Open Access Article
Open Access ArticleTiO2@Sn3O4 nanorods vertically aligned on carbon fiber papers for enhanced photoelectrochemical performance
Weiwei Xia†
a,
Haoyu Qian†a,
Xianghua Zeng *ab,
Jiawei Suna,
Pengdi Wanga,
Min Luoa and
Jing Donga
*ab,
Jiawei Suna,
Pengdi Wanga,
Min Luoa and
Jing Donga
aCollege of Physics Science and Technology, Institute of Optoelectronic Technology, Yangzhou University, Yangzhou 225002, P. R. China. E-mail: xhzeng@yzu.edu.cn; wwxia@yzu.edu.cn
bCollege of Electrical, Energy and Power Engineering, Yangzhou 225127, P. R. China
First published on 29th July 2019
Abstract
Semiconductor heterostructures are regarded as an efficient way to improve the photocurrent in photoelectrochemical cell-type (PEC) photodetectors. To better utilize solar energy, TiO2@Sn3O4 arrays vertically aligned on carbon fiber papers were synthesized via a hydrothermal route with a two-step method and used as photoanodes in a self-powered photoelectrochemical cell-type (PEC) photodetector under visible light. TiO2@Sn3O4 heterostructures exhibit a stable photocurrent of 180 μA, which is a 4-fold increase with respect to that of the Sn3O4 nanoflakes on carbon paper, and a two-order increase with respect to that of the TiO2 NRs arrays. The evolution of hydrogen according to the photo-catalytic water-splitting process showed that Sn3O4/TiO2 heterostructures have a good photocatalytic hydrogen evolution activity with the rate of 5.23 μmol h−1, which is significantly larger than that of Sn3O4 nanoflakes (0.40 μmol h−1) and TiO2 nanorods (1.13 μmol h−1). Furthermore, the mechanism behind this was discussed. The detector has reproducible and flexible properties, as well as an enhanced photosensitive performance.
Introduction
TiO2, as one of the widest band gap semiconductors (∼3.0 eV), is widely used in photocatalysts and photoelectrodes due to its strong optical absorption, favorable band edge positions, and abundant availability.1 For example, oriented, single-crystalline rutile TiO2 nanorod films on transparent conductive fluorine-doped tin oxide (FTO) substrates or carbon fiber were used as dye-sensitized solar cells (DSSCs) and exhibited an improved cell performance due to a direct connection of the point of photogeneration with the collection electrode.2,3 However, the energy unitization efficiency of TiO2 is limited because of its wide band gap and the rapid recombination of photogenerated electron–hole pairs. Metal/nonmetal element doping or decoration was developed to increase its photoactivity, for example, TiO2 modification with carbon nanolayers used as a photocatalyst led to an enhanced efficiency due to the increased substrate adsorption in the vicinity of the photocatalytic sites and high migration efficiency of photoinduced electrons at the carbon/TiO2 interface.4,5 And metal modification of TiO2 to increase photocatalytic properties of nanoparticles were reported also.6,7Among their methods, heterogeneous photocatalysts were regarded as an effective way to improve photocatalytic activity, since heterojunctions can promote charge migration across both the semiconductor/electrolyte and the semiconductor/photoelectrode interface effectively separating photogenerated electrons and holes.8–10 P-SnO/n-TiO2 composite electrodes revealed a better PEC performance as reported by Naeem et al.11 And tree-like TiO2 architectures assembled with CdS and reduced graphene oxide can improve photoelectrochemical performance as reported by Pathak et al.12
On the other hand, a mixed-valence tin oxide (Sn2+ and Sn4+) has a strong resistance against acidic/alkaline solutions and exhibits a good photocatalytic activity without generating secondary pollutants under irradiation with visible light. It is a desirable material for PEC-type photodetectors and an efficient visible-light-driven photocatalyst.13–16 To improve the photoelectric properties, Sn3O4 nanomaterials combined with others have been used as electrodes and photocatalysts. For example, integrated Sn3O4 nanosheets and rGO nanosheets as hybrid nanostructures were used as high-performance photocatalysts.17 With the combination of visible light active Sn3O4 and ultraviolet (UV) light active TiO2, this composite can be a broad spectrum photocatalytic material from the UV to visible region as well as having enhanced separation of photogenerated electrons and holes,18 therefore people paid more attention to TiO2@Sn3O4 nanostructures. For example, 3D lupinus-like TiO2@Sn3O4 nanostructures on a transparent F-doped SnO2 (FTO) glass substrate were prepared and used as photoanode for PEC water splitting as reported by Zhu et al.19 TiO2@Sn3O4 hybrid nanobelts exhibited markedly enhanced photo-electrochemical (PEC) response, which caused higher photocatalytic hydrogen evolution even without the assistance of Pt as a co-catalyst, and enhanced the degradation ability of organic pollutants under both UV and visible light irradiation.20
Recently, self-powered photodetectors (SPPD) are becoming a promising candidate for application in high-sensitivity and high-speed SPPDs because they do not require batteries as an external power source.21,22 For example, TiO2 based self-powered photochemical UV detectors have been reported, but they are restricted to UV light.23–25 To better use solar light for energy conversion, it is very important to extend the spectrum to the visible region. Although there are some discussions on the TiO2@Sn3O4 electrodes, flexible SPPDs based on TiO2@Sn3O4 haven't been reported yet.
Herein, TiO2 nanorod arrays (NRs) vertically aligned on the carbon fiber papers were synthesized via a hydrothermal route, where TiO2 nanorods have a diameter of about 500–1000 nm; then Sn3O4 nanoflakes (NFs) with a thickness of less than 100 nm and width of 100–300 nm were grown on the surface of TiO2 nanorod. Furthermore, hybrid PEC-type photodetectors were designed using three prepared 3-D (three dimensional) hierarchical arrays, TiO2, Sn3O4 and TiO2@Sn3O4 as photoanodes, respectively. As a visible light SPPD device, TiO2@Sn3O4 heterostructures exhibit a stable photocurrent of 180 μA, which is a 4-fold increase with respect to that of Sn3O4 NFs on carbon paper, and a two-order increase with respect to that of TiO2 NRs on carbon paper. The detector exhibits reproducible and flexible properties, as well as an enhanced photosensitive performance. Finally, the photocatalytic properties of the pure Sn3O4, TiO2 NRs and their combinations have been studied, where the evolution of hydrogen was measured according to the photo-catalytic water-splitting process. The results show that Sn3O4/TiO2 heterostructures have hydrogen evolution activity with the rate of 5.23 μmol h−1, significantly larger than that of others. The mechanism behind has been discussed.
Experimental section
Materials
All chemicals were analytical-grade and used without further purification. Titanium butoxide (Ti(OC4H9)4), tin dichloride dehydrate (SnCl2·2H2O), sodium citrate dihydrate (Na3C6H5O7·2H2O, 99%), ethanol, and hydrochloric acid (HCl, 36.5–38% by weight) were purchased from Sinopharm Chemical Reagent Co., Ltd. Commercial carbon fiber papers were provided by FuelCellStore.Synthesis of TiO2 NRs
TiO2 nanorod arrays (NRs) were prepared on the carbon fiber papers by the one-step hydrothermal method. Firstly, the commercial carbon fiber papers were cut into the desired sizes and ultrasonically washed using deionized water and ethanol. Then, 12 mL of deionized water was mixed with 12 mL of hydrogen chloride (36.5–38% by weight). The mixture was stirred for 5 min before the addition of 400 μL of titanium butoxide (97% Aldrich). After stirring for another 5 min, the mixture was transferred into a 50 mL Teflon-lined stainless steel autoclave with the treated carbon fiber paper vertically immersed in the reaction solution. The hydrothermal reaction was carried out at 180 °C for 18 h and cooled naturally to room temperature. Finally, the carbon fiber paper substrates covered by TiO2 NRs were washed with deionized water.Synthesis of Sn3O4 NFs
Sn3O4 nanoflakes (NFs) were prepared on the carbon fiber papers by a modified hydrothermal approach, which is similar to the reported in our previous study.26 In detail, 1.073 g of SnCl2·2H2O and 2.940 g Na3C6H5O7·2H2O were dissolved in the mixture of 20 mL de-ionized water and 20 mL alcohol under constant magnetic stirring for approximately 60 min. The obtained homogeneous solution was transferred into a Teflon liner of 50 mL capacity with the carbon fiber paper vertical immersed into the reaction solution and keeping sealed under 180 °C for 18 h. After the reaction, the carbon fiber paper coated with a brown product was further washed with deionized water and absolute ethanol for several times. The final sample dried in an oven before characterization.Synthesis of TiO2@Sn3O4 hierarchical heterostructured arrays
Details of growth process of the TiO2@Sn3O4 hierarchical heterostructured arrays were described previously. 0.35 g of SnCl2·2H2O and 1.14 g of Na3C6H5O7·2H2O were dissolved in a mixture of 25 mL of deionized water and 25 mL of alcohol under constant magnetic stirring for approximately 60 min. Then, the mixture was transferred to a Teflon-lined autoclave (50 mL). Subsequently, the carbon fiber paper covered by TiO2 NRs was vertically immersed into the aforementioned mixture. The hydrothermal reaction was carried out at 180 °C for 18 h and cooled naturally to room temperature. Finally, the resulting carbon fiber papers with TiO2@Sn3O4 hierarchical heterostructured arrays were rinsed with deionized water and dried at 60 °C in air.Characterization
The morphology and microstructure of the TiO2@Sn3O4 heterostructured nanowire arrays on carbon fiber papers were examined by a Hitachi S-4800 field emission scanning electron microscope (FESEM) and a Tecanai G2 F30 field emission transmission electron microscope (TEM) operated at an accelerating voltage of 300 kV. The phase and composition of samples were analyzed by powder X-ray diffraction (XRD, Bruker D8 advance) with Cu Kα (λ = 1.5406 Å). UV-visible absorption spectra (200–1200 nm) were measured using a Varian Cary 50 UV-visible spectrophotometer. X-ray photoelectron spectroscopy (XPS) was conducted on an ESCALAB-250Xi photoelectron spectroscope to obtain information on the valence state of the Ti and O ions.PEC measurements
To perform the PEC tests, the Zanner CIMPS electrochemical workstation (Germany) was used to examine the photocurrent densities' curves and electrochemical impedance spectroscopy (EIS) plots in a three-electrode mode. PEC measurements were performed in 0.1 M Na2SO4 using Pt wire as the counter electrode and Ag/AgCl in saturated KCl as a reference electrode. The carbon fiber papers with TiO2@Sn3O4 hierarchical heterostructured arrays were acted as the working electrode and placed in the cell with an area of 1 × 1 cm2 exposed to the electrolyte. A 500 W Xe lamp (CEL-HXF 300, Beijing Au-light, China) was employed as incident light source to study the PEC response of the samples. The H2 evolution experiments were carried out in a gas-closed circulation system under a 300 W Xe lamp.Results and discussion
Morphology and structure
The schematic diagram of the formation process of the TiO2@Sn3O4 hierarchical heterostructured nanowire arrays on carbon fiber papers is shown in Fig. 1a, which involves two major steps. In the first step, large-scale well-aligned TiO2 nanowires are grown on the carbon fiber papers by the one-step hydrothermal method. High-density TiO2 NRs are grown uniformly on the carbon fiber papers, which also serve as stems to provide a platform for later Sn3O4 NFs growth. In the second step, the Sn3O4 NFs were conformably coated onto TiO2 nanowire stems by a facile and effective hydrothermal method. Fig. 1b reveals the crystal structure of the as-synthesized TiO2, Sn3O4 and TiO2@Sn3O4 nanowires heterostructure arrays on carbon fiber papers. The diffraction peaks in each pattern at 26.22° and 54.72° are indexed to the carbon fiber paper substrate. For TiO2 NRs on the carbon fiber paper substrate, the diffraction peaks at 2θ values of 27.45°, 36.08°, 41.22°, 44.05°, 56.64°, 62.74°, 64.04°, 69.01° and 69.79° can be well identified as (110), (101), (111), (210), (220), (002), (310), (301) and (112) planes of the tetragonal rutile TiO2 phase (JCPDS 21-1276). Diffraction peaks at about 33.01°, 37.07° and 51.69° can be indexed to (210), (130) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 2) phases of Sn3O4 (JCPDS 16-0737). Besides, the XRD pattern of as-synthesized TiO2@Sn3O4 hierarchical nanowire arrays on carbon fiber papers is also shown in Fig. 1c. All the major diffraction peaks of TiO2 and Sn3O4 can also be identified in the pattern of TiO2@Sn3O4 hierarchical structure and no extra peak was detected.
2) phases of Sn3O4 (JCPDS 16-0737). Besides, the XRD pattern of as-synthesized TiO2@Sn3O4 hierarchical nanowire arrays on carbon fiber papers is also shown in Fig. 1c. All the major diffraction peaks of TiO2 and Sn3O4 can also be identified in the pattern of TiO2@Sn3O4 hierarchical structure and no extra peak was detected.
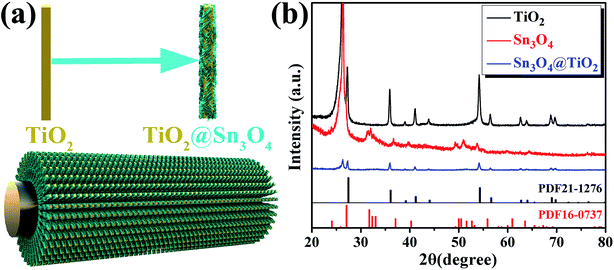 | ||
| Fig. 1 (a) Schematic of fabrication process and (b) XRD patterns of TiO2 NRs, Sn3O4 NFs and TiO2@Sn3O4 heterostructure arrays grown on carbon fiber papers. | ||
To observe the morphology and microstructure of the samples, the FESEM images of TiO2 NRs and TiO2@Sn3O4 hierarchical structure are shown in Fig. 2. Fig. 2a–c show FESEM images of TiO2 NRs on carbon fiber paper substrate with different magnifications. With the hydrothermal synthesis, TiO2 NRs with a size of 500–1000 nm grow vertically along the surface of the carbon fiber paper substrate forming nanowire arrays with good uniformity, each TiO2 NR has a square shape. After the growth of Sn3O4 nanoflake branches by the followed hydrothermal method, it can be obviously noted that high-density secondary Sn3O4 nanoflake branches are successfully grown on the surface of the TiO2 nanowires with good uniformity, which lead to a thicker and rougher surface of TiO2 nanowires. The magnified image (Fig. 2f) reveals that Sn3O4 NFs have a thickness of less than 100 nm and width of 100–300 nm. Because of the existence of convenient diffusion pathways, these TiO2@Sn3O4 nanowire array hierarchical structures are extraordinarily accessible to electrolytes during the electrochemical measurement process. In addition, the compact nanoflakes interlaced with each other can also greatly increase the capture of light. Further morphological and structural characterizations of the TiO2@Sn3O4 hierarchical structure are performed using HRTEM. The low-magnification TEM image (Fig. 3a) reveals the typical heterostructured nanowire taken from hierarchical TiO2@Sn3O4 nanowire arrays, which can be evidently observed that the individual TiO2 nanowire with a length of several micrometers is covered by compact Sn3O4 nanoflake branches. A close examination of the exposed profile (inset of Fig. 3a) reveals that thickness of the outer Sn3O4 NFs is around several tens nm. The high resolution TEM examination shown in Fig. 3b reveals a distinct set of visible lattice fringes of 0.33 nm, corresponding to the (111) plane of triclinic Sn3O4 (JCPDS 16-0737). To determine the atomic distribution in TiO2@Sn3O4 hierarchical structure, EDS element mapping analysis of the individual hybrid nanowire was performed (Fig. 3c–f), Ti, Sn and O elements are found clearly in the hierarchical heterostructure of TiO2@Sn3O4 nanowire.
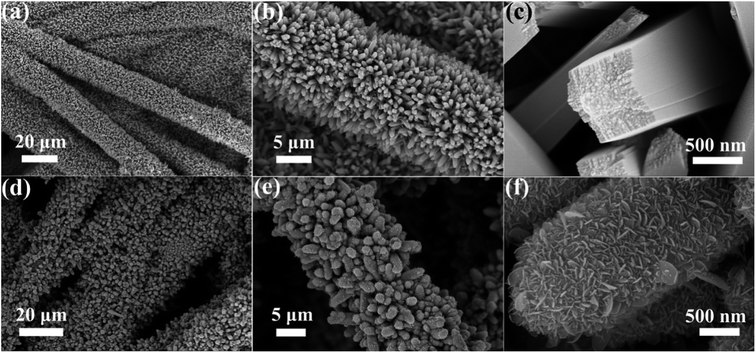 | ||
| Fig. 2 FESEM images of (a–c) TiO2 NRs and (d–f) TiO2@Sn3O4 hierarchical structure grown on carbon fiber paper. | ||
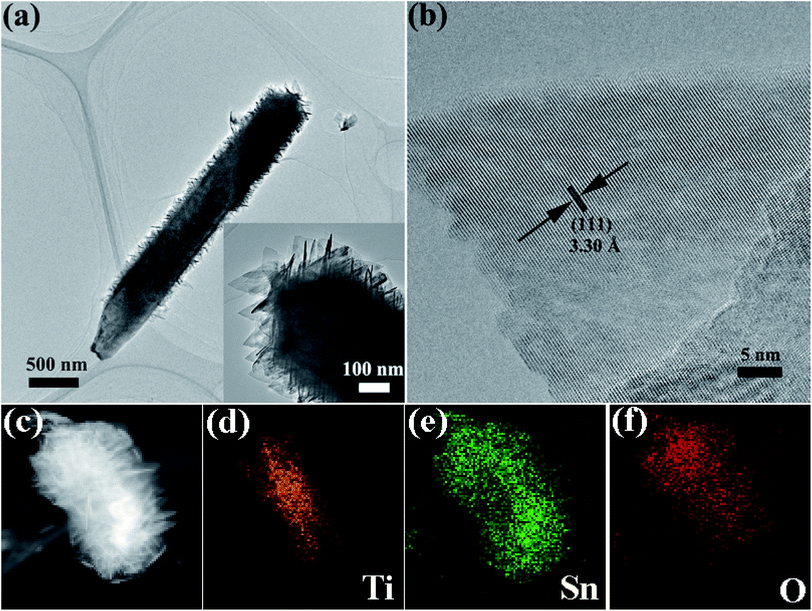 | ||
| Fig. 3 TEM image (a) and HRTEM image (b) of a representative TiO2@Sn3O4 heterostructured nanowire. (c–f) Elemental mapping images of an individual hybrid nanowire. | ||
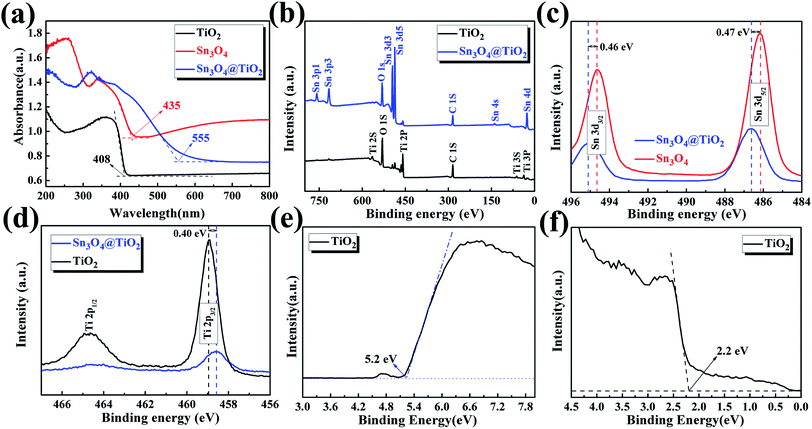
Optical properties of the as-prepared TiO2 NRs, Sn3O4 NFs and TiO2@Sn3O4 hierarchical structure grown on carbon fiber paper were investigated by diffuse reflectance UV-vis spectra (ultraviolet-visible spectroscopy) (Fig. 4a). Compared with bare TiO2 NRs, TiO2@Sn3O4 hierarchical structure shows significantly increased light absorption in the visible-light region. The absorption edge of the TiO2 NRs (black line) is located at 408 nm, which indicates the energy gap is 3.04 eV. The absorption band edge of the Sn3O4 NFs (red) is closed to 435 nm (with the energy gap of 2.85 eV), which can absorb the visible light. For the 3D TiO2@Sn3O4 hierarchical structure (blue line), its absorption band edge is at ∼558 nm, showing a significant redshift compared with the bare TiO2 NRs. Therefore, coupling TiO2 NRs with Sn3O4NFs can significantly broaden the range of photo-response, which can result in an enhancement of utilization of solar light and improved PEC performance. XPS measurements were used to investigate the surface composition of the as-synthesized products. Fig. 4b shows the XPS survey spectra of Sn3O4 NFs and TiO2@Sn3O4 hierarchical structure. Chemical state binding energy of Sn 3d in the Sn3O4 NFs and TiO2@Sn3O4 hierarchical structure was carried by XPS measurements as shown in Fig. 4c. Consistent with our previous study, the binding energies of Sn 3d3/2 and Sn 3d5/2 peaks in the pure Sn3O4 NFs locate at 494.64 eV and 486.15 eV, respectively. After growing onto the TiO2 NRs, however, the Sn 3d3/2 and Sn 3d5/2 peaks of TiO2@Sn3O4 hierarchical structure obviously shifted to higher binding energies by 0.46 and 0.47 eV, respectively. Therefore, the observed peak shift indicates that the electrons are injected from Sn3O4 NFs to the TiO2 NRs in TiO2@Sn3O4 hierarchical structure. This result can be further supported by the shift by 0.4 eV of the Ti 2p3/2 peak in Fig. 4d. The TiO2@Sn3O4 hierarchical structure is possibly advantageous because of their electronic band structure.
UPS measurements were carried out to study the surface electron behavior of TiO2 NRs, as shown in Fig. 4e and f. Fig. 4e is a view of the secondary electron edge (SEE) energy corresponding to the left spectra in UPS data. The work function (ϕ) can be by observing the low energy secondary electron cutoff, which is ∼5.20 eV for TiO2 NRs. Fig. 4f is a view of the valence band maximum (VBM) region corresponding to the right spectra in UPS data and the VBM can be extracted from the Fig. 4f, which is about 2.2 eV for TiO2 NRs. The energy band of Sn3O4 NFs has been discussed previously.22 Then, the conduction band and valence band (vs. vacuum) of TiO2 NRs and Sn3O4 NF scan be obtained, as shown in Table 1.
| Samples | Eg (eV) | EC (eV vs. vacuum) | EF (eV vs. vacuum) | Ev (eV vs. vacuum) |
|---|---|---|---|---|
| TiO2 | 3.04 | 4.43 | 5.20 | 7.47 |
| Sn3O4 | 2.85 | 3.7 | 3.9 | 6.55 |
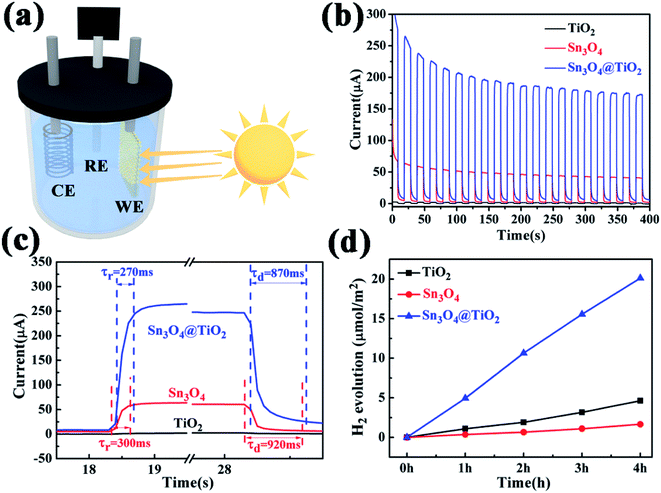
The PEC measurements were carried out in a three-electrode configuration in 0.1 M Na2SO4 using Pt wire as a counter electrode, Ag/AgCl in saturated KCl as a reference electrode, and carbon-paper-supported Sn3O4 NFs, TiO2 NRs and TiO2@Sn3O4 hierarchical structures as active photoanodes, respectively, as shown in the Fig. 5a. The incident radiation is switched with an on/off interval of 20 s. Twenty repeated cycles are displayed in Fig. 5b, in which TiO2 NRs display a quite weak photocurrent under visible-light irradiation. The as-obtained Sn3O4 NFs exhibit a much enhanced photocurrent density of ∼40 μA cm−2 in the visible-light spectral region. However, the photocurrent exhibited nearly 40% decrease after 20 cycles. The TiO2@Sn3O4 hierarchical electrode shows the highest photocurrent and a decrease of 30% compared with the initial photocurrent. From the magnified rising and decaying edges of photocurrent shown in Fig. 5c, a fast photo-response can be seen clearly. The rising time (τr, defined as the time to increase from 10% to 90% of the maximum photocurrent) and the decaying time (τd, defined as the time to recover from 90% to 10% of the maximum photocurrent) of TiO2@Sn3O4 hierarchical electrodes are about 0.27 s and 0.87 s, respectively, faster than 0.30 s and 0.92 s for Sn3O4 NFs. The mechanism of reason for enhanced PEC performance will be explained in detail later. PEC measurements that the 3D TiO2@Sn3O4 photoelectrode exhibited a stable photocurrent of 180 μA, which is 4-fold increase with respect to that of Sn3O4 NFs, and two-order increase respect to that of TiO2 NRs. In comparison to the report of ref. 19, the formation of the heterostructure on the photocurrent is much larger without external power source.
Furthermore, the photocatalytic property of the pure Sn3O4, TiO2 NRs and their combinations have been studied under visible light, where the evolution of hydrogen was measured according to the photo-catalytic water-splitting process, as shown in Fig. 5d. The results show that Sn3O4 NFs show negligible photocatalytic hydrogen evolution activity with the rate of 0.40 μmol h−1 cm−2 and the rate of the TiO2 NRs is 1.13 μmol h−1 TiO2 NRs, which is much lower than that of Sn3O4/TiO2 heterostructures of 5.23 μmol h−1, this value is comparable with the results reported by Kodiyath et al.27 Contrary to the photocurrents, the H2 generation of Sn3O4 is less than that of TiO2. Several aspects, such as the density of the photoanode catalysts, electron transportation and carrier's concentration etc., will influence the H2 generation. In the further work we will study them.
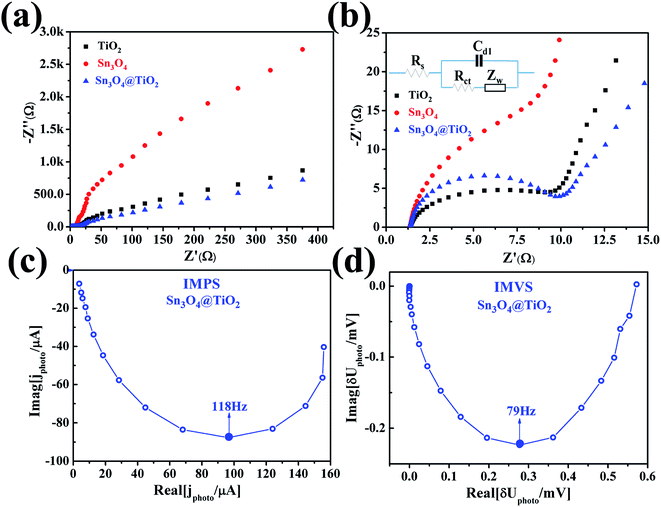
To further understand the reasons of the charge transfer process occurring at the interface of photoelectrode/electrolyte, electrochemical impedance spectroscopy (EIS)28,29 was carried out and presented in Fig. 6a and b. The equivalent RC circuit was used to interpret the EIS results (see inset of Fig. 6b). As shown in Fig. 6a and b, each Nyquist plots are composed of one semicircle and a slope line. For deeper analysis, all Nyquist plots display a semicircle at high frequencies whose diameter represents the charge-transfer resistance (Rct), which reflects the electron transfer kinetics of the redox probe at the interface. The slope line at low frequency is related to the diffusion process. The corresponding equivalent circuit is depicted in the inset of Fig. 6b, where Rs denotes the series resistance at the interface of the photoelectrode material and the carbon fiber paper substrate, Cd1 reflects the constant phase element that models capacitance of the double layer, and Zw stands for the Warburg impedance originated from the diffusion process at the electrode surface. After being decorated with Sn3O4 NFs, the value Rct of TiO2@Sn3O4 hierarchical photoelectrode is almost the same, which is smaller than the corresponding value of Sn3O4 NFs. The above result demonstrates that TiO2 NRs provide a conduction path and rapidly transfer the photoelectrons coming from Sn3O4 NFs to the carbon fiber paper substrate along the vertically oriented TiO2 NRs, which is also correlated to the enhanced PEC performance. To obtain deep insights into the enhanced PEC properties of the TiO2@Sn3O4 hierarchical electrodes, intensity modulated photocurrent spectroscopy (IMPS) and intensity modulated photovoltage spectroscopy (IMVS) have been employed to investigate further the electron transport and recombination process, as shown in Fig. 6c and d. The IMPS and IMVS plots display a semicircle in the complex plane under the incident light source was an LED with a wavelength of 564 ± 60 nm. From the IMPS measurement (Fig. 6c), the transport time (τd) of injected electrons from Sn3O4 NFs to TiO2 NRs can be calculated from the following equation:
| τd = 1/(2πfd,min), |
| τn = 1/(2πfn,min), |
Based on the above measurements and analyses, by combining both the band gap estimated from optical absorption and the conduction band and valence band (vs. vacuum) obtained by UPS, the energy band alignment of TiO2 NRs and Sn3O4 NFs is displayed in Fig. 7a. When Sn3O4 NFs contact with TiO2 NRs to form a heterojunction, the band structure of TiO2@Sn3O4 is reconfigured. The bandgap energy of Sn3O4 (2.85 eV) is smaller than TiO2 (3.04 eV) and both the potentials of VB and CB of Sn3O4 are higher than those of TiO2, so TiO2@Sn3O4 heterostructure belongs to a typical type-II heterojunction. Under visible-light irradiation, photogenerated holes and electrons appear in the VB and CB of Sn3O4 NFs. Due to type-II heterostructure, the photogenerated electrons in Sn3O4 CB were easily injected into the TiO2 CB. Effective separation of photoexcited electron–hole pairs could be accomplished by a longer electron lifetime. To explain the enhanced PEC performance of TiO2@Sn3O4 photoelectrode in the visible-light spectral region, we present the scheme of the electron–hole pair separation and the transfer process in TiO2@Sn3O4 hierarchical photoelectrode (Fig. 7b). Generally, enhanced PEC performance of TiO2@Sn3O4 photoelectrode may be attributed to the reasonable design of TiO2NRs @Sn3O4NFs heterostructure. On one hand, the aligned TiO2 NRs can provide a conduction path and rapidly transfer electrons to carbon fiber paper substrate along the vertically oriented TiO2 NRs. On the other hand, the type-II heterojunction of TiO2@Sn3O4 heterostructure can drive photoexcited electron transfer from Sn3O4 NFs to TiO2 NRs. In one word, for our TiO2@Sn3O4 photoelectrode under the synergistic effects between unique band structures and morphology, which is crucial for the enhancement of PEC performance.
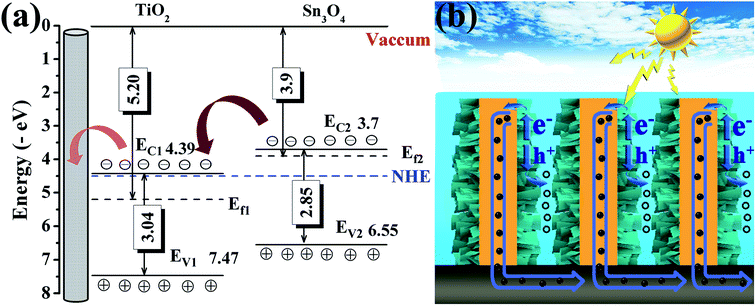 | ||
| Fig. 7 (a) Proposed energy band alignment and (b) scheme of the electron–hole pair separation and the transfer process in TiO2@Sn3O4 photoelectrode. | ||
Conclusion
In this paper, TiO2@Sn3O4 arrays vertically aligned on the carbon fiber papers were synthesized and used as photoanodes to improve the photocurrent performance under visible light. Because of the 3-D structure, the TiO2@Sn3O4 arrays can absorb more incident lights which have been reflected among Sn3O4 NFs, at same time there are more interfacial states to adsorb electrolyte molecules. The TiO2@Sn3O4 arrays not only extend the spectra from UV to visible region with respect to TiO2, but also increase the separation of photogenerated electrons and hole due to the good band alignment of type-II heterostructure for TiO2@Sn3O4. Therefore, as photoanodes in self-powered photoelectrochemical cell-type (PEC) photodetector under visible light, TiO2@Sn3O4 heterostructures exhibit a stable photocurrent of 180 μA, which is 4-fold increase with respect to that of Sn3O4 NFs, and two-order increase respect to that of TiO2 NRs arrays. Sn3O4/TiO2 heterostructures also have a good photocatalytic hydrogen evolution activity with the rate of 5.23 μmol h−1, which is quite larger than that of Sn3O4 nanoflakes (0.40 μmol h−1) and TiO2 NRs (1.13 μmol h−1). By the way, the detector exhibits reproducible and flexible properties, as well as an enhanced photosensitive performance. The results will be helpful for the construction of hierarchical structures used in the PEC detectors.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2017YFB0403101), the National Natural Science Foundation of China (Grant 61604127, 61474096).References
- W. A. Gil, A. M. Mahadeo, S. C. Weon, G. K. Hyun, C. Min and S. J. Jum, Enhanced solar photoelectrochemical conversion efficiency of the hydrothermally-deposited TiO2 nanorod arrays: Effects of the light trapping and optimum charge transfer, Appl. Surf. Sci., 2018, 440, 688–699 CrossRef.
- L. Bin and S. A. Eray, Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells, J. Am. Chem. Soc., 2009, 131, 3985–3990 CrossRef PubMed.
- W. X. Guo, C. Xu, X. Wang, S. H. Wang, C. F. Pan, C. J. Lin and Z. L. Wang, Rectangular bunched rutile TiO2 nanorod arrays grown on carbon fiber for dye-sensitized solar cells, J. Am. Chem. Soc., 2012, 134, 4437–4441 CrossRef CAS PubMed.
- G. Q. Chang, Z. Cheng, R. Warren, G. X. Song, J. Y. Shen and L. W. Lin, Highly efficient photocatalysts for surface hybridization of TiO2 nanofibers with carbon films, ChemPlusChem, 2015, 80, 827–831 CrossRef CAS.
- X. Cai, H. W. Wu, H. C. Hou, M. Peng, X. Yu and D. C. Zou, Dye-sensitized solar cells with vertically aligned TiO2 nanowire arrays grown on carbon fibers, ChemSusChem, 2014, 7, 474–482 CrossRef CAS PubMed.
- P. K. Sharma, M. A. L. R. M. Cortes, J. W. J. Hamiltona, Y. S. Han, J. A. Byrne and M. Nolan, Surface modification of TiO2 with copper clusters for band gap narrowing, Catal. Today, 2019, 321–322, 9–17 CrossRef CAS.
- Z. M. Zhao, J. Sun, G. J. Zhang and L. J. Bai, The study of microstructure, optical and photocatalytic properties of nanoparticles (NPs)-Cu/TiO2 films deposited by magnetron sputtering, J. Alloys Compd., 2015, 652, 307–312 CrossRef CAS.
- H. Masai, T. Miyazaki, K. Mibu, Y. Takahashi and T. Fujiwara, Phase separation and the effect of SnO addition in TiO2-precipitated glass-ceramics, J. Eur. Ceram. Soc, 2015, 35, 2139–2144 CrossRef CAS.
- L. X. Liu, G. C. Fan, J. R. Zhang and J. J. Zhu, Ultrasensitive cathode photoelectrochemical immunoassay based on TiO2 photoanode-enhanced 3D Cu2O nanowire array photocathode and signal amplification by biocatalytic precipitation, Anal. Chim. Acta, 2018, 1027, 33–40 CrossRef CAS PubMed.
- G. D. Moon, J. B. Joo, I. Lee and Y. Yin, Decoration of size-tunable CuO nanodots on TiO2 nanocrystals for noble metal-free photocatalytic H2 production, Nanoscale, 2014, 6, 12002–12008 RSC.
- R. Naeem, M. A. Ehsan, A. Rehman, Z. H. Yamani, A. S. Hakeem and M. Mazhar, Single step aerosol assisted chemical vapor deposition of p–n Sn(II) oxide–Ti(IV) oxide nanocomposite thin film electrodes for investigation of photoelectrochemical properties, New J. Chem., 2018, 42, 5256–5266 RSC.
- P. Pathak, S. Gupta, K. Grosulak, H. Imahori and V. Subramanian, Nature-inspired tree-like TiO2 architecture: a 3D platform for the assembly of CdS and reduced graphene oxide for photoelectrochemical processes, J. Phys. Chem. C, 2015, 119, 7543–7553 CrossRef CAS.
- O. M. Berengue, R. A. Simon, A. J. Chiquito, C. J. Dalmaschio, E. R. Leite, H. A. Guerreiro and F. E. G. Guimaraes, Semiconducting Sn3O4 nanobelts: growth and electronic structure, J. Appl. Phys., 2010, 107, 033717 CrossRef.
- W. W. Xia, H. B. Wang, X. H. Zeng, J. Han, J. Zhu, M. Zhou and S. D. Wu, High-efficiency photocatalytic activity of type II SnO/Sn3O4 heterostructures via interfacial charge transfer, CrystEngComm, 2014, 16, 6841–6847 RSC.
- Y. H. He, D. Z. Li, J. Chen, Y. Shao, J. J. Xian, X. Z. Zheng and P. Wang, Sn3O4: a novel heterovalent-tin photocatalyst with hierarchical 3D nanostructures under visible light, RSC Adv., 2014, 4, 1266–1269 RSC.
- H. Song, S. Y. Son, S. K. Kim and G. Y. Jung, A facile synthesis of hierarchical Sn3O4 nanostructures in an acidic aqueous solution and their strong visible light-driven photocatalytic activity, Nano Res., 2015, 8, 3553–3561 CrossRef CAS.
- X. Yu, Z. H. Zhao, N. Ren, J. Liu, D. H. Sun, L. H. Ding and H. Liu, Top or Bottom, Assembling modules determine the photocatalytic property of the sheet like nanostructured hybrid photocatalyst composed with Sn3O4 and rGO (GQD), ACS Sustainable Chem. Eng., 2018, 6, 11775–11782 CrossRef CAS.
- X. Yu, L. F. Wang, J. Zhang, W. B. Guo, Z. H. Zhao, Y. Qin, X. N. Mou, A. X. Li and H. Liu, Hierarchical hybrid nanostructures of Sn3O4 on N doped TiO2 nanotubes with enhanced photocatalytic performance, J. Mater. Chem. A, 2015, 3, 19129–19136 RSC.
- L. P. Zhu, H. Lu, D. Hao, L. L. Wang, Z. H. Wu, L. J. Wang, P. Li and J. H. Ye, Three-dimensional lupinus-like TiO2 nanorod@Sn3O4 nanosheet hierarchical heterostructured arrays as photoanode for enhanced photoelectrochemical performance, ACS Appl. Mater. Interfaces, 2017, 9, 38537–38544 CrossRef CAS PubMed.
- G. H. Chen, S. Z. Ji, Y. H. Sang, S. J. Chang, Y. N. Wang, P. Hao, J. Claverie, H. Liu and G. W. Yu, Synthesis of scaly Sn3O4/TiO2 nanobelt heterostructures for enhanced UV-visible light photocatalytic activity, Nanoscale, 2015, 7, 3117 RSC.
- W. J. Lee and M. H. Hon, An ultraviolet photo-detector based on TiO2/water solid–liquid heterojunction, Appl. Phys. Lett., 2011, 99, 251102 CrossRef.
- W. W. Xia, H. Y. Qian, X. H. Zeng, J. Dong, J. Wang and Q. Xu, Visible-light self-powered photodetector and recoverable photocatalyst fabricated from vertically aligned Sn3O4 nanoflakes on carbon paper, J. Phys. Chem. C, 2017, 121, 19036–19043 CrossRef CAS.
- X. D. Li, C. T. Gao, H. G. Duan, B. G. Lu, X. J. Pan and E. Q. Xie, Nanocrystalline TiO2 film based photoelectrochemical cell as selfpowered UV-photodetector, Nano Energy, 2012, 1, 640–645 CrossRef CAS.
- Z. R. Wang, S. H. Ran, B. Liu, D. Chen and G. Z. Shen, Multilayer TiO2 nanorod cloth/nanorod array electrode for dye-sensitized solar cells and self-powered UV detectors, Nanoscale, 2012, 4, 3350–3358 RSC.
- C. T. Gao, X. D. Li, X. P. Zhu, L. L. Chen, Y. Q. Wang, F. Teng, Z. X. Zhang, H. G. Duan and E. Q. Xie, High performance, self-powered UV-photodetector based on ultrathin, transparent, SnO2–TiO2 core–shell electrodes, J. Alloys Compd., 2014, 616, 510–515 CrossRef CAS.
- W. W. Xia, H. Y. Qian, X. H. Zeng, J. Dong, J. Wang and Q. Xu, Visible-light self-powered photodetector and recoverable photocatalyst fabricated from vertically aligned Sn3O4 nanoflakes on carbon paper, J. Phys. Chem. C, 2017, 121, 19036–19043 CrossRef CAS.
- R. Kodiyath, J. J. Wang, T. Hara, A. Dakshanamoorthy, S. Ishihara, K. Ariga, J. H. Ye, N. Umezawa and H. Abe, et al., Photocatalytic water splitting under visible light by mixed-valence Sn3O4, ACS Appl. Mater. Interfaces, 2014, 6, 3790–3793 CrossRef PubMed.
- P. A. DeSario, J. J. Pietron, D. H. Taffa, R. Compton, S. Schünemann, R. Marschall, T. H. Brintlinger, R. M. Stroud, M. Wark, J. C. Owrutsky and D. R. Rolison, Correlating changes in electron lifetime and mobility on photocatalytic activity at network-modified TiO2 aerogels, J. Phys. Chem. C, 2015, 119, 17529–17538 CrossRef CAS.
- L. M. Peter and K. G. U. Wijayantha, Intensity dependence of the electron diffusion length in dye-sensitised nanocrystalline TiO2 photovoltaic cells, Electrochem. Commun., 1999, 1, 576–580 CrossRef CAS.
Footnote |
| † W. X. and H. Q. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |