Open Access Article
Open Access ArticleSubmicron fibers as a morphological improvement of amorphous zirconium oxide particles and their utilization in antimonate (Sb(V)) removal†
Satu Lönnrot *,
Valtteri Suorsa
*,
Valtteri Suorsa ,
Johanna Paajanen,
Timo Hatanpää
,
Johanna Paajanen,
Timo Hatanpää ,
Mikko Ritala and
Risto Koivula
,
Mikko Ritala and
Risto Koivula
Department of Chemistry, FI-00014 University of Helsinki, P. O. box 55, Finland. E-mail: satu.lonnrot@helsinki.fi
First published on 18th July 2019
Abstract
Mesoporous and large surface area zirconium oxide aggregate granules with good adsorption properties were synthesized using a simple precipitation method. Since utilization of these small and fragile particles is considered rather difficult in larger scale column operation, the product was formed into a fibrous form to improve its usability. The submicron fibers were obtained from an optimized electroblowing synthesis that resulted in elastic and uniform fibers with a tetragonal structure and high length-to-diameter ratio. In antimonate (Sb(V)) adsorption experiments, the higher calcination temperature (350 °C) of the fibers did not seem to decrease the Sb(V) adsorption capacity excessively since the high theoretical adsorption capacities were 113 mg g−1 and 58 mg g−1 for the aggregate and fibers, respectively. Both materials had fast kinetics, fibers being faster in the beginning of the reaction. Moreover, both materials offered efficient Sb(V) removal in the studied pH range from 1 to 11 by reaching over 99.9% adsorption in the optimal pH range. X-ray absorption near edge spectroscopy (XANES) revealed that Sb(V) stays as pentavalent antimony after being adsorbed by these materials and based on the isoelectric point shifts in the zeta potential measurement, adsorption occurs mainly by an inner-sphere complexation reaction. Finally, our study showed that pressure buildup in a flow-through column packed with zirconium oxide fibers was significantly lower than in a column packed with aggregates. Thus, zirconium oxide aggregates can be formed into submicron fibers with enhanced column operation properties without a too large compromise in the adsorption properties.
1. Introduction
Zirconium oxide is a versatile and well-studied material with multiple applications, including as catalysts, gas sensors, ceramics and fuel cells.1–4 Zirconium oxide can be synthesized in amorphous, cubic, tetragonal and monoclinic forms, the stability and occurrence of which depend on crystallite size, dopants, synthesis pH and temperature.5–7 Monoclinic zirconium oxide is found to be stable below 1170 °C and cubic above 2370 °C whereas temperatures between 1170 °C and 2370 °C stabilize the tetragonal form.8 The tetragonal zirconium oxide can however exist at room temperature in a metastable state that can be achieved for instance by limiting the crystal size to below 30 nm.9 In ceramics, the tetragonal structure is often preferred due to its superior mechanical properties such as fracture toughness, and strength.10In addition to different crystal structures, zirconium oxide can be prepared in different morphologies, such as nanopowders, microspheres, grains, films and fibers.7,11–15 One method to produce nanocrystalline zirconium oxide is a precipitation method in which zirconium precursor, which is first dissolved into acid, is precipitated with base causing the formation of small colloids that can stay monodispersed or form aggregates during a drying process.16 Precipitated materials usually have a large specific surface area and porosity but the use of such small particles is rather challenging in some applications, for example, in fixed-bed columns.17 In industrial scale columns, pressure surges are breaking these fragile aggregate particles leading to column blockages and leakages of nanoparticles out of the columns. This problem could be avoided by formatting zirconium oxide into a fiber. Nano- and submicron fibers are often produced by a spinning technique including electro- and blowspinning, and eletroblowing that are applicable to the synthesis of various materials including zirconium oxide.18,19 The electroblowing synthesis enhances the production rate of fibers compared to electrospinning since additional gas flow induces faster evaporation of solvents that enables greater solution feeding rates.20 The production rate of fibers can be further increased by adding needles to the synthesis unit. Nanofibers in general show some excellent properties such as high specific surface area and good mechanical strength, and handling of woven fiber mats is simple compared to small particles.14 The forementioned different morphologies and structures have a determining impact on surface properties, such as specific surface area, porosity and pore size, which are the main factors contributing to the adsorption through available sorption sites.11,21
Contamination of toxic Sb in natural waters have raised concerns of its effects on human health and environment. Weathering of rock minerals, soil runoff and anthropogenic sources such as mining and smelting industries are releasing antimony into the natural waters.22 In non-polluted areas, a typical antimony concentration in water is less than 1 μg L−1 but near industrial areas the concentration can be over 100 times that of the natural level.22 Antimony is a metalloid, resembling arsenic and phosphorus, that occurs in two oxidation states: antimonite (Sb(III)) and antimonate (Sb(V)), in natural waters from which the latter is the prevalent in oxidizing conditions. In the oxidizing environment, Sb(III) is thermodynamically unstable, but Sb(V) have also been found from anoxic systems.22 Sb(V) has two hydroxyl species: Sb(OH)5 that exists below pH 2.7 and Sb(OH)6− that is a prevalent above this pH-value.23 This makes Sb(OH)6− a more important species from the uptake point of view. Respectively, three hydroxyl species of Sb(III) in the order of increasing pH are Sb(OH)2+, Sb(OH)3 and Sb(OH)4−.23 As a more soluble and mobile species, Sb(V) was selected for this study.
Because of the hazardous nature of antimony, several authorities have set maximum concentration limits of antimony for drinking water. Commonly, the maximum level for antimony in drinking water is regulated to 5–6 μg L−1.24–26 Therefore, several methods for separation of antimony have been suggested including coagulation, liquid extraction, membrane separation, electrochemical methods, adsorption and bioremediation.27–32 For years, antimony separation has been conducted with coagulation method that combines Fe or Al salt precipitation and adsorption. The more common Fe precipitation has effective particularly for Sb(III) but coexisting anions such as PO43− and SO42− are hampering already weaker separation of Sb(V).33 The removal efficiency could be improved by using selective adsorption since adsorption is a simple, efficient and inexpensive method for metal separation from water, especially on trace level concentrations.27
Some zirconium based materials such as zirconium oxide,12,26 iron-zirconium bimetal oxide,26 zirconium oxide decorated carbon nanofibers,14 zirconium-based metal–organic framework34 and Zr-loaded saponified orange waste35 are proven to be efficient Sb(V) adsorbents. However, most of these materials are composites or hybrids that chemical and physical properties can greatly differ from those of pure zirconium oxide. In fact, zirconium oxide possesses several excellent properties as an adsorbent. It is practically insoluble over a wide range of pH and is resistant to strong reducing and oxidizing conditions. Moreover, as a metal oxide, zirconium oxide should have a vast amount of surface hydroxyl groups that are proposed to strongly interact with anions.21,26 The non-toxicity of zirconium oxide further enables its applicability in antimony removal, from industrial wastewater to daily drinking water purification.
Thus, in this study, we synthesized granular zirconium oxide aggregate material by using easy and inexpensive precipitation method that is known to result in material with excellent adsorption capabilities. The morphology of the product was then improved by electroblowing zirconium oxide into submicron fibers. The electroblowing synthesis is more challenging but fibers should provide a high specific surface area indicating good adsorption capacity, and better usability in columns due to its mechanical performance. Hence, our goal is to find out, if a well-working precipitated zirconium oxide grains can be formatted into submicron fibers without losing too much of its great adsorption capabilities. Thus, Sb(V) adsorption properties of granular and fibrous zirconium oxides are compared with each other by the same author resulting a coherent comparison between materials. The main focus is to study morphology, structure and surface properties of these two materials and their influence on Sb(V) adsorption capability. Our study investigated the effect of pH, contact time and competing ions on Sb(V) adsorption and determined the Sb(V) adsorption capacities of the materials. In addition, pressure buildups in the columns packed with fibers and aggregate grains was examined.
2. Materials and methods
2.1 Chemicals
Sb2O5 (99.995% trace metal basis) was purchased from Sigma-Aldrich and NaNO3 (>99%) from VWR Chemicals. NaHCO3, Na2SO4 and H2NaPO4 used in competition experiments were analytical grade. A stock solution of 1000 mg L−1 Sb(V) was made by dissolving Sb2O5 in 4.5 M hydrochloric acid. Nitric acid (Romil SpA super purity, 67–69 wt%) and hydrochloric acid (Merck suprapur, 30%) were used for sample dilution for ICP-MS measurement. In all samples and solutions deionized water (Millipore, 18.2 MΩ cm at 25 °C) was used. Chemicals used in synthesis are listed as ESI, Section S1.†2.2 Synthesis
2.3 Characterization
2.4 Batch adsorption methods
Results of the pH and kinetic studies were presented as distribution coefficients, Kd, as a function of pH or time. The distribution coefficient describes the distribution of elements between solid and solution phases. Kd was calculated using eqn (1):
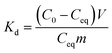 | (1) |
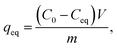 | (2) |
2.5 Pressure test
Pressure buildup in the columns packed with 0.25 g of GZR or FZR was determined with solution feeding rates of 5, 25, 50 and 75 mL h−1 that were controlled with a peristaltic pump. Both GZR and FZR were packed into low-pressure Bio-Rad Econo-columns that were 0.7 cm in diameter. Columns were connected to a pressure sensor and pressure buildup was measured 30 minutes after starting the peristaltic pump. Pressure was released before moving on the next feeding rate.3. Results and discussion
3.1 Structural and chemical analysis
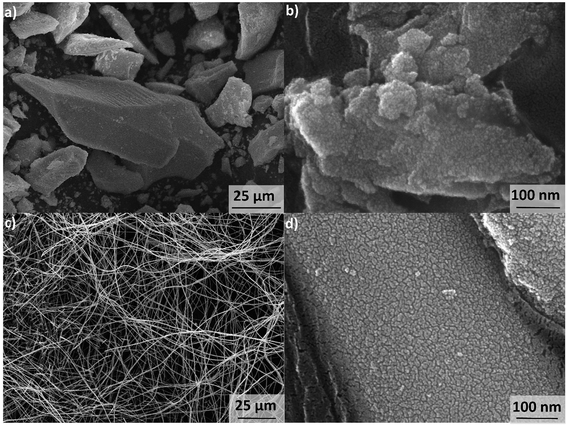
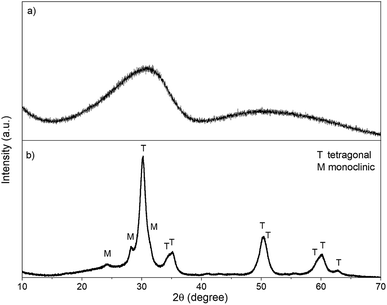
The synthesis pH of GZR was set to 7.8 that would favor the formation of tetragonal structure of zirconia which is well known to be the active phase considering the materials sorption properties.38 The random agglomeration of small crystals resulted in precipitation of a course, hard cake in a slightly pink color (Fig. 1a and b). The obtained 30 g of hydrous zirconium dioxide (ZrO2·1.1H2O according to TG analysis, Fig. S1†) released some CO2 that was most probably absorbed from the air during the synthesis (TGA-MS graph, Fig. S1†). A clear exothermic peak at 450 °C appears in DSC due to the formation and orientation of tetragonal crystallites and broader peak above 800 °C indicating phase transformation to monoclinic.39The spun fiber mats retained their physical form after the calcination (350 °C) and formation of zirconium dioxide. The mats showed some elasticity when bended indicating mechanical strength, but the product was relatively easy to grind into a powder. The low magnification SEM image points out that fibers are intact, smooth and uniform with a mean diameter from 300 to 500 nm (Fig. 1c). The fibers have a cylindrical shape with a high length to diameter ratio, and no beads or fused fibers are visible. However, with high magnification the fiber shows surface roughness that could arise from crystallite structure of the fiber (Fig. 1d). The total mass loss of the calcined fiber in TGA is only 2.7% from which approximately a half is the loss of readsorbed water between 40 and 200 °C (TGA from 40 to 200 °C, Fig. S2†). Above 400 °C, where PVP starts to decompose, a 1.5% weight loss was measured that is linked to burning of polymer residues.40 Moreover, FZR shows CO2 releases: first at 500 °C and later at 900 °C and 1000 °C (TGA-MS graph, Fig. S2†). Since the total mass loss during the analysis is rather small, the product can be regarded as relatively pure. Nevertheless, this minor residue could weaken materials adsorption properties by blocking the material's surface. However, a relatively high porosity and good adsorption capacity in the isotherm experiment was observed indicating that residual polymer did not significantly interfere with adsorption. A strong increase in the DSC was observed above 800 °C indicating transformation of tetragonal crystallites to monoclinic (Fig. S2†).
≡ZrOH2+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ≡ZrOH + H+ ≡ZrOH + H+
| (3) |
≡ZrOH ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ≡ZrO− + H+ ≡ZrO− + H+
| (4) |
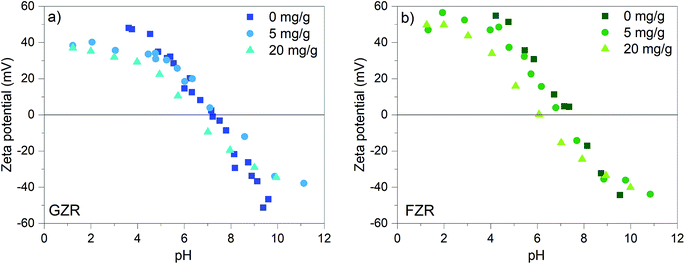 | ||
| Fig. 4 Zeta potential (mV) of GZR and FZR loaded with 0, 5, and 20 mg g−1 Sb(V) as a function of pH in 0.01 M NaNO3, after 24 h equilibration time. | ||
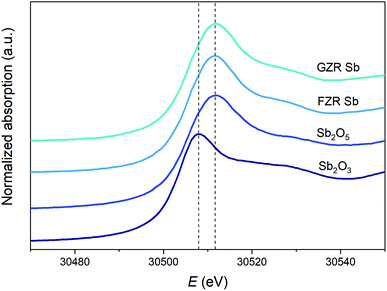
At low pH, the surface groups are positive ≡ZrOH2+ and in alkaline conditions the surface charge is negative because of ≡ZrO− groups. At the isoelectric points (IEP), the surface group charges are in balance and the net charge of the material is zero. The isoelectric point of FZR and GZR in 0.01 M NaNO3 are at pH 7.4 and 7.2, respectively, which are in the typical range from 4 to 11 observed for ZrO2.51 By loading the materials with 5 mg g−1 of Sb(V), the IEP of FZR decreases slightly compared to unloaded material but no difference can be observed in the case of GZR. When materials were loaded with 20 mg g−1 of Sb(V), the IEP shift is more obvious and visible for both materials. The IEP of FZR with 20 mg g−1 Sb(V) loading is 6.0 and that of GZR is 6.2. The observed decrease of the IEP value during adsorption is proposed to be due to an inner-sphere complexation of Sb(OH)6−.26,52,53 Essington and Stewart54 suggested that Sb(V) adsorption on gibbsite, Al(OH)3, would happen through both inner- and outer-sphere complexation reactions (5) and (6), respectively, presented below for zirconia:
≡ZrOH + Sb(OH)6− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ≡ZrOSb(OH)5− + H2O ≡ZrOSb(OH)5− + H2O
| (5) |
≡ZrOH + H+ + Sb(OH)6− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) ≡ZrOH2+–Sb(OH)6− ≡ZrOH2+–Sb(OH)6−
| (6) |
3.2 Sb(V) uptake
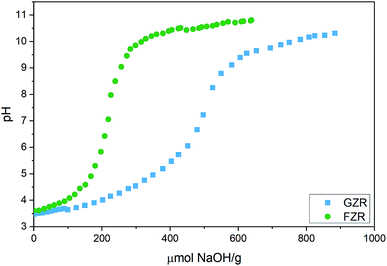
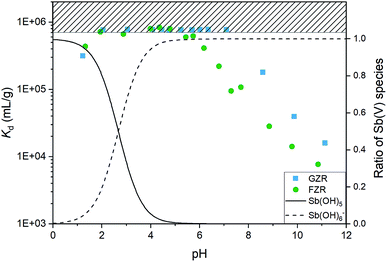
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1. In fact, the equilibrium Sb(V) concentration was below the ICP-MS detection limit of 0.07 μg L−1 with almost all the samples in this pH range. At this optimal pH range, adsorption is facilitated by electrostatic attraction between positively charged adsorbent surface and negatively charged Sb(OH)6−. Below pH 2, Kd value decreases towards more acidic conditions, that can be attributed to electrically neutral Sb(OH)5 species that becomes prevalent instead of Sb(OH)6−. A decrease of the Kd as a function pH in the alkaline end can be explained by gradually emerging negative surface charge of the materials that repulses the prevailing Sb(OH)6− species, Fig. 4 and 6. However, the rising pH do not totally prevent adsorption but probably increases an energy barrier between Sb(OH)6− and the negatively charged surface slowing down the adsorption reaction.
000 mL g−1. In fact, the equilibrium Sb(V) concentration was below the ICP-MS detection limit of 0.07 μg L−1 with almost all the samples in this pH range. At this optimal pH range, adsorption is facilitated by electrostatic attraction between positively charged adsorbent surface and negatively charged Sb(OH)6−. Below pH 2, Kd value decreases towards more acidic conditions, that can be attributed to electrically neutral Sb(OH)5 species that becomes prevalent instead of Sb(OH)6−. A decrease of the Kd as a function pH in the alkaline end can be explained by gradually emerging negative surface charge of the materials that repulses the prevailing Sb(OH)6− species, Fig. 4 and 6. However, the rising pH do not totally prevent adsorption but probably increases an energy barrier between Sb(OH)6− and the negatively charged surface slowing down the adsorption reaction.
The decrease of the Kd value in GZR system starts at the IEP when the net charge of the surface becomes negative. Even though the Kd value decreases from pHIEP onwards, GZR performs on good level since it is able to uptake still 99.8% (Kd 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1) of Sb(V) at pH 8 and even in the most alkaline sample 97.0% (Kd 16
000 mL g−1) of Sb(V) at pH 8 and even in the most alkaline sample 97.0% (Kd 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1). Different from GZR, the Sb(V) uptake of FZR decreases already below material's pHIEP. The decrease could be attributed to larger surface coverage on FZR surface causing greater repulsion between adsorbed and solute Sb(OH)6−. Despite the possible repulsion, FZR is able to uptake even 99.5% (Kd 108
000 mL g−1). Different from GZR, the Sb(V) uptake of FZR decreases already below material's pHIEP. The decrease could be attributed to larger surface coverage on FZR surface causing greater repulsion between adsorbed and solute Sb(OH)6−. Despite the possible repulsion, FZR is able to uptake even 99.5% (Kd 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1) at pH 8 and 93.8% (Kd 7700 mL g−1) at pH 11.
000 mL g−1) at pH 8 and 93.8% (Kd 7700 mL g−1) at pH 11.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1) adsorption in 2 min and GZR in 11 min, and they reach the detection limit, 0.025 μg L−1, after 7 and 2 hours, respectively. On this basis, both materials are suitable for a continuous purification process where fast kinetics is essential for an effective separation. At low Sb concentrations where high capacity is unnecessary, FZR probably performs better than GZR, but when a higher adsorption capacity is required, GZR should provide more efficient removal.
000 mL g−1) adsorption in 2 min and GZR in 11 min, and they reach the detection limit, 0.025 μg L−1, after 7 and 2 hours, respectively. On this basis, both materials are suitable for a continuous purification process where fast kinetics is essential for an effective separation. At low Sb concentrations where high capacity is unnecessary, FZR probably performs better than GZR, but when a higher adsorption capacity is required, GZR should provide more efficient removal.
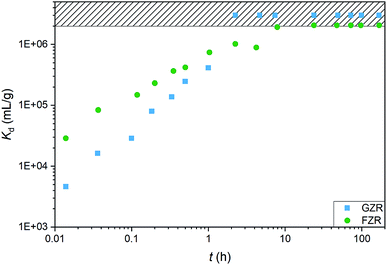 | ||
| Fig. 7 The Sb(V) adsorption kinetics on GZR and FZR presented as Kd (mL g−1) as a function of time (h) in 10 ppm Sb(V) and 0.01 M NaNO3 at pH 4.0 ± 0.2. | ||
The kinetic data was analyzed according to the pseudo-first and pseudo-second-order rate laws in their nonlinear forms.57 The pseudo-first-order equation can be presented as
| qt = qeq[1 − exp(−k1t)] | (7) |
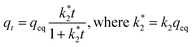 | (8) |
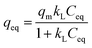 | (9) |
| qeq = kFC1/neq, | (10) |
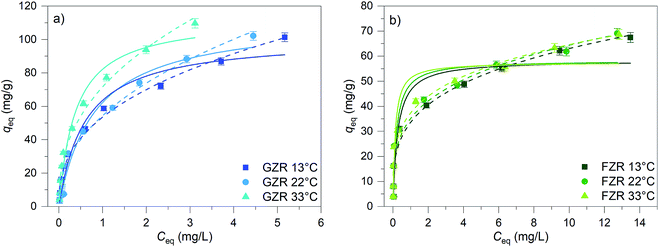
| T (°C) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm (mg g−1) | kL (L mg−1) | R2 | kF (g mg−1 L−1) | n | R2 | ||
| GZR | 13 | 101 | 1.7 | 0.956 | 54 | 2.6 | 0.989 |
| 22 | 113 | 1.2 | 0.947 | 54 | 2.3 | 0.971 | |
| 33 | 113 | 2.6 | 0.969 | 72 | 2.5 | 0.982 | |
| FZR | 13 | 58 | 3.9 | 0.885 | 34 | 3.7 | 0.950 |
| 22 | 58 | 5.1 | 0.887 | 35 | 3.8 | 0.959 | |
| 33 | 58 | 8.1 | 0.873 | 37 | 4.2 | 0.966 | |
A high correlation with the Langmuir model is an indication of constant adsorption site energy and asymptotic approach of the full monolayer surface coverage at high concentrations whereas the empirical multilayer Freundlich model assumes that the adsorbent has different adsorption sites.62 The R2 values of model fittings show that Freundlich model describes Sb(V) adsorption better than Langmuir model suggesting heterogeneous adsorption. However, as Freundlich model cannot be used to obtain the theoretical maximum adsorption capacity, the theoretical capacities were based on the Langmuir parameter qm that is 113 mg g−1 (0.928 mmol g−1) for GZR and 58 mg g−1 (0.476 mmol g−1) for FZR at 22 °C. Because of the rather low correlation with the Langmuir model, an experimental adsorption capacity of FZR, 69 mg g−1, exceeds the result of Langmuir model fitting slightly. The capacity of FZR is in a good agreement with earlier Sb(V) adsorption studies on zirconium based materials such as zirconium oxide26 at pH 7.0, 55 mg g−1, zirconium oxide decorated carbon nanofibers14 at pH 7.0, 57 mg g−1, and iron-zirconium bimetal oxide26 at pH 7.0, 51 mg g−1. However, GZR shows even greater capacity towards Sb(V) being approximately twice that reported in literature for zirconium oxide.
From the results of the adsorption capacity experiment and the BET surface area measurement, a surface site density can be calculated. The surface site density of GZR is 2.3 sites per nm2 and that of FZR is 4.0 sites per nm2. The site density of FZR is higher but the larger specific surface area of GZR dominates the capacity difference observed in the adsorption isotherms. When the surface site densities are compared with the hydroxyl site densities presented before (1.2 sites per nm2 for GZR and 1.8 sites per nm2 for FZR) a significant difference can be observed. On this basis, adsorption cannot be explained only with reactions between Sb(V) and hydroxyl groups. It is likely that there are other kinds of reactive surface groups or mechanisms taking part in the adsorption process such as nucleation, reaction with non-hydrolyzed zirconium or oxygen vacancies.
3.3 Pressure test
The pressure buildup in the GZR and FZR columns were measured to determine their functioning in packed bed columns since evolution of high pressures can cause severe problems in the purification process, Fig. 10. At the feeding rate of 5 mL h−1, which can be considered as a normal feeding rate for packed bed column of this size, the overpressure is low (50 mbar) for GZR and even lower for FZR. However, at higher feeding rates the column packed with GZR is generating significantly more backpressure than FZR. At the highest feeding rate, the overpressure in the GZR column is 930 mbar whereas the overpressure in the FZR column is only 70 mbar that is only small fraction compared to GZR. The difference originates from the physical form of the materials. Small <74 μm GZR particles are packing tightly in the column creating notably higher flow resistance than loosely packed self-supported FZR fibers that are forming 3D webs. Due to the lower flow resistance, formatting zirconia into the fiber could improve the column functioning by retaining low pressures even at higher flow rates. Furthermore, no breaking or fragmentation of the fibers was observed during the experiment that indicates material to be suitable for column separation even with high flow rates.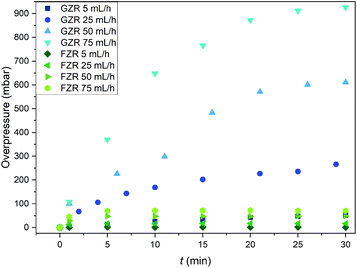 | ||
| Fig. 10 The overpressure (mbar) generated in the columns packed with 0.25 g of GZR or FZR at solution feed rates of 5, 25, 50 and 75 mL h−1 during 30 min period. | ||
4. Conclusions
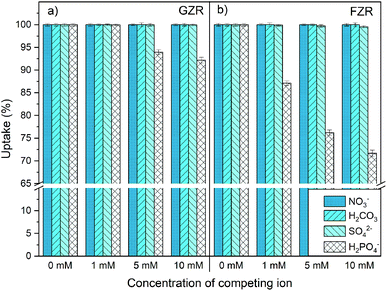
In this study, two zirconium oxide materials grains and fibers were synthesized by precipitation and electroblowing methods, respectively, and products compared with each other. The precipitated zirconium oxide formed uneven aggregate grains with broad variation in size and shape that showed amorphous or nanocrystalline structure in XRD. In addition, elastic fibers with cylindrical shape and dominating tetragonal structure were obtained from electroblowing synthesis with an average diameter of 300–500 nm. Both synthesized zirconium oxide materials showed efficient Sb(V) removal in a wide pH range enabling their use in various conditions. Furthermore, good adsorption capacities were observed in the isotherm experiment in which adsorption capacity of GZR was higher than that of FZR. The good adsorption capacities are probably due to the large specific surface areas and porosities of the materials that were higher for GZR. Both materials had fast kinetics FZR being faster in the beginning of the reaction whereas GZR reached the detection limit earlier because of GZR's higher adsorption capacity. The materials followed pseudo-second-order rate model indicating chemical interaction between surface and Sb(V) ions that is supported by the IEP shift, negligible competition between NO3− and Sb(V) and the efficient Sb(V) uptake also in alkaline conditions where surface charge of the products are negative. This indicates that even though the product morphologies are greatly different from each other the suggested adsorption mechanism stays the same.Hence, well-working zirconium oxide aggregate can be formatted into submicron fibers but the adsorption capacity is slightly weakened because of higher calcination temperature of fibers that decreases the specific surface area and porosity. The major benefit of FZR was its better mechanical stability, lower pressure buildup in the column and faster adsorption kinetics that could benefit antimony removal in dynamic column operation. Nonetheless, when considering application of these materials in industrial scale, further study is needed to determine optimal conditions for such process by column experiments. In addition, regeneration of the materials would extend materials usability and therefore should be investigated in the future. Overall, these two materials have a great potential to uptake Sb(V) from different water solutions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A XANES experiment of this study was carried out at PETRA III P64 at DESY, a member of the Helmholtz Association (HGF). We thank W. Caliebe for assistance in measurements at P64 beamline. This work was supported by University of Helsinki (Doctoral School in Chemistry and Molecular Sciences). The authors also acknowledge the support of Fortum Power and Heat Oy.References
- Y. Shao, Z. Xu, H. Wan, H. Chen, F. Liu, L. Li and S. Zheng, J. Hazard. Mater., 2010, 179, 135–140 CrossRef CAS
.
- A. V. Borhade, D. R. Tope and J. A. Agashe, J. Mater. Sci.: Mater. Electron., 2018, 29, 7551–7561 CrossRef CAS
.
- R. Zhang, R. He, W. Zhou, Y. Wang and D. Fang, Mater. Des., 2014, 62, 1–6 CrossRef CAS
.
- A. Malolepszy, M. Mazurkiewicz, L. Stobinski, B. Lesiak, L. Kover, J. Toth, B. Mierzwa, A. Borodzinski, F. Nitze and T. Wagberg, Int. J. Hydrogen Energy, 2015, 40, 16724–16733 CrossRef CAS
.
- E. Djurado, P. Bouvier and G. Lucazeau, J. Solid State Chem., 2000, 149, 399–407 CrossRef CAS
.
- M. Kogler, E. Koeck, S. Vanicek, D. Schmidmair, T. Goetsch, M. Stoeger-Pollach, C. Hejny, B. Kloetzer and S. Penner, Inorg. Chem., 2014, 53, 13247–13257 CrossRef CAS
.
- D. G. Lamas, A. M. Rosso, M. S. Anzorena, A. Fernandez, M. G. Bellino, M. D. Cabezas, N. E. Walsoe de Reca and A. F. Craievichc, Scr. Mater., 2006, 55, 553–556 CrossRef CAS
.
- M. Li, Z. Feng, G. Xiong, P. Ying, Q. Xin and C. Li, J. Phys. Chem. B, 2001, 105, 8107–8111 CrossRef CAS
.
- R. Garvie, J. Phys. Chem., 1965, 69, 1238–1243 CrossRef CAS
.
- K. Tsukuma and M. Shimada, J. Mater. Sci., 1985, 20, 1178–1184 CrossRef CAS
.
- H. Tel, Y. Altas, F. Gur and A. Ugur, Radiochim. Acta, 2010, 98, 215–219 CAS
.
- R. Koivula, R. Harjula and H. Manni, EP2243547A1, 2010
.
- S. Starschich, T. Schenk, U. Schroeder and U. Boettger, Appl. Phys. Lett., 2017, 110, 182905 CrossRef
.
- J. Luo, X. Luo, J. Crittenden, J. Qu, Y. Bai, Y. Peng and J. Li, Environ. Sci. Technol., 2015, 49, 11115–11124 CrossRef CAS
.
- Y. Zhao, Y. Tang, Y. Guo and X. Bao, Fibers Polym., 2010, 11, 1119–1122 CrossRef CAS
.
- H. Cui, Q. Li, S. Gao and J. K. Shang, J. Ind. Eng. Chem., 2012, 18, 1418–1427 CrossRef CAS
.
- H. Cui, Y. Su, Q. Li, S. Gao and J. K. Shang, Water Res., 2013, 47, 6258–6268 CrossRef CAS
.
- Z. Shi, J. Ju, Y. Liang, W. Huang, W. Kang and B. Cheng, Chem. Lett., 2017, 46, 131–134 CrossRef CAS
.
- H. Liu, B. Liu, X. Wang, L. Zhu, C. Feng, G. Zhang and D. Xu, J. Sol-Gel Sci. Technol., 2015, 76, 482–491 CrossRef CAS
.
- M. Pokorny, V. Rassushin, L. Wolfova and V. Velebny, Polym. Eng. Sci., 2016, 56, 932–938 CrossRef CAS
.
- Y. Su, W. Yang, W. Sun, Q. Li and J. K. Shang, Chem. Eng. J., 2015, 268, 270–279 CrossRef CAS
.
- M. Filella, N. Belzile and Y. Chen, Earth-Sci. Rev., 2002, 57, 125–176 CrossRef CAS
.
- S. C. Wilson, P. V. Lockwood, P. M. Ashley and M. Tighe, Environ. Pollut., 2010, 158, 1169–1181 CrossRef CAS
.
- USEPA, National Primary Drinking Water Regulations, 2009 Search PubMed
.
- Council of the European Union, Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, 1998 Search PubMed
.
- X. Li, X. Dou and J. Li, J. Environ. Sci., 2012, 24, 1197–1203 CrossRef CAS
.
- G. Ungureanu, S. Santos, R. Boaventura and C. Botelho, J. Environ. Manage., 2015, 151, 326–342 CrossRef CAS
.
- B. Sargar, M. Rajmane and M. Anuse, J. Serb. Chem. Soc., 2004, 69, 283–298 CrossRef CAS
.
- X. Guo, Z. Wu and M. He, Water Res., 2009, 43, 4327–4335 CrossRef CAS PubMed
.
- Y. Zhu, M. Wu, N. Gao, W. Chu, N. An, Q. Wang and S. Wang, J. Hazard. Mater., 2018, 341, 36–45 CrossRef PubMed
.
- M. E. H. Bergmann and A. S. Koparal, J. Hazard. Mater., 2011, 196, 59–65 CrossRef CAS
.
- Z. Qi, T. P. Joshi, R. Liu, Y. Li, H. Liu and J. Qu, J. Hazard. Mater., 2018, 343, 36–48 CrossRef CAS
.
- M. Arnold, P. Kangas, A. Mäkinen, E. Lakay, N. Isomäki, G. Laven, M. Gericke, P. Pajuniemi, T. Kaartinen and L. Wendling, Mine Water Environ., 2019, 38, 431–446 CrossRef CAS
.
- J. Li, X. Li, T. Hayat, A. Alsaedi and C. Chen, ACS Sustainable Chem. Eng., 2017, 5, 11496–11503 CrossRef CAS
.
- B. K. Biswas, J. Inoue, H. Kawakita, K. Ohto and K. Inoue, J. Hazard. Mater., 2009, 172, 721–728 CrossRef CAS PubMed
.
- J. Holopainen and M. Ritala, J. Eur. Ceram. Soc., 2016, 36, 3219–3224 CrossRef CAS
.
- W. A. Caliebe, V. Murzin, A. Kalinko and M. Görlitz, AIP Conf. Proc., 2019, 2054, 060031 CrossRef
.
- S. Ardizzone and C. Bianchi, J. Electroanal. Chem., 1999, 465, 136–141 CrossRef CAS
.
- V. G. Deshmane and Y. G. Adewuyi, Microporous Mesoporous Mater., 2012, 148, 88–100 CrossRef CAS
.
- Y. Du, P. Yang, Z. Mou, N. Hua and L. Jiang, J. Appl. Polym. Sci., 2006, 99, 23–26 CrossRef CAS
.
- R. Ruiz-Rosas, J. Bedia, J. M. Rosas, M. Lallave, I. G. Loscertales, J. Rodriguez-Mirasol and T. Cordero, Catal. Today, 2012, 187, 77–87 CrossRef CAS
.
- Z. Yu, C. Xu, K. Yuan, X. Gan, C. Feng, X. Wang, L. Zhu, G. Zhang and D. Xu, J. Hazard. Mater., 2018, 346, 82–92 CrossRef CAS
.
- M. Alvarez and M. Torralvo, Colloids Surf., A, 1994, 83, 175–182 CrossRef CAS
.
- V. Reddy, D. Hwang and J. Lee, Korean J. Chem. Eng., 2003, 20, 1026–1029 CrossRef CAS
.
- W. Xia, F. Wang, X. Mu and K. Chen, Fuel Process. Technol., 2017, 166, 140–145 CrossRef CAS
.
- J. Y. Koo, S. Hwang, M. Ahn, M. Choi, D. Byun and W. Lee, J. Am. Ceram. Soc., 2016, 99, 3146–3150 CrossRef CAS
.
- Y. Sun, J. Qu, Q. Guo, J. Song, G. Wei, X. Xi, G. Hou and T. Qi, Ceram. Int., 2017, 43, 12551–12556 CrossRef CAS
.
- H. Wang, Y. Duan and W. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 26414–26420 CrossRef CAS
.
- J. Blackwell and P. Carr, J. Liq. Chromatogr., 1991, 14, 2875–2889 CrossRef CAS
.
- S. Muhammad, S. T. Hussain, M. Waseem, A. Naeem, J. Hussain and M. T. Jan, Iran. J. Sci. Technol., Trans. A: Sci., 2012, 36, 481–486 CAS
.
- D. Konrad, Ion exchangers, De Gruyter, Berlin, 1991 Search PubMed
.
- S. Goldberg and C. Johnston, J. Colloid Interface Sci., 2001, 234, 204–216 CrossRef CAS
.
- R. Liu, W. Xu, Z. He, H. Lan, H. Liu, J. Qu and T. Prasai, Chemosphere, 2015, 138, 616–624 CrossRef CAS
.
- M. E. Essington and M. A. Stewart, Soil Sci. Soc. Am. J., 2016, 80, 1197–1207 CrossRef CAS
.
- C. Dai, Z. Zhou, X. Zhou and Y. Zhang, Water, Air, Soil Pollut., 2014, 225, 1799 CrossRef
.
- P. Dorjee, D. Arnarasiriwardena and B. Xing, Microchem. J., 2014, 116, 15–23 CrossRef CAS
.
- J. Simonin, Chem. Eng. J., 2016, 300, 254–263 CrossRef CAS
.
- Y. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS
.
- D. Robati, J. Nanostruct. Chem., 2013, 3, 55 CrossRef
.
- Y. Ho and G. McKay, Process Saf. Environ. Prot., 1998, 76, 332–340 CrossRef CAS
.
- J. Febrianto, A. N. Kosasih, J. Sunarso, Y. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 2009, 162, 616–645 CrossRef CAS
.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra04211c |
| This journal is © The Royal Society of Chemistry 2019 |