr1H, the significant overlap in the electron densities of the adsorbed H2 and that of the CrGNF (seen in Fig. 3b) meant that there were certain electrons transferred between the gas molecule and the substrate, implying their strong interaction with each other.26 All the calculated results provided clear clues that the H2 molecule only exhibited a weak physical adsorption on the PGNF but a strong chemical adsorption on the CrGNF. This different adsorption of H2 molecule on PGNF or CrGNF could also be confirmed through the analysis of their PDOS shown in Fig. 3c and d. The PDOS of the Cr-doped system showed that the peaks of the H2 molecule undertook a stronger overlap with those of the Cr atom in CrGNF than those of the PGNF system due to the hybridization of σ and σ* orbitals of H2 molecule with d orbitals of the Cr atom, further indicating the more effective interaction between the adsorbed H2 molecule and the graphene nanoflake doped with Cr atom.48
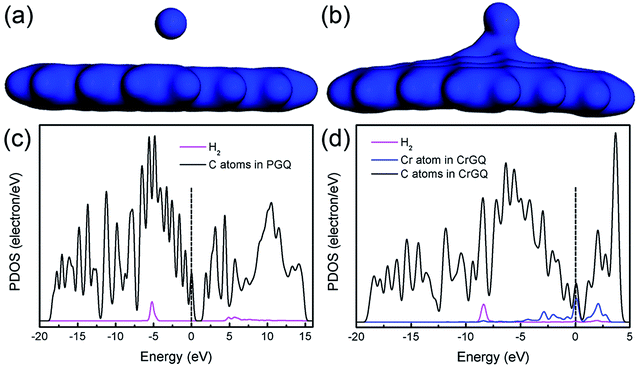 | ||
| Fig. 3 The electron density and PDOS of the systems of one H2 adsorbed on PGNF (a and c) and CrGNF (b and d). | ||
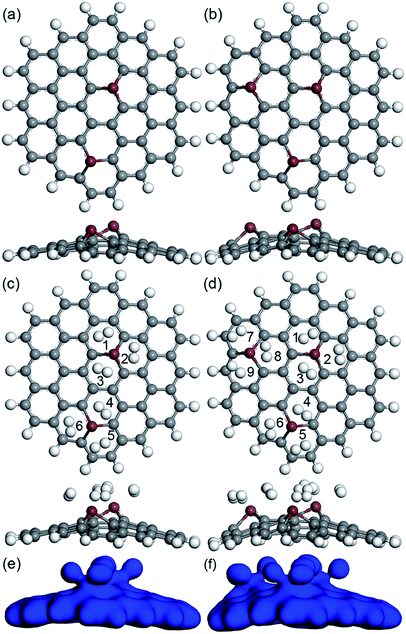
Also, more H2 molecules were also introduced on the CrGNF to fully investigate its hydrogen storage property. Fig. 4 displays the calculated optimized geometries of the adsorption systems in which two, three, four and five H2 molecules were adsorbed on CrGNF, and they were defined as the modes Cr2H, Cr3H, Cr4H and Cr5H, respectively. The corresponding H2 molecules were labeled with different numbers in the above modes. The d between the H2 molecules and the active Cr atom in the doped nanoflake were studied in detail, which was listed in Table 1. As can be seen in Fig. 4a, the two molecules adsorbed on CrGNF in mode Cr2H exhibited a bilaterally symmetric structure with the d of 1.922 Å and 1.942 Å for H2 molecule numbered #1 and #2, respectively, which were comparable to the d of the one H2 molecule adsorbed on CrGNF. In mode Cr3H, the H2 molecules numbered #1, #2 and #3 presented a symmetric structure of a triangle with slightly larger d being 2.177 Å, 1.971 Å and 2.081 Å, respectively.49 However, when the fourth H2 molecule was introduced on the surface of the CrGNF, the obtained results showed that this H2 molecule numbered #4 in mode Cr4H stayed farther away from the substrate than the other three molecules (as seen in Fig. 4c and listed in Table 1), exhibiting a d of ∼3.682 Å. Moreover, the H2 molecules number #4 and #5 in mode Cr5H also showed similar tendencies to exhibit weak interaction with the CrGNF, staying away from the substrate with the d of 3.610 Å and 3.418 Å, respectively. The bond lengths (l) of the H2 molecules in all the built modes were also studied and listed in Table 1. The results showed that the l of the H2 molecule numbered #4 in mode Cr4H and the H2 molecules numbered #4 or #5 in mode Cr5H almost retained the original value of free H2 molecule (approximate 0.754 Å), while the l of the other stored H2 molecules in the studied modes expanded to larger values of over 0.766 Å. Our calculated research is well consistent with the studies of the hydrogen molecules stored on the 8B transition metal-doped silicon carbide nanotubes and graphene quantum dots.27,49
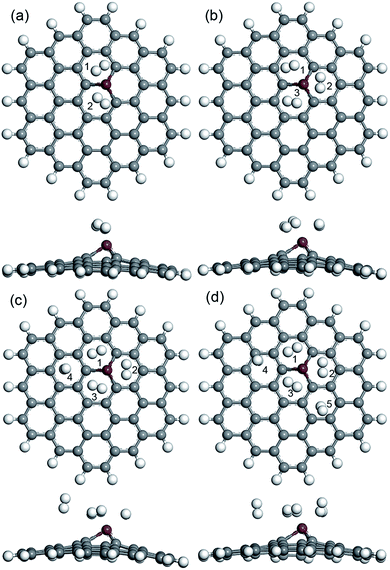 | ||
| Fig. 4 The optimized geometries (top view and side view) of two (a), three (b), four (c) and five (d) H2 molecules adsorbed on CrGNF. | ||
| Sensing material | Mode | Numbers of H2 | l (Å) | d (Å) | Eab (eV) | Ēab (eV) |
|---|---|---|---|---|---|---|
| CrGNF | Cr1H | H2#1 | 0.846 | 1.756 | −0.574 | −0.574 |
| Cr2H | H2#1 | 0.802 | 1.922 | — | −0.363 | |
| H2#2 | 0.792 | 1.942 | −0.160 | |||
| Cr3H | H2#1 | 0.770 | 2.177 | — | −0.310 | |
| H2#2 | 0.815 | 1.971 | — | |||
| H2#3 | 0.779 | 2.081 | −0.182 | |||
| Cr4H | H2#1 | 0.766 | 2.092 | — | −0.261 | |
| H2#2 | 0.799 | 1.942 | — | |||
| H2#3 | 0.779 | 2.087 | — | |||
| H2#4 | 0.754 | 3.682 | −0.115 | |||
| Cr5H | H2#1 | 0.766 | 2.096 | — | −0.227 | |
| H2#2 | 0.784 | 2.024 | — | |||
| H2#3 | 0.780 | 2.045 | — | |||
| H2#4 | 0.755 | 3.610 | — | |||
| H2#5 | 0.755 | 3.418 | −0.093 |
The Ead and the Ēad of the H2 molecules adsorbed on CrGNF were also calculated according to the eqn (2) and (3), as listed in Table 1. It was found that there was a negative relationship between the calculated Ead (or Ēad) and the number of the H2 molecules adsorbed on the CrGNF, which agreed with the results of the hydrogen stored on the Y or Ti decorated graphene-based materials.50,51 The Ead of the second (or third) H2 molecule in the mode Cr2H (or Cr3H) from eqn (2) were calculated to be −0.160 eV (or −0.182 eV). However, the Ead of the fourth H2 molecule was calculated to be only −0.114 eV, which was lower than the Ēad of four H2 molecules (−0.261 eV) in the mode Cr4H obtained with the eqn (3). A similar difference between the Ead of the fifth H2 molecule (−0.093 eV) and the Ēad of the five H2 molecules (−0.227 eV) was also found in mode Cr5H. Generally speaking, the low Ead of gas molecules usually means the weak interaction between the adsorbed gas molecules with the substrates, whereas the high Ēad of gas molecule adsorbed on substrate indicates a strong interaction between them.48 Then, according to Ead and Ēad, it was difficult to confirm whether the physical or chemical interaction took place when the fourth (or fifth) H2 molecule was adsorbed on the CrGNF. To solve this problem leading to the misunderstanding in the interaction between the stored H2 molecules and the CrGNF, the electron densities of the modes Cr2H, Cr3H, Cr4H and Cr5H were further studied, which were displayed in the Fig. 5. As shown, there was a distinct overlap among the electron densities of the two (or three) H2 molecules and that of the Cr atom in the CrGNF in the mode Cr2H (or Cr3H), confirming the strong chemical adsorption of the H2 molecules on CrGNF. In the case of the Cr4H, we found that the electron densities of three H2 molecules numbered #1, #2 and #3 presented a similarly strong overlap with that of the Cr atom, but the electron density of the H2 molecule numbered #4 presented extremely weaker interactions with those of other H2 molecules or Cr atom in the adsorption system. For the H2 molecules numbered #4 and #5 in mode Cr5H, their electron densities were also discovered to be distributed mainly just within them, interacting weakly with other molecules or Cr atom. Based on all the calculated results above, it could be inferred that the H2 molecule numbered #4 in mode Cr4H (numbered #4 or #5 in Cr5H) exhibited only weak physical interaction with the CrGNF, which consisted well with the reported results in the previous literatures.27,49 Therefore, it was reasonable to speculate that there were three H2 molecules in maximum being chemisorbed and stored stably on the monolayer graphene nanoflake doped with one Cr atom, indicating the promising hydrogen storage property of the Cr doped graphene-based nanoflake.
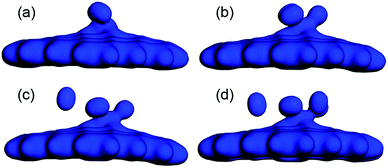 | ||
| Fig. 5 The electrons distributions of two (a), three (b), four (c) and five (d) H2 molecules adsorbed on CrGNF. | ||
Based on the research discussed above, another C atom in the CrGNF was further replaced by the Cr atom to establish 2CrGNF to improve the hydrogen gas storage performance of the doped graphene nanoflakes. The optimized geometry of the built 2CrGNF was studied and we numbered the two Cr atoms as #1 and #2, respectively, as shown in Fig. 6a. Similar with the CrGNF, the second doped Cr atom (numbered #2) also moved up from the plan of the graphene nanoflake with the highness of 1.736 Å. The highness of the first doped Cr atom (numbered #1) in the 2CrGNF was calculated to be 1.783 Å, little affected by the doped Cr atom numbered #2. The average binding energy (Ēb) of two doped Cr atoms was calculated to be −3.86 eV, indicating the stability of the 2CrGNF. Based on the studies of mode Gr3H, six H2 molecules were introduced on the 2CrGNF with three ones above each Cr atom (mode 2Cr6H). The calculated results showed that all the H2 molecules could be adsorbed and stored on the 2CrGNF, as presented in Fig. 6c. The three H2 molecules numbered #1, #2 and #3 interacted with the Cr atom numbered #1 with the d of 2.083 Å, 1.938 Å and 2.028 Å, respectively. For the three H2 molecules stored on the Cr atom numbered #2, the corresponding d were studied to be 2.103 Å, 1.911 Å and 2.033 Å for H2 molecules numbered #4, #5 and #6, respectively. The calculated distances between the three stored H2 molecules and each Cr atom in the 2CrGNF were comparable with those obtained in the mode Cr3H. The bond lengths of the H2 molecules numbered #1, #2, #3, #4, #5 and #6 were 0.777 Å, 0.798 Å, 0.783 Å, 775 Å, 0.804 Å and 0.781 Å, respectively, which were also similar with those in mode Cr3H, as shown in Fig. 6c and listed in Table 2. Meanwhile, we have simulated the electronic density of the mode 2Cr6H to systematically study the H2 storage performance of graphene nanoflake doped with two Cr atoms, as shown in Fig. 6e. The overlaps between the electronic densities of the three H2 molecules and each Cr atom indicated the extremely strong interactions within them, clearly implying that the six H2 molecules could be chemically stored on the 2CrGNF. Moreover, the Ēab of the H2 molecules in modes 2Cr6H was calculated to be −0.305 eV, further confirming the possibility of the storage of the six H2 molecules on the 2CrGNF. As expected, it was found that there were nine H2 molecules stored on the graphene quantum doped with 3 Cr atoms (3CrGNF), which could be proved by the stable optimized geometries (seen in Fig. 6b and d) and the strong overlap in the electron densities (seen in Fig. 6f) of this storage system as well as the detailed parameters of the d or l (listed in b) in this storage system. From our research, it is reasonable to imply that the H2 storage performance of the graphene-based material could be significantly enhanced through modification with more than one metal atom.
| Sensing material | Active site | Numbers of H2 | l (Å) | d (Å) | Ēab (eV) |
|---|---|---|---|---|---|
| 2CrGNF | Cr#1 | H2#1 | 0.777 | 2.083 | −0.305 |
| H2#2 | 0.798 | 1.938 | |||
| H2#3 | 0.783 | 2.028 | |||
| Cr#2 | H2#4 | 0.775 | 2.103 | ||
| H2#5 | 0.804 | 1.911 | |||
| H2#6 | 0.781 | 2.033 | |||
| 3CrGNF | Cr#1 | H2#1 | 0.788 | 2.006 | −0.303 |
| H2#2 | 0.785 | 2.023 | |||
| H2#3 | 0.782 | 2.053 | |||
| Cr#2 | H2#4 | 0.797 | 1.946 | ||
| H2#5 | 0.791 | 1.977 | |||
| H2#6 | 0.778 | 2.071 | |||
| Cr#3 | H2#7 | 0.785 | 2.033 | ||
| H2#8 | 0.796 | 1.962 | |||
| H2#9 | 0.771 | 2.174 |
4. Conclusions
The monolayer graphene nanoflake and the nanoflakes doped with Cr atoms were constructed to investigate their hydrogen storage performance based on the density functional theory. The calculated results presented that the hydrogen molecule exhibited weak interaction with the pristine graphene nanoflake. However, the hydrogen molecule could be chemically adsorbed on the Cr-doped graphene nanoflake with the high adsorption energy of −0.574 eV. The further systematical studies of two to five hydrogen molecules adsorbed on the graphene nanoflake doped with one Cr atom showed that the maximum number of hydrogen molecules stored on this modified nanoflake was three. The hydrogen storage capacity of the modified graphene nanoflake could be successfully improved to be six (or nine) hydrogen molecules through doping graphene nanoflake with two (or three) Cr atoms. Our investigation reveals that the graphene nanoflakes doped with Cr atoms have great potential to be the outstanding hydrogen storage materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Chunqi Xiang and Ao Li contributed equally to this work. This work was financially supported by the National Natural Science Foundation of China (Grant no. 51802109), the Science and Technology Research Project for Young Professionals of Education Department of Hubei Province (Grant no. Q20182903) and the ChuTian Scholars Program of Hubei Province.References
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef PubMed.
- J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Adv. Mater., 2010, 22, 4877–4880 CrossRef CAS PubMed.
- F. Banhart, J. Kotakoski and A. V. Krasheninnikov, ACS Nano, 2011, 5, 26–41 CrossRef CAS PubMed.
- J. Arasteh and M. Naseh, Struct. Chem., 2019, 30, 97–105 CrossRef CAS.
- M. D. Esrafili and N. Saeidi, New J. Chem., 2017, 41, 13149–13155 RSC.
- L. Noerochim, J.-Z. Wang, D. Wexler, Z. Chao and H.-K. Liu, J. Power Sources, 2013, 228, 198–205 CrossRef CAS.
- L. Zhang and Z. Xia, J. Phys. Chem. C, 2011, 115, 11170–11176 CrossRef CAS.
- O. Faye, J. A. Szpunar, B. Szpunar and A. C. Beye, Appl. Surf. Sci., 2017, 392, 362–374 CrossRef CAS.
- S. S. Samantaray, S. R. Mangisetti and S. Ramaprabhu, J. Alloys Compd., 2019, 789, 800–804 CrossRef CAS.
- M. D. Ganji, N. Sharifi, M. Ardjmand and M. G. Ahangari, Appl. Surf. Sci., 2012, 261, 697–704 CrossRef CAS.
- F. Wang, T. Zhang, X. Hou, W. Zhang, S. Tang, H. Sun and J. Zhang, Int. J. Hydrogen Energy, 2017, 42, 10099–10108 CrossRef CAS.
- M. Chi and Y.-P. Zhao, Comput. Mater. Sci., 2009, 46, 1085–1090 CrossRef CAS.
- F. Nasehnia and M. Seifi, Mod. Phys. Lett. B, 2014, 28, 1450237 CrossRef CAS.
- D. Cortés-Arriagada, N. Villegas-Escobar and D. E. Ortega, Appl. Surf. Sci., 2018, 427, 227–236 CrossRef.
- S. Yang, G. Lei, H. Xu, B. Xu, H. Li, Z. Lan, Z. Wang and H. Gu, Appl. Surf. Sci., 2019, 480, 205–211 CrossRef CAS.
- L. Lin, W. Zhou, R. Gao, S. Yao, X. Zhang, W. Xu, S. Zheng, Z. Jiang, Q. Yu, Y.-W. Li, C. Shi, X.-D. Wen and D. Ma, Nature, 2017, 544, 80–83 CrossRef CAS PubMed.
- B. Sharma, A. Sharma and J.-S. Kim, Sens. Actuators, B, 2018, 262, 758–770 CrossRef CAS.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 CrossRef CAS PubMed.
- J. Fu, Y. Liu, Y. Tian and J. Wu, J. Phys. Chem. C, 2015, 119, 5374–5385 CrossRef CAS.
- Y. Zhou, W. Chu, F. Jing, J. Zheng, W. Sun and Y. Xue, Appl. Surf. Sci., 2017, 410, 166–176 CrossRef CAS.
- J. Li, H. Zhang and G. Yang, J. Phys. Chem. C, 2015, 119, 19681–19688 CrossRef CAS.
- S. Seenithurai, R. K. Pandyan, S. V. Kumar, C. Saranya and M. Mahendran, Int. J. Hydrogen Energy, 2014, 39, 11016–11026 CrossRef CAS.
- M. M. Zhong, H. K. Yuan, C. Huang and G. Wang, J. Alloys Compd., 2018, 766, 104–111 CrossRef CAS.
- S. Yang, Z. Lan, H. Xu, G. Lei, W. Xie and Q. Gu, Int. J. Nanotechnol., 2018, 2018, 1–5 CrossRef.
- Z. Luo, X. Fan, R. Pan and Y. An, Int. J. Hydrogen Energy, 2017, 42, 3106–3113 CrossRef CAS.
- H.-p. Zhang, X.-g. Luo, X.-y. Lin, X. Lu and Y. Leng, Int. J. Hydrogen Energy, 2013, 38, 14269–14275 CrossRef CAS.
- C. Tabtimsai, V. Ruangpornvisuti, S. Tontapha and B. Wanno, Appl. Surf. Sci., 2018, 439, 494–505 CrossRef CAS.
- Q. Zhou, X. Yang, Z. Fu, C. Wang, L. Yuan, H. Zhang and Y. Tang, Phys. E, 2015, 65, 77–83 CrossRef CAS.
- X.-Y. Liu and J.-M. Zhang, Appl. Surf. Sci., 2014, 293, 216–219 CrossRef CAS.
- D. Zhao, X. Fan, Z. Luo, Y. An and Y. Hu, Phys. Lett. A, 2018, 382, 2965–2973 CrossRef CAS.
- J. I. G. Enriquez and A. R. C. Villagracia, Int. J. Hydrogen Energy, 2016, 41, 12157–12166 CrossRef CAS.
- T. T. Li, C. He and W. X. Zhang, Appl. Surf. Sci., 2018, 427, 388–395 CrossRef CAS.
- D. Cortés-Arriagada and N. Villegas-Escobar, Appl. Surf. Sci., 2017, 420, 446–455 CrossRef.
- A. S. Rad and E. Abedini, Appl. Surf. Sci., 2016, 360, 1041–1046 CrossRef CAS.
- J. Feng, H. Dong, B. Pang, F. Shao, C. Zhang, L. Yu and L. Dong, Phys. Chem. Chem. Phys., 2018, 20, 15244–15252 RSC.
- H. Tachikawa, Appl. Surf. Sci., 2017, 396, 1335–1342 CrossRef CAS.
- A. Omidvar and A. Mohajeri, RSC Adv., 2015, 5, 54535–54543 RSC.
- N. Yodsin, C. Rungnim, V. Promarak, S. Namuangruk, N. Kungwan, R. Rattanawan and S. Jungsuttiwong, Phys. Chem. Chem. Phys., 2018, 20, 21194–21203 RSC.
- L. Chen, X. Chen, C. Duan, Y. Huang, Q. Zhang and B. Xiao, Phys. Chem. Chem. Phys., 2018, 20, 30304–30311 RSC.
- S. Thakur, S. M. Borah and N. C. Adhikary, Optik, 2018, 168, 228–236 CrossRef CAS.
- F. Montejo-Alvaro, J. Oliva, A. Zarate, M. Herrera-Trejo, H. M. Hdz-García and A. I. Mtz-Enriquez, Phys. E, 2019, 110, 52–58 CrossRef CAS.
- M. Vatanparast and Z. Shariatinia, J. Fluorine Chem., 2018, 211, 81–93 CrossRef CAS.
- P. Buasaeng, W. Rakrai, B. Wanno and C. Tabtimsai, Appl. Surf. Sci., 2017, 400, 506–514 CrossRef CAS.
- P. Olsson, J. Wallenius, C. Domain, K. Nordlund and L. Malerba, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 214119 CrossRef.
- L. Ma, J.-M. Zhang, K.-W. Xu and V. Ji, Appl. Surf. Sci., 2015, 343, 121–127 CrossRef CAS.
- M. Gallouze, A. Kellou and M. Drir, Int. J. Hydrogen Energy, 2016, 41, 5522–5530 CrossRef CAS.
- I. Cabria, M. J. Lopez and J. A. Alonso, J. Chem. Phys., 2017, 146, 214104 CrossRef CAS PubMed.
- Z. Ao, S. Dou, Z. Xu, Q. Jiang and G. Wang, Int. J. Hydrogen Energy, 2014, 39, 16244–16251 CrossRef CAS.
- C. Tabtimsai, W. Rakrai and B. Wanno, Vacuum, 2017, 139, 101–108 CrossRef CAS.
- L. Yuan, L. Kang, Y. Chen, D. Wang, J. Gong, C. Wang, M. Zhang and X. Wu, Appl. Surf. Sci., 2018, 434, 843–849 CrossRef CAS.
- L. Yuan, Y. Chen, L. Kang, C. Zhang, D. Wang, C. Wang, M. Zhang and X. Wu, Appl. Surf. Sci., 2017, 399, 463–468 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |