Open Access Article
Open Access ArticleFabrication of NiFe2O4@carbon fiber coated with phytic acid-doped polyaniline composite and its application as an electromagnetic wave absorber
Ailing Fenga,
Mingliang Mab,
Zirui Jia*c,
Meng Zhang d and
Guanglei Wu
d and
Guanglei Wu *ce
*ce
aInstitute of Physics & Optoelectronics Technology, Baoji University of Arts and Sciences, Baoji 721016, P. R. China
bResearch Institute of Functional Materials, School of Civil Engineering, Qingdao University of Technology, Qingdao 266033, P. R. China
cInstitute of Materials for Energy and Environment, State Key Laboratory of Bio-fibers and Eco-textiles, College of Materials Science and Engineering, Qingdao University, Qingdao 266071, P. R. China. E-mail: jiazirui@mail.nwpu.edu.cn; wuguanglei@mail.xjtu.edu.cn; wuguanglei@qdu.edu.cn; Fax: +86 532 85951496; Tel: +86 532 85951496
dCollege of Electromechanical Engineering, Key Laboratory of Polymer Material Advanced Manufacturing's Technology of Shandong Province, Qingdao University of Science and Technology, Qingdao, 266061, China
eKey Laboratory of Engineering Dielectrics and Its Application, Ministry of Education, Harbin University of Science and Technology, Harbin 150080, PR China
First published on 19th August 2019
Abstract
In this work, a novel CF@NiFe2O4 composite coated with phytic acid-doped polyaniline (CF@NiFe2O4@p-PANI) was facilely synthesized. First, a typical solvothermal reaction was applied to obtain the CF@NiFe2O4 composite, and then the phytic acid-doped polyaniline was grown in situ on the surface of the CF@NiFe2O4 composite. The morphological structure, chemical composition, and surface functional group distribution of this hybrid were systematically evaluated. The magnetic saturation (Ms) value of the hybrid is 29.9 emu g−1, which represents an improvement in the magnetic loss. According to its reflection loss curve, the hybrid exhibits a superior EM wave absorption capacity, with a minimum reflection loss value and effective absorbing bandwidth of −46 dB when the sample thickness is 2.9 mm, and an effective absorption bandwidth of 5 GHz when the sample thickness is 1.5 mm. The excellent performance of this hybrid can mainly be attributed to its ideal matching of magnetic loss and dielectric loss, interfacial polarizations, eddy current loss and interface relaxation. This new material has the potential to be a superior electromagnetic wave absorber or applied as a functional filler to modify resin matrices.
1. Introduction
With the rapid development of science and technology in the contemporary society, the popularization and application of communication, broadcasting, electromagnetic medicine and computer technology has made electromagnetic (EM) waves pervasive in our daily lives.1–6 Therefore, the nuisance to humans caused by EM waves and the interference from related equipment are receiving increasing attention from countries around the world.7–10 Meanwhile, in order to achieve EM wave protection, research on EM wave-absorbing materials has rapidly developed. In the light of the dielectric loss and magnetic loss mechanisms of microwaves, an ideal electromagnetic wave absorber should both possess select dielectric properties and magnetic properties. Thus, the combination of dielectric materials (such as carbon-based materials, conductive polymers, and transition metal oxides) and magnetic materials (metallic iron, cobalt, nickel and their compounds) through chemical reactions has been a classic research method to obtain predominant EM wave-absorbing composites.11–15Due to its special conjugated molecular chain structure, polyaniline (PANI) possesses excellent electrical conductivity, a unique doping mechanism, ideal stability, high design capacity and many other advantages; therefore, PANI has been widely utilized in EM wave-absorbing and anti-electromagnetic shielding materials.16–19 PANI-based EM absorption composite materials frequently show high absorption efficiency and wide absorption bandwidths. Previous research results have shown that PANI can enhance the absorptive capacity of EM wave absorption composites through dielectric loss and eddy current loss among some other ways.20–24 Besides, its structure has an important influence on its EM wave-absorbing performance; special structural features such as its rod shape and spherical shape have received extensive attention.25–29
As a typical magnetic material, NiFe2O4 has been widely applied in the synthesis of efficient EM wave-absorbing composites. Chen30 and his co-workers developed a novel urchin-like PANI@carbon@NiFe2O4 hybrid, which shows excellent EM wave-absorbing performance with a minimum reflection loss value of −37 dB. Another ideal EM wave absorber, a NiFe2O4@MnO2@graphene composite synthesized by Wang et al., exhibits a minimum reflection loss value of −47.4 dB with a sample thickness of 3.0 mm.31 Furthermore, the PANI@NiFe2O4@graphite nanosheet,32 NiFe2O4-RGO-elastomer33 and PANI/graphene/NiFe2O4 (ref. 34) also exhibited superior EM wave absorption properties.
In this study, a novel CF@NiFe2O4 composite coated with phytic acid-doped polyaniline (CF@NiFe2O4@p-PANI) was facilely prepared. First, a typical solvothermal reaction was adopted to prepare the CF@NiFe2O4 composite. Then, the as-synthesized CF@NiFe2O4 composite was further coated by p-PANI via in situ polymerization to obtain the CF@NiFe2O4@p-PANI hybrid. The morphological structure, chemical composition, magnetic properties and EM wave-absorbing features of the sample were systematically evaluated. The results prove that the hybrid possesses an excellent EM wave-absorbing capacity.
2. Experimental
2.1 Materials
The carbon fiber used in this experiment is T300B and was supplied by Japan TORAY industries. Other reagents including ferric chloride (FeCl3·6H2O), nickel chloride (NiCl2·6H2O), ammonium acetate (NH4Ac), glycol, phytic acid, ammonium persulfate (APS) and aniline were purchased from Aladdin Industrial Corporation and used without further purification.2.2 Synthesis of CF@NiFe2O4 composite
The CF@NiFe2O4 composite was facilely synthesized via a typical solvothermal reaction.24 200 mg CF, 10 mmol FeCl3·6H2O, 5 mmol NiCl2·4H2O and 80 mmol ammonium acetate (NH4COOH) were homogeneously dispersed in 80 mL ethylene glycol phase by ultrasonication for 5 min and stirring for 25 min. Then, the liquid mixture was transferred into a 100 mL hydrothermal reactor and kept at 200 °C for 720 min. After naturally cooling to room temperature, the CF@NiFe2O4 composite was collected by 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm centrifugation and washed with deionized water and ethanol several times.
000 rpm centrifugation and washed with deionized water and ethanol several times.
2.3 Preparation of CF@NiFe2O4@p-PANI hybrid
The CF@NiFe2O4@p-PANI hybrid was synthesized via the in situ polymerization of aniline monomer in a liquid phase system. Before the reaction, the CF@NiFe2O4 composite was soaked in a mixed solution of absolute ethanol, deionized water and KH550 (wt% = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 4 h to obtain the surface-aminated CF@NiFe2O4 (n-CF@NiFe2O4). 200 mg as-prepared n-CF@NiFe2O4 composite, 5 mL phytic acid and 100 μm aniline were uniformly dispersed in 40 mL deionized water by 30 min high-speed stirring. Then, 20 mL ammonium persulfate solution (APS) was slowly dripped into the mixed turbid liquid. After stirring for about 6 hours, the CF@NiFe2O4@p-PANI hybrid was separated from the liquid by 10
1) for 4 h to obtain the surface-aminated CF@NiFe2O4 (n-CF@NiFe2O4). 200 mg as-prepared n-CF@NiFe2O4 composite, 5 mL phytic acid and 100 μm aniline were uniformly dispersed in 40 mL deionized water by 30 min high-speed stirring. Then, 20 mL ammonium persulfate solution (APS) was slowly dripped into the mixed turbid liquid. After stirring for about 6 hours, the CF@NiFe2O4@p-PANI hybrid was separated from the liquid by 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm centrifugation and washed with deionized water and ethanol several times.35–38
000 rpm centrifugation and washed with deionized water and ethanol several times.35–38
2.4 Characterization
The samples' surface structural features, morphologies and elemental composition proportions were identified using a scanning electron microscope (FESEM, Quanta 600FEG) equipped with an energy dispersive X-ray (EDS) accessory. Furthermore, X-ray powder diffraction (ESCALAB 250) was used to study the crystal forms of the carbon fiber, NiFe2O4, CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid. The binding energy of the CF@NiFe2O4@p-PANI hybrid was examined by X-ray photoelectron spectroscopy (XPS, Thermal Scientific Kα). The distribution of functional groups on the surface of the specimens was studied using Fourier infrared spectroscopy (FT-IR, WQF-310, China, with KBr pellets). The hysteresis loops of the magnetic specimens were measured via a vibrating sample magnetometer (VSM, Lake Shore7307). A coaxial method was adopted to obtain the complex permeability (εr) and permittivity (μr) of the samples in the range of 2–18 GHz using a vector network analyzer (HP8720ES). First, the specimens were uniformly distributed in a paraffin phase (the wt% of paraffin and sample is 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); then, they were pressed into a cylindrical mold (φin = 3.04 mm, φout = 7.00 mm). Based on the obtained parameters of the sample, their EM wave absorption properties were further calculated.39–41
3); then, they were pressed into a cylindrical mold (φin = 3.04 mm, φout = 7.00 mm). Based on the obtained parameters of the sample, their EM wave absorption properties were further calculated.39–41
3. Results and discussions
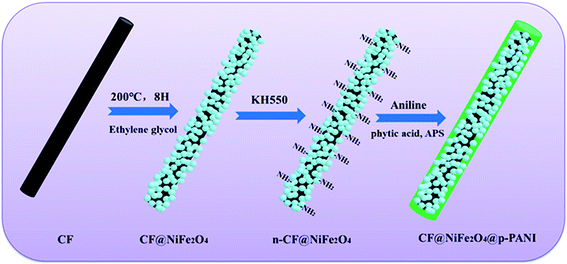
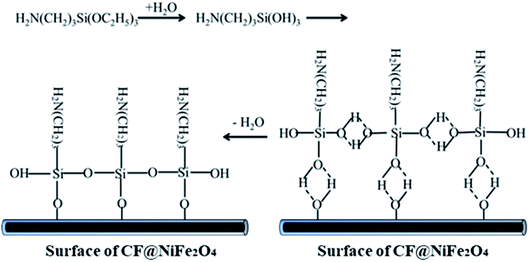
The preparation process of CF@NiFe2O4@p-PANI hybrid is briefly depicted in Fig. 1. First, a typical solvothermal reaction in a glycol phase was adopted to synthesize the CF@NiFe2O4 composite. Then, in order to polymerize the aniline monomer on the surface of the CF@NiFe2O4 composite, the sample was subjected to surface amination using KH550, and the resulting chemical reaction is shown in Fig. 2. Owing to the silane coupling agent's complexation with H2O molecules, after the hydration and dehydration reactions, the amino groups that had attached to the other end of the KH550 were adsorbed onto the surface of the CF@NiFe2O4 composite. Finally, aniline monomers were grown in situ alongside the amino groups on the surface of the CF@NiFe2O4 composite in a mixed system of phytic acid and water, and the CF@NiFe2O4@p-PANI hybrid was obtained.42–44Fig. 3 shows the SEM images of CF (a), NiFe2O4 (b), the CF@NiFe2O4 composite (b) and the CF@NiFe2O4@p-PANI hybrid (c); the corresponding EDX elemental mapping images of the hybrid are also exhibited (Fig. 3d–i). It can be observed from the images that the pre-reaction carbon fiber has a smooth surface, and its diameter is about 6–7 μm. After the solvothermal reaction, the surface of the CF was uniformly cladded by NiFe2O4 nanospheres. Fig. 3c reveals the morphology of the CF@NiFe2O4@p-PANI hybrid, the CF@NiFe2O4 composite that was further coated by PANI after the in situ polymerization. The sample's EDX elemental mapping images indicate that the hybrid includes the chemical elements C, N, Ni, Fe and O, which convincingly prove the chemical composition (C, NiFe2O4 and PANI) of the obtained hybrid.
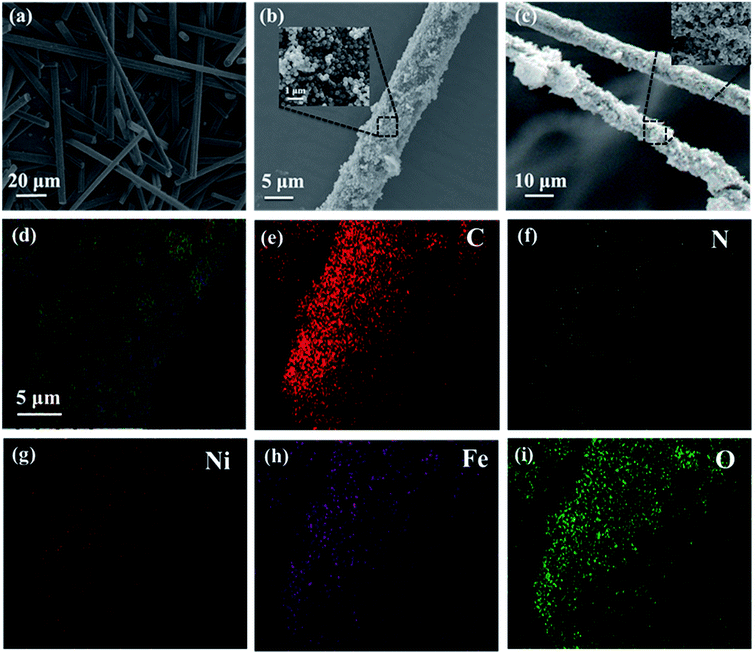 | ||
| Fig. 3 SEM images of CF (a), NiFe2O4 (b), CF@NiFe2O4 composite (b) and CF@NiFe2O4@p-PANI hybrid (c); EDX elemental mapping images (d–i) of CF@NiFe2O4@p-PANI hybrid. | ||
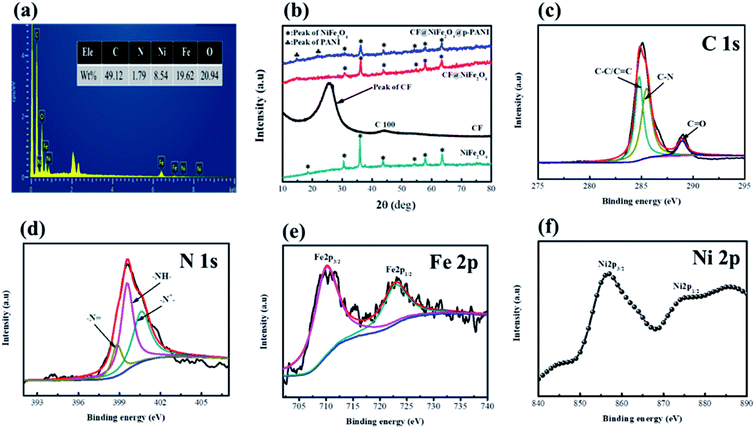
Fig. 4 exhibits the EDS spectra (a), XPS spectra (c–f) of CF@NiFe2O4@p-PANI hybrid and XRD patterns of the samples. From Fig. 4a, the elemental composition ratios (wt%) of C, N, Ni, Fe and O in the hybrid are 49.12%, 1.79%, 8.54%, 19.62% and 20.94%, respectively, and these results are consistent with its EDX elemental mapping results. Fig. 4b displays the XRD patterns of NiFe2O4, CF, the CF@NiFe2O4 composite and the CF@NiFe2O4@p-PANI hybrid, which can provide information about the samples' crystal structure. The broad peak between 20–40° represents the weak crystallinity of the CF. After the solvothermal reaction, 6 diffraction peaks appeared at 2θ = 30.2°, 35.7°, 43.4°, 54.0°, 57.3° and 62.9°, which signify the (220), (311), (400), (422), (511) and (440) planes of NiFe2O4 (JCPDS card no. 10-0325), respectively. Compared with the CF@NiFe2O4 composite, the additional two peaks before 30° belong to PANI. The above conclusion further proves the successful synthesis of the CF@NiFe2O4@p-PANI hybrid. Fig. 4c–f exhibits the XPS pattern of the CF@NiFe2O4@p-PANI hybrid. The three peaks located at 284.6 eV, 285.3 eV and 288.3 eV represent the C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and C
C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O binding energies of the C element, respectively (Fig. 4c). In Fig. 4d, the three peaks of –N
O binding energies of the C element, respectively (Fig. 4c). In Fig. 4d, the three peaks of –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) , –NH– and –N+– represent the three different valence states of the N element in the polyaniline molecular chain, which indicate that the as-synthesized polyaniline is in the doped state. In addition, the two peaks situated at 711 eV and 724.6 eV are assigned to the F2p3/2 and Fe2p1/2 binding energies, respectively (Fig. 4e). The Ni2p3/2 and Ni2p1/2 peaks can be observed in the narrow sweep spectra in Fig. 4f. These results further verify the chemical composition of the CF@NiFe2O4@p-PANI hybrid.45–47
, –NH– and –N+– represent the three different valence states of the N element in the polyaniline molecular chain, which indicate that the as-synthesized polyaniline is in the doped state. In addition, the two peaks situated at 711 eV and 724.6 eV are assigned to the F2p3/2 and Fe2p1/2 binding energies, respectively (Fig. 4e). The Ni2p3/2 and Ni2p1/2 peaks can be observed in the narrow sweep spectra in Fig. 4f. These results further verify the chemical composition of the CF@NiFe2O4@p-PANI hybrid.45–47
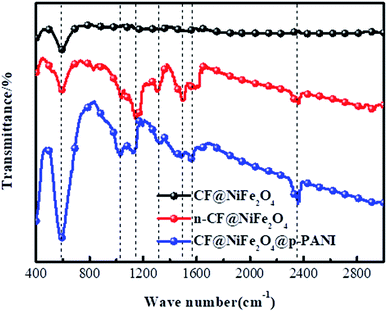
The distribution of functional groups on the surface of CF@NiFe2O4, n-CF@NiFe2O4 and the CF@NiFe2O4@p-PANI hybrid were investigated using FT-IR spectroscopy, and the results are shown in Fig. 5. Compared with CF@NiFe2O4, vibration peaks belonging to C–C, C–O, C–N appear in the n-CF@NiFe2O4 sample's spectra, which illustrates the effective surface treatment of KH550. The vibration peak located at 1032 cm−1 can be assigned to the P–O–C of phytic acid, indicating the successful doping of phytic acid into the PANI molecular chain. Besides, the other corresponding adsorption peaks and assigned various modes are illustrated in Table 1.48
| CF@NiFe2O4, cm−1 | n-CF@NiFe2O4, cm−1 | CF@NiFe2O4@p-PANI, cm−1 | Assignment of adsorption peaks (stretching vibration and deformation vibration) |
|---|---|---|---|
| 595 | 595 | 595 | Fe–O and NH2 (from NiFe2O4, KH550 and PANI) |
| — | — | 1032 | P–O–C (from phytic acid) |
| — | 1157 | 1157 | C–C (from KH550, phytic acid and PANI backbone) |
| — | 1333 | 1336 | C–O (from KH550 and phytic acid) |
| — | 1500 | 1500 | C–N (from KH550 and PANI) |
| — | — | 1571 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (from PANI) N (from PANI) |
| — | 2369 | 2369 | Unsaturated chemical bond (from the surface of the samples and PANI) |
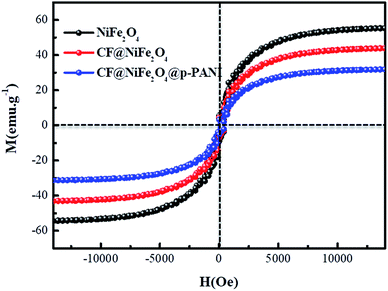
Based on the magnetic loss mechanism of electromagnetic waves, the magnetic behaviors of materials have an important influence on their electromagnetic wave absorption performance; thus, the magnetic samples' hysteresis loops were measured by utilizing VSM and the results are exhibited in Fig. 6. The NiFe2O4, CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid all perform typical paramagnetic soft magnetic behavior, which is very beneficial for the absorption of electromagnetic waves. The magnetic saturation values of NiFe2O4, CF@NiFe2O4 and CF@NiFe2O4@p-PANI hybrid are 56.1 emu g−1, 41.2 emu g−1 and 29.9 emu g−1, respectively. The reduction in these samples' magnetic saturation is mainly due to the decrease in the mass ratio of the magnetic material NiFe2O4.
Normally, upon the incidence of microwaves onto the sample surface, the waves are reflected or transmitted; meanwhile, the contained microwave energy is absorbed by magnetic loss and dielectric loss or transforms into heat energy and scatters and disappears in the air. Considering the loss mechanism of microwaves, the absorbing mode of microwaves can mainly be divided into magnetic loss and dielectric loss, which can be calculated using the permeability μ′, μ′′ and permittivity ε′, ε′′, respectively. The parameter's real part μ′ and ε′ represent the storage capability of the material, while the imaginary part μ′′ and ε′′ stand for the dissipation capability of magnetic energy and electric energy, respectively. Based on μ′, μ′′, ε′ and ε′′, the corresponding reflection loss curve of the measured sample can be calculated by using eqn (1)–(4).49–51
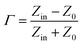 | (1) |
| Z0 = (μ0/ε0)1/2 | (2) |
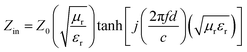 | (3) |
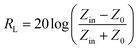 | (4) |
In these formulas, Zin represents the normalized input impedance of the absorbing material, Z0 represents the impedance of free space, parameter d stands for the thickness of the absorber, parameter c represents the light velocity in vacuum, and the parameter f signifies the frequency of the input microwave. Thus, the measured sample's thickness has a very important influence on its electromagnetic wave-absorbing capacity. The material's electromagnetic wave-absorbing performance is co-determined by its magnetic properties and dielectric properties. The electromagnetic parameters of CF, NiFe2O4, the CF@NiFe2O4 composite and the CF@NiFe2O4@p-PANI hybrid are exhibited in Fig. 7, and the corresponding dielectric losses (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δe = ε′′/ε′) and magnetic losses (tan
δe = ε′′/ε′) and magnetic losses (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δm = μ′′/μ′) are also shown. Comparing the four samples' electromagnetic parameters, as a conductive material, the CF sample possesses the highest dielectric parameter value (including both the real and imaginary parts), while the insulating material NiFe2O4 possess the lowest dielectric parameter values. However, as a nonmagnetic material, the magnetic parameter values of CF are extremely low, which is unfavorable to the magnetic loss of electromagnetic waves. The dielectric parameter values and tan
δm = μ′′/μ′) are also shown. Comparing the four samples' electromagnetic parameters, as a conductive material, the CF sample possesses the highest dielectric parameter value (including both the real and imaginary parts), while the insulating material NiFe2O4 possess the lowest dielectric parameter values. However, as a nonmagnetic material, the magnetic parameter values of CF are extremely low, which is unfavorable to the magnetic loss of electromagnetic waves. The dielectric parameter values and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δe of the CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid are between those of CF and NiFe2O4; meanwhile, the magnetic parameter values are higher than those of NiFe2O4. The results indicate that the CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid may possess a more reasonable electromagnetic matching effect. It is worth mentioning that the electromagnetic parameters of NiFe2O4, CF@NiFe2O4 and the CF@NiFe2O4@p-PANI samples all displayed resonance effects in various degrees, which can mainly be attributed to the gyromagnetic effect of NiFe2O4.
δe of the CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid are between those of CF and NiFe2O4; meanwhile, the magnetic parameter values are higher than those of NiFe2O4. The results indicate that the CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid may possess a more reasonable electromagnetic matching effect. It is worth mentioning that the electromagnetic parameters of NiFe2O4, CF@NiFe2O4 and the CF@NiFe2O4@p-PANI samples all displayed resonance effects in various degrees, which can mainly be attributed to the gyromagnetic effect of NiFe2O4.
 | ||
| Fig. 7 Electromagnetic parameters of CF (a), NiFe2O4 (b), CF@NiFe2O4 (c) and CF@NiFe2O4@p-PANI hybrid (d), dielectric loss (e) and magnetic loss (f). | ||
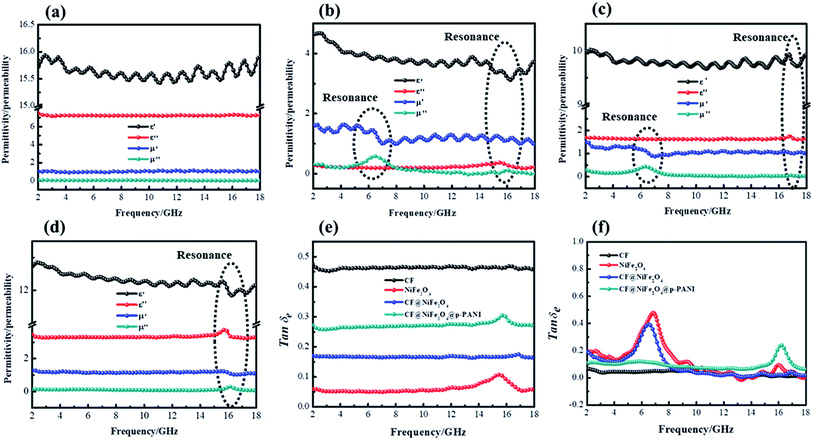
According to the measured ε′, ε′′, μ′, μ′′ parameters, the reflection loss patterns ranging from 2–18 GHz of CF, NiFe2O4, CF@NiFe2O4 composite and CF@NiFe2O4@p-PANI hybrid samples were calculated, and the results are exhibited in Fig. 8. As can be observed in the figure, as a nonmagnetic material, the EM wave-absorbing capacity of the pure carbon fiber is poor, and its minimum reflection loss (RLmin) value is −15.6 dB with a specimen thickness of 1.6 mm. Conversely, due to the extremely poor conductivity, the NiFe2O4 sample's EM wave performance is also inferior, with a minimum reflection loss value of −12 dB. However, the CF@NiFe2O4 composite possesses a strong EM wave-absorption capacity, which can reach up to −37 dB. When further combined with phytic acid-doped PANI, the EM wave-absorbing performance of the CF@NiFe2O4@p-PANI hybrid is enhanced, achieving −46 dB, which can mainly be attributed to its fair electromagnetic matching.
 | ||
| Fig. 8 Reflection loss patterns of CF (a), NiFe2O4 (b), CF@NiFe2O4 (c) and CF@NiFe2O4@p-PANI hybrid (d). | ||
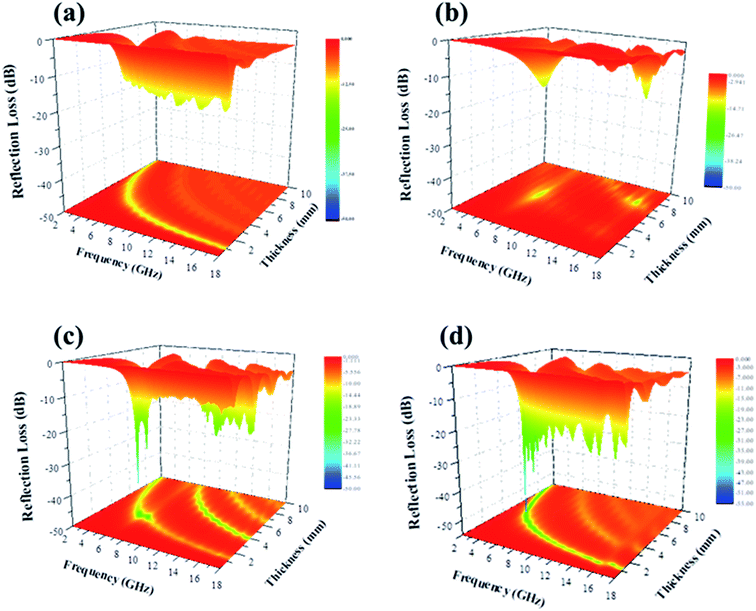
To further investigate the EM wave absorption performance of CF, NiFe2O4, CF@NiFe2O4 and CF@NiFe2O4@p-PANI hybrid, their |Zin/Z0| values were calculated to estimate their impedance matching, which are shown in Fig. 9a. A value of |Zin/Z0| that is closer to one indicates that the material possesses better impedance matching characteristics. Compared with the CF and NiFe2O4 samples, the CF@NiFe2O4 and CF@NiFe2O4@p-PANI hybrid samples' |Zin/Z0| values are closer to one, which illustrates that superior impedance matching can be obtained with CF@NiFe2O4 and CF@NiFe2O4@p-PANI hybrid. The attenuation constant α can be expressed as eqn (5), which quantitatively represents the attenuation effect in the frequency range. The corresponding α values of the four samples are exhibited in Fig. 9b. Compared with NiFe2O4 and CF@NiFe2O4, the hybrid shows a higher attenuation constant α, which is beneficial for enhancing its EM wave absorption capability.52–54
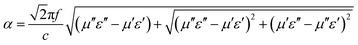 | (5) |
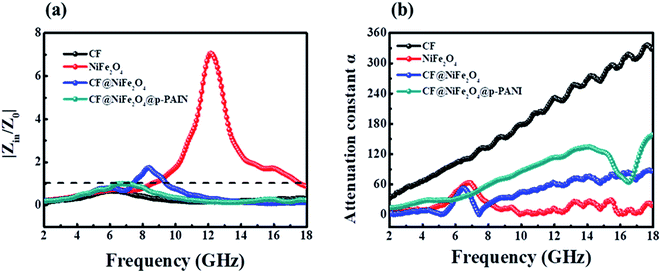 | ||
| Fig. 9 The impedance matching values of samples at different frequencies (a) and their attenuation constant α (b). | ||
The EM wave-absorbing performance of the as-synthesized CF@NiFe2O4@p-PANI hybrid was investigated in detail, and the relative results are shown in Fig. 10. In Fig. 10a and b, the hybrid's RLmin can reach up to −46 dB with a sample thickness of 2.9 mm, which is smaller than any other thickness, indicating that the hybrid possesses the highest absorption efficiency at this thickness. Fig. 10c exhibits the effective absorption bandwidth (EAB, RL < −10 dB) at 1.5 mm, 2.0 mm, 2.5 mm, 2.8 mm, 2.9 mm, 3.0 mm and 3.5 mm when the thickness is 1.5 mm; the hybrid's EAB width extends up to 5 GHz, from 12.5 GHz to 17.5 GHz, almost covering all of the Ku band. These results indicate that the CF@NiFe2O4@p-PANI hybrid has excellent electromagnetic wave-absorbing performance, both in terms of its absorbing efficiency and EAB width. A sketch illustration of the mechanism of the electromagnetic wave absorption by the CF@NiFe2O4@p-PANI hybrid is displayed in Fig. 10d. First, the conductive material CF, PANI and the magnetic material NiFe2O4 achieve reasonable electromagnetic matching in the hybrid. Materials with good electrical conductivity may produce skin effects and additional reflection at the interface between the material and air; this offers effective electromagnetic attenuation. Secondly, the multiple interfaces that emerged due to its layer-by-layer cladding structure can increase the absorption times of EM waves. Thirdly, PANI is an effective coating that prevents NiFe2O4 from further oxidation, thus enhancing the stability of NiFe2O4 in the heterostructures. Moreover, the introduction of lightweight PANI may improve the interface between the carbon fibers and matrix, which may result in lightweight composites with better matrix–filler compatibility. Besides, the complexation between phytic acid and NiFe2O4 and the adsorption effect of KH550 can further enhance the interfacial interaction, which can further enhance the hybrid's EM wave performance by its eddy current loss, interfacial polarizations and interface relaxation. When compared with other reported analogous EM wave absorbers, including PANI@p-C@NiFe2O4, NanoG/NiFe2O4/PANI, PANI/MnO2/CF, NiFe2O4@MnO2, PANI/NiFe2O4, PANI@Ni@CF and RGO@NiFe2O4@SiO2 (Table 2), the as-synthesized CF@NiFe2O4@p-PANI hybrid shows excellent EM wave absorption properties.55–59
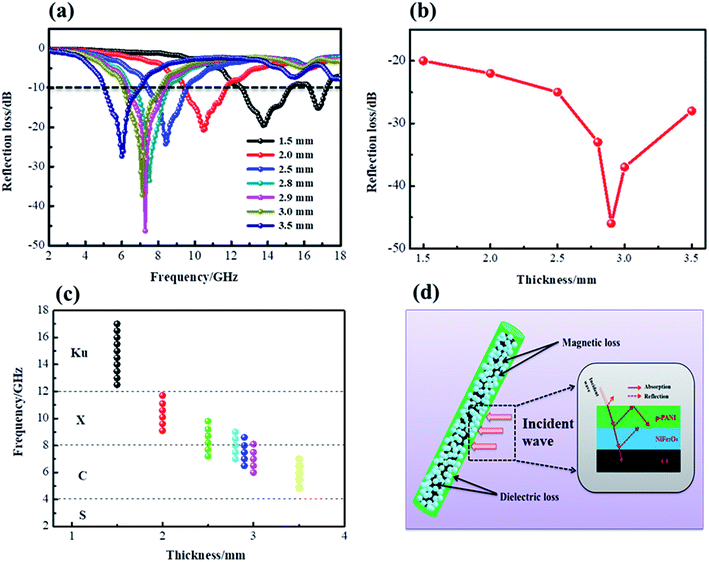 | ||
| Fig. 10 RL patterns (a), RLmin values (b), EAB at different thicknesses (c) and sketch illustration of the mechanism of the EM wave absorption (d) for the CF@NiFe2O4@p-PANI hybrid. | ||
| Sample | Frequency range (GHz) | Weight percent of filler | Adhesive | Thickness | RLmin | EAB width (GHz) | Ref. |
|---|---|---|---|---|---|---|---|
| PANI@p-C@NiFe2O4 | 2–18 | 30% | Paraffin wax | 2.5 mm | −37 dB | 3.9 | 30 |
| NanoG/NiFe2O4/PANI | 8.2–12.4 | 30% | Paraffin wax | 2.5 mm | −30 dB | 2.8 | 32 |
| PANI/MnO2/CF | 8.2–12.4 | 30% | Paraffin wax | 2.5 mm | −22 dB | 3.0 | 60 |
| NiFe2O4@MnO2 | 2–18 | 30% | Paraffin wax | 2.0 mm | −25 dB | 2.7 | 31 |
| PANI/NiFe2O4 | 1–6 | 66% | Epoxy | N.A | −20.3 dB | 2.8 | 61 |
| PANI@Ni@CF | 8.2–12.4 | 20% | Paraffin wax | 2.0 mm | −12.4 dB | 1.2 | 62 |
| RGO@NiFe2O4@SiO2 | 2–18 | 30% | Paraffin wax | 3.0 mm | −42 dB | 11.5 | 63 |
| CF@NiFe2O4@PANI | 2–18 | 30% | Paraffin wax | 2.9 mm | −46 dB | 2.0 | This work |
4. Conclusion
In summary, a uniform CF@NiFe2O4@p-PANI hybrid was facilely fabricated via a two-step method involving a solvothermal reaction and in situ polymerization. The morphological structure, chemical composition, magnetic behavior, surface functional group distribution and EM wave-absorbing properties of the as-prepared hybrid were systematically evaluated. The results indicate that the hybrid possesses excellent EM wave-absorbing performance, and its reflection loss value can reach up to −46 dB with a specimen thickness of 2.9 mm. The superior EM performance of the hybrid can be attributed to its fair electromagnetic matching, multiple interfaces and eddy current loss.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51407134, No. 51801001), China Postdoctoral Science Foundation (No. 2016M590619, No. 2016M601878), Natural Science Foundation of Shandong Province (No. ZR2019YQ24), Qingdao Postdoctoral Application Research Project, Key Laboratory of Engineering Dielectrics and Its Application (Harbin University of Science and Technology), Ministry of Education (No. KFZ1803), Provincial Key Research and Development Program of Shaanxi (No. 2019GY-197), Key Project of Baoji University of Arts and Sciences (No. ZK2018051), Baoji Science and Technology Project (No. 16RKX1-29) and Baoji Engineering Technology Research Center for Ultrafast Optics and New Materials (No. 2015CXNL-1-3). Feng AL is supported by the Thousand Talents Plan for Young Professionals of Shaanxi Province.References
- S. Ji, C. Li, Z. Zhang, X. Jiang and L. Yu, Synth. Met., 2018, 239, 59–65 CrossRef CAS.
- Z. Jia, Z. Gao, A. Feng, Y. Zhang, C. Zhang, G. Nie, K. Wang and G. Wu, Composites, Part B, 2019, 176, 107246 CrossRef.
- M. Chen, W. Zhao, M. Zhu, X. Li, C. Xu, H. Chen and J. Xu, Chem. Sci., 2019, 10, 4141–4147 RSC.
- Z. Gao, Z. Jia, J. Zhang, A. Feng, Z. Huang and G. Wu, J. Mater. Sci.: Mater. Electron., 2019, 30, 13474–13487 CrossRef CAS.
- J. Liu, H. Liang, Y. Zhang, G. Wu and H. Wu, Composites, Part B, 2019, 176, 107240 CrossRef.
- I. Abdalla, A. Salim, M. Zhu, J. Yu, Z. Li and B. Ding, ACS Appl. Mater. Interfaces, 2018, 10, 44561–44569 CrossRef CAS PubMed.
- X. L. Chen, Z. R. Jia, A. L. Feng, B. B. Wang, X. H. Tong, C. H. Zhang and G. L. Wu, J. Colloid Interface Sci., 2019, 553, 465–474 CrossRef CAS PubMed.
- J. Fang, T. Liu, Z. Chen, Y. Wang, W. Wei, X. Yue and Z. Jiang, Nanoscale, 2016, 8, 8899–8909 RSC.
- C. Huang, Y. Ding, C. Hao, S. Zhou, X. Wang, H. Gao, L. Zhu and J. Wu, Chem. Eng. J., 2019, 378, 122202 CrossRef CAS.
- J. Fang, Z. Chen, W. Wei, Y. Li, T. Liu, Z. Liu and X. Yue, RSC Adv., 2016, 6, 4695–4704 RSC.
- I. Abdalla, J. Yu, Z. Li and B. Ding, Composites, Part B, 2018, 155, 397–404 CrossRef CAS.
- Y. Wang, X. Gao, C. Lin, L. Shi, X. Li and G. Wu, J. Alloys Compd., 2019, 785, 765–773 CrossRef CAS.
- J. Fang, Z. Chen, W. Wei, Y. Li, T. Liu, Z. Liu and X. Yue, RSC Adv., 2015, 5, 50024–50032 RSC.
- I. Abdalla, J. Shen, J. Yu, Z. Li and B. Ding, Sci. Rep., 2018, 8, 12402 CrossRef PubMed.
- F. M. Alanagha, A. B. Khiabanib and A. A. Asl, Compos. Sci. Technol., 2017, 150, 65–78 CrossRef.
- G. Wu, Z. Jia, Y. Cheng, H. Zhang, X. Zhou and H. Wu, Appl. Surf. Sci., 2019, 10, 472–478 CrossRef.
- C. Huang, C. Hao, Z. Ye, S. Zhou, X. Wang, L. Zhu and J. Wu, Nanoscale, 2019, 11, 10114–10128 RSC.
- A. Feng, G. Wu, C. Pan and Y. Wang, J. Nanosci. Nanotechnol, 2017, 17, 3786–3791 CrossRef CAS.
- J. Ma, W. Liu, B. Quan, X. Liang and G. Ji, J. Colloid Interface Sci., 2017, 504, 479–484 CrossRef CAS PubMed.
- X. Xue, H. Yan and Y. Fu, Solid State Ionics, 2019, 335, 1–6 CrossRef CAS.
- A. Feng, G. Wu, C. Pan and Y. Wang, J. Nanosci. Nanotechnol., 2017, 17, 3859–3863 CrossRef CAS.
- W. Wu, C. Yu, J. Chen and Q. Yang, Int. J. Environ. Anal. Chem., 2019 DOI:10.1080/03067319.2019.1636977.
- X. Yang, Y. Guo, Y. Han, Y. Li, T. Ma, M. Chen, J. Kong, J. Zhu and J. Gu, Composites, Part B, 2019, 175, 107070 CrossRef.
- M. Sabet, H. Jahangiri and E. Ghashghaei, Synth. Met., 2017, 224, 18–26 CrossRef CAS.
- X. Chen, T. Shi, K. Zhong, G. Wu and Y. Lu, Chem. Eng. J., 2020, 379, 122240 CrossRef CAS.
- H. L. Xu, X. W. Yin, M. Zhu, M. H. Li, H. Zhang, H. J. Wei, L. T. Zhang and L. F. Cheng, Carbon, 2019, 142, 346–353 CrossRef CAS.
- X. Zhou, Z. Jia, X. Wang, J. Liu, M. Zhang, H. Cao and G. Wu, Carbon, 2019, 152, 827–836 CrossRef CAS.
- Z. Jia, B. Wang, A. Feng, J. Liu, M. Zhang, Z. Huang and G. Wu, J. Alloys Compd., 2019, 799, 216–223 CrossRef CAS.
- Y. Sun, J. W. Zhang, Y. Zong, X. Deng, H. Y. Zhao, J. Feng, M. He, X. H. Li, Y. Peng and X. L. Zheng, ACS Appl. Mater. Interfaces, 2019, 11, 6374–6383 CrossRef CAS PubMed.
- X. Chen, K. Zhong, T. Shi, X. Meng, G. Wu and Y. Lu, Synth. Met., 2019, 248, 59–67 CrossRef CAS.
- Y. Wang, Y. Fu, X. Wu, W. Zhang, Q. Wang and J. Li, Ceram. Int., 2017, 43, 11367–11375 CrossRef CAS.
- X. L. Chen and S. H. Qi, J. Sol-Gel Sci. Technol., 2017, 81, 824 CrossRef CAS.
- R. S. Yadav, I. Kuřitka, J. Vilcakova and D. Skoda, Composites, Part B, 2019, 166, 95–111 CrossRef CAS.
- H. Yan, Y. Fu, X. Wu, X. Xue, C. Li and L. Zhang, Solid State Ionics, 2019, 336, 95–101 CrossRef CAS.
- J. Feng, Y. Zong, Y. Sun, Y. Zhang, X. Wang, G. Long, Y. Wang, X. Li and X. Zheng, Chem. Eng. J., 2018, 345, 441–451 CrossRef CAS.
- Y. Cheng, J. M. Cao, Y. Li, Z. Y. Li, H. Q. Zhao, G. B. Ji and Y. W. Du, ACS Sustainable Chem. Eng., 2018, 6, 1427–1435 CrossRef CAS.
- K. Nasouri and A. M. Shoushtari, Compos. Sci. Technol., 2017, 145, 46–54 CrossRef CAS.
- X. Chen and S. Qi, J. Mater. Sci.: Mater. Electron., 2016, 27, 13099–13104 CrossRef CAS.
- J. T. Kim, C. W. Park and B. J. Kim, Synth. Met., 2017, 223, 212–217 CrossRef CAS.
- Z. Jia, B. Wang, A. Feng, J. Liu, C. Zhang, M. Zhang and G. Wu, Ceram. Int., 2019, 13, 15854–15859 CrossRef.
- J. Liu, R. Che, H. Chen, F. Zhang, F. Xia, Q. Wu and M. Wang, Small, 2012, 8, 1214–1221 CrossRef CAS PubMed.
- D. Lan, M. Qin, R. Yang, H. Wu, Z. Jia, K. Kou, G. Wu, Y. Fan, Q. Fu and F. Zhang, J. Mater. Sci.: Mater. Electron., 2019, 30, 8771–8776 CrossRef CAS.
- J. Wu, Z. Ye, H. Ge, J. Chen, W. Liu and Z. Liu, J. Colloid Interface Sci., 2017, 506, 217–226 CrossRef CAS PubMed.
- H. L. Lv, Z. H. Yang, S. J. H. Ong, C. Wei, H. B. Liao, S. B. Xi, Y. H. Du, G. B. Ji and Z. C. J. Xu, Adv. Funct. Mater., 2019, 14, 1900163 CrossRef.
- Y. Wang, W. Z. Zhang, X. M. Wu, C. Y. Luo, Q. G. Wang, J. H. Li and L. Hu, Synth. Met., 2017, 228, 18–24 CrossRef CAS.
- H. Zhang, B. Wang, A. Feng, N. Zhang, Z. Jia, Z. Huang, X. Liu and G. Wu, Composites, Part B, 2019, 167, 690–699 CrossRef CAS.
- H. L. Lv, Z. H. Yang, P. L. Wang, G. B. Ji, J. Z. Song, L. R. Zheng, H. B. Zeng and Z. J. Xu, Adv. Mater., 2018, 30, 1706343 CrossRef PubMed.
- Z. Wang, M. Yang, Y. Cheng, J. Liu, B. Xiao, S. Chen, J. Huang, Q. Xie, G. Wu and H. Wu, Composites, Part A, 2019, 118, 302–311 CrossRef CAS.
- G. Wu, Y. Cheng, Z. Yang, Z. Jia, H. Wu, L. Yang, H. Li, P. Guo and H. Lv, Chem. Eng. J., 2018, 333, 519–528 CrossRef CAS.
- T. Hou, B. Wang, Z. Jia, H. Wu, D. Lan, Z. Huang, A. Feng, M. Ma and G. Wu, J. Mater. Sci.: Mater. Electron., 2019, 30, 10961–10984 CrossRef CAS.
- A. Feng, Z. Jia, Y. Zhao and H. Lv, J. Alloys Compd., 2018, 745, 547–554 CrossRef CAS.
- S. Gupta, C. Chang, C. H. Lai and N. H. Tai, Composites, Part B, 2019, 164, 447–457 CrossRef CAS.
- J. Li, J. Ma, S. Chen, Y. Huang and J. He, Mater. Sci. Eng., C, 2018, 89, 25–32 CrossRef CAS PubMed.
- H. Zhao, L. Hou, S. Bi and Y. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 33059–33070 CrossRef CAS.
- G. Wu, H. Zhang, X. Luo, L. Yang and H. Lv, J. Colloid Interface Sci., 2019, 536, 548–555 CrossRef CAS.
- Y. Wang, X. Gao, L. Zhang, X. Wu, Q. Wang, C. Luo and G. Wu, Appl. Surf. Sci., 2019, 480, 830–838 CrossRef CAS.
- W. Wu, C. Yu, Q. Wang, F. Zhao, H. He, C. Liu and Q. Yang, Crit. Rev. Food Sci., 2019 DOI:10.1080/03067319.2019.1636977.
- S. Y. Chen, Y. H. Cheng, Q. Xie, B. Xiao, Z. D. Wang, J. Y. Liu and G. L. Wu, Composites, Part A, 2019, 120, 84–94 CrossRef CAS.
- M. Cao, J. Yang, W. Song, D. Zhang, B. Wen, H. Jin, Z. Hou and J. Yuan, ACS Appl. Mater. Interfaces, 2012, 4, 6949–6956 CrossRef CAS PubMed.
- J. Wang, B. Cheng, H. Qiu and S. Qi, J. Electron. Mater., 2018, 47, 5564–5571 CrossRef CAS.
- Y. C. Du, W. W. Liu, R. Qiang, Y. Wang, X. J. Han, J. Ma and P. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12997–13006 CrossRef CAS PubMed.
- X. L. Chen, X. W. Wang, L. D. Li and S. H. Qi, J. Mater. Sci.: Mater. Electron., 2016, 27, 5607–5612 CrossRef CAS.
- Y. Wang, W. Zhang, C. Luo, X. Wu, Q. Wang and W. Chen, Ceram. Int., 2016, 42, 17374–17381 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |