Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of cobalt addition on the structure and properties of Ni–MCM-41 for the partial oxidation of methane to syngas
Yufeng Lia,
Junwen Wang *a,
Chuanmin Ding*a,
Lichao Maa,
Yanan Xuea,
Jing Guoa,
Shunqiang Wanga,
Yuanyuan Menga,
Kan Zhangb and
Ping Liub
*a,
Chuanmin Ding*a,
Lichao Maa,
Yanan Xuea,
Jing Guoa,
Shunqiang Wanga,
Yuanyuan Menga,
Kan Zhangb and
Ping Liub
aCollege of Chemistry & Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, PR China. E-mail: wangjunwen@tyut.edu.cn; dingchuanmin@tyut.edu.cn; Fax: +86 351 6014 498; Tel: +86 351 6014 498
bInstitute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, PR China
First published on 15th August 2019
Abstract
A one-step hydrothermal crystallization method was used to synthesize Co–Ni–MCM-41 catalysts for the partial oxidation of methane to syngas reaction. Co was added as an assistant in the synthesis process. The formation of a Ni–Co alloy decreased the damage of Ni ions to the framework of MCM-41. The Ni–Co alloy introduced more Ni into the channel exposing more active sites. The properties of the synthesized catalysts were characterized by XRD, N2 adsorption–desorption, TEM, ICP, FT-IR, H2-TPR, XPS and TGA techniques. Co–Ni–MCM-41 catalysts showed superior catalytic performance and sintering resistance than Ni–MCM-41 catalyst without Co. The Ni–Co alloy inhibited the formation of the NiO, thus reducing the sintering of the catalyst. The result was attributed to higher metal dispersion and more regular pore structure of the Co–Ni–MCM-41 catalysts. When the Co content was 1%, a conversion of 88% and selectivity of 87% was achieved.
1. Introduction
In recent years, methane (CH4) has attracted enormous attention as an efficient and clean energy source. The partial oxidation of methane reaction (POM) provides an important intermediate (syngas) for chemical processes.1,2 Compared with conventional steam methane reforming (CSMR), POM is a mild exothermic reaction with a rapid reaction rate, high CH4 conversion and requires a smaller reactor. In addition, the suitable H2/CO ratio of 2 is beneficial to methanol synthesis and Fischer–Tropsch synthesis.3,4Ni-based catalysts have been the subject of many POM research studies due to their excellent performance, high abundance and low cost.5 However, Ni-based catalysts have the problems of deactivation due to carbon deposition and sintering at high temperatures.6 Much effort has been made to improve the anti-carbon and anti-sintering properties of the Ni-based catalysts, such as increasing metal dispersion, decreasing the metal particle size, confining the active components within porous materials.7–9 The confinement of the zeolite or mesoporous materials can effectively control the agglomeration and sintering of the nanoparticles. Iglesia developed a strategy to encapsulate a series of metal clusters (Pt, Pd, Ru, and Rh) in different aluminosilicate zeolites such as SOD, GIS, ANA and LTA zeolites.10–12 The confinement effect of zeolite improved the catalytic performance and stability of the catalyst. However, the small pores and the acidity of these zeolites are not conducive to the POM reaction.
The MCM-41 mesoporous molecular sieve has been widely studied for its high specific surface area, regular pore structure and high thermal stability.13 The ordered mesoporous structure is more favorable for POM reaction compared with the microporous molecular sieve. Nickel has been successfully encapsulated in the MCM-41 mesoporous materials.14,15 However, a large amount of nickel entered the framework, leading to the destruction of the molecular sieve structure and the reduction of the active sites.
Previous studies have shown that the addition of additives can effectively improve the catalytic performance of nickel-based catalysts, prevent the loss of Ni nanoparticles and inhibit the formation of carbon deposition.16–18 A CoNi@SiO2 catalyst was prepared by Li and applied in the POM reaction.19 Compared with Ni@SiO2 and Co@SiO2 catalysts, it showed better catalytic activity and anti-carbon deposition performance due to the formation of a Co–Ni alloy. The alloy improved the reduction temperature of the catalyst thus inhibiting carbon deposition over the catalyst at high temperatures.
The Ni–Co alloy confined within MCM-41 may improve the dispersibility of Ni and avoid destruction of the molecular sieve structure. At the same time, the alloy may be beneficial to enhance the stability of nickel. Herein, the MCM-41 zeolite was used to encapsulate metallic nickel and cobalt under direct hydrothermal conditions. The role of introduced Co on catalytic performance and stability was investigated further.
2. Experimental section
2.1 Catalyst preparation
The Co–Ni–MCM-41 catalysts were synthesized via one-step hydrothermal crystallization method.14 Cetyltrimethylammonium bromide (CTAB) was used as the structural template and tetraethylorthosilicate (TEOS) was used as silicon source. Typically, 2.345 g CTAB was dissolved in the 100 mL distilled water and stirred for 30 min at ambient temperature. Appropriate amount of Ni(NO3)2·6H2O was dissolved in 20 mL distilled water. Ultrasonic oscillation was conducted for 5 min until the Ni(NO3)2·6H2O was fully dissolved and ammonia solution was added slowly to obtain a complex Ni(NH3)62+ solution with pH of 10. Then the two solutions were mixed and stirred for 30 min. 10 mL TEOS was dropped into the mixture and ammonia solution was used to adjust the pH of the solution to 10. The molar ratio of the composition was 1.0SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.152CTAB
0.152CTAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8NH3
2.8NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1Ni
0.1Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xCo
xCo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141.2H2O (x = 0.005, 0.01, 0.015). After continuous stirring for 6 h, the mixture was transferred into a Teflon-lined stainless steel autoclave and crystallized at 110 °C for 48 h. The resulting solid was filtered, washed with deionized water until neutral, dried for 24 h at 70 °C and finally calcined at 550 °C for 6 h. The obtained catalysts were denoted as 0.5Co–Ni–MCM-41, 1Co–Ni–MCM-41 and 1.5Co–Ni–MCM-41. Additionally, Ni–MCM-41 and Co–MCM-41 was prepared by similar method.
141.2H2O (x = 0.005, 0.01, 0.015). After continuous stirring for 6 h, the mixture was transferred into a Teflon-lined stainless steel autoclave and crystallized at 110 °C for 48 h. The resulting solid was filtered, washed with deionized water until neutral, dried for 24 h at 70 °C and finally calcined at 550 °C for 6 h. The obtained catalysts were denoted as 0.5Co–Ni–MCM-41, 1Co–Ni–MCM-41 and 1.5Co–Ni–MCM-41. Additionally, Ni–MCM-41 and Co–MCM-41 was prepared by similar method.
2.2 Catalyst characterization
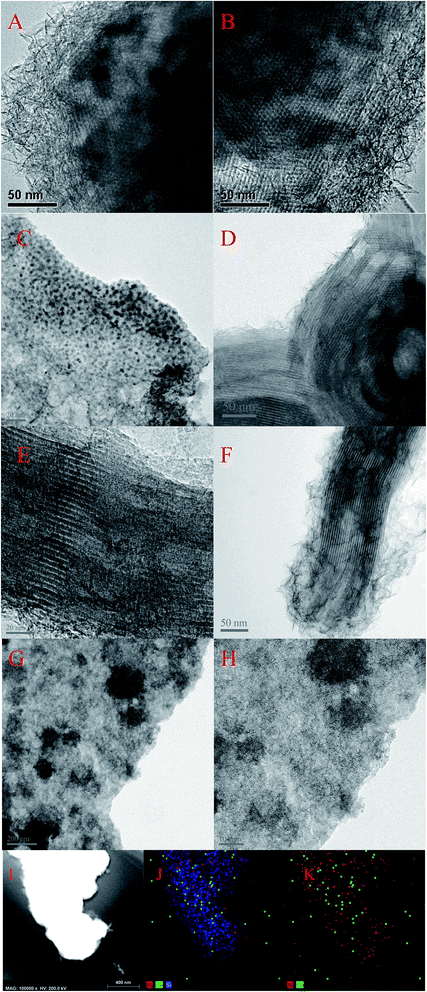
The as-prepared catalyst was characterized by X-ray diffraction using a BRUKER AXS D8 ADVANCE diffractometer with Cu Kα radiation (40 kV, 40 mA) at a scanning rate of 2° min−1 in both small angle (2θ range 0.5–10°) and wide angle (2θ range 10–80°). N2 adsorption–desorption isotherms were measured on a Micrometrics ASPA 2000 gas adsorption analyzer. Before test, the sample was degassed under high vacuum at 200 °C for 12 h. The TEM images were taken over a Tecnai G2-F30 instrument. The content of active components of the catalyst was determined by Thermo ICAP6300 inductively coupled plasma atomic emission spectrometer. The framework of the catalyst was measured using a Nicolet-Impact 400 FT-IR spectrometer. Temperature programmed reduction of H2 (H2-TPR) was performed on a chemical adsorption analyzer (TP-5676) equipped with a thermal conductive detector to study the reducibility of the catalyst. 50 mg samples were heated to 400 °C at a rate of 10 °C min−1 under N2 flow of 50 mL min−1 and kept at this temperature for 1 h to remove adsorbed water. After cooled down to room temperature, the samples were switched to a 25% H2/N2 (v/v, 60 mL min−1) mixture. The sample temperature was programmed to 900 °C at rate of 10 °C min−1. The surface oxidation states of Ni and Co were analyzed using X-ray photoelectron spectroscopy (XPS) on a Thermo ESCALAB 250 spectrometer. TG data was recorded on a SETARAM thermal analyzer in a 50 mL min−1 air flow from room temperature to 1000 °C with heating rate of 10 °C min−1.2.3 Catalyst evaluation
The catalytic activity was tested in the fixed bed quartz tubular reactor (inner diameter: 10 mm) at atmospheric pressure. 0.5 g catalyst sandwiched by two silica wool was placed in the center of the quartz tube. A thermocouple fixed in the furnace was used to monitor and measure the temperature of the catalyst bed. Before the reaction, the catalysts were reduced by H2 with a flow rate of 70 mL min−1 at 750 °C for 2 h. Then CH4 and O2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molar ratio) with a total flow of 150 mL min−1 (GHSV = 18 L g−1 h−1) were fed into the reactor. The catalysts were tested in the temperature of 750 °C. The catalytic activity tests of different GHSV (10.8–32.4 L g−1 h−1) were operated at 750 °C. The outlet mixture products were analyzed by a GC-920 gas chromatograph equipped with TCD and FID detectors.
1, molar ratio) with a total flow of 150 mL min−1 (GHSV = 18 L g−1 h−1) were fed into the reactor. The catalysts were tested in the temperature of 750 °C. The catalytic activity tests of different GHSV (10.8–32.4 L g−1 h−1) were operated at 750 °C. The outlet mixture products were analyzed by a GC-920 gas chromatograph equipped with TCD and FID detectors.
In this work, methane conversion (XCH4), CO selectivity (SCO) and H2 selectivity (SH2) were investigated to determine the catalytic performance of the catalysts. All the performance data of catalysts were calculated using the following equations:
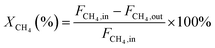 | (1) |
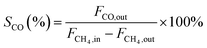 | (2) |
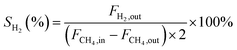 | (3) |
3. Results and discussion
3.1 Characterization
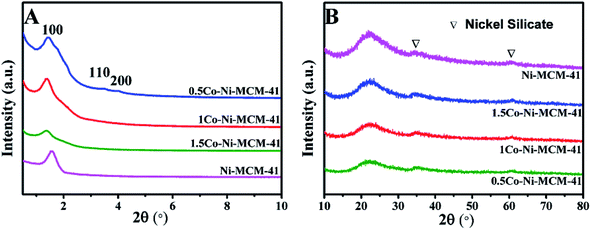 | ||
| Fig. 1 Small angle XRD patterns (A) and wide angle XRD patterns (B) of Ni–MCM-41 and Co–Ni–MCM-41 catalysts. | ||
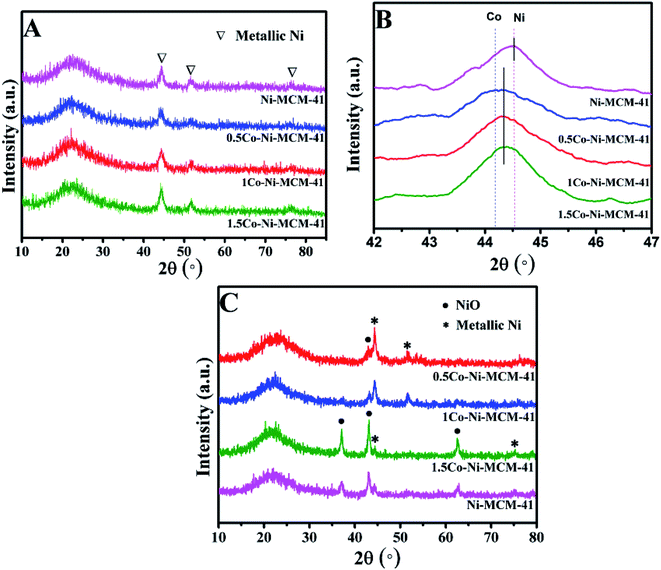
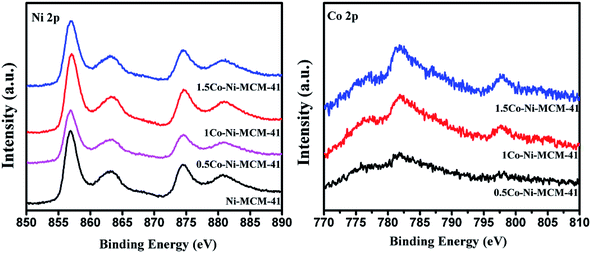
Fig. 1B showed the wide angle XRD of the as-prepared catalysts. There were no obvious diffraction peaks of metal species from the XRD patterns, indicating that most metal ions were inserted into the framework or highly dispersed in the channel of the molecular sieve. It was worth noting that nickel silicate reflections of 0.5Co–Ni–MCM-41 was lower than those of Ni–MCM-41, which suggested that there were less nickel silicate existing in 0.5Co–Ni–MCM-41.22 This may be due to that a part of Ni ions formed alloy with Co ions, which reduced the content of Ni ions connected to the framework. The results were in accord with the small angle XRD analysis.
The diffraction patterns of the reduced catalysts were displayed in Fig. 2A and B. The diffraction peaks of Ni phase could be observed in all the reduced catalysts. Fig. 2B presented the XRD analysis of the reduced catalysts in the range of 2 theta between 42° and 47°. The diffraction peak at 2 theta of 44.52° in Ni–MCM-41 was assigned to Ni (File no. 04-0850). With the addition of Co, it could be seen that the maxima of the metallic peak were shifted to low angle (44.38°) while the diffraction peak of Co at 44.22° (File no. 15-806) was absent. Occurrence of the new peak at 44.38° indicated the formation of a Ni–Co alloy in Co–Ni–MCM-41 catalysts. Similar conclusions were reported over different support materials in the previous studies.19,23,24 Takanabe et al.25 found that the diffraction peak at 2 theta of 44.22° (Co) were shifted to 44.51° (Ni) as the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni increased from 0
Ni increased from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 100
100 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 in the titania supported bimetallic catalysts. XRD patterns of SBA-15 loaded with Ni and Co catalysts also supported this conclusion.26 In addition, these studies proved the Ni–Co alloy in bimetallic catalysts suppressed the oxidation of cobalt.27,28 The formation of Ni–Co alloy was further discussed in the following sections. The low diffraction peak of metallic particles in the 0.5Co–Ni–MCM-41 catalyst indicated that the smaller metal particles were well dispersed in the catalyst. With the increasing of Co content, the diffraction peak intensity of the metal gradually increased, accompanied by the narrowing of the half-peak width, which indicating the growth of the metallic particles. It might be the fact that the formation of Ni–Co alloy decreased the Ni species entering the framework. The interaction between metal Ni and molecular sieve was weakened, leading to the metal agglomeration at high temperature. In addition, it was found that the influence on the size of Ni particles was small when the content of Co was less than 1% according to Debye–Scherrer formula.29
0 in the titania supported bimetallic catalysts. XRD patterns of SBA-15 loaded with Ni and Co catalysts also supported this conclusion.26 In addition, these studies proved the Ni–Co alloy in bimetallic catalysts suppressed the oxidation of cobalt.27,28 The formation of Ni–Co alloy was further discussed in the following sections. The low diffraction peak of metallic particles in the 0.5Co–Ni–MCM-41 catalyst indicated that the smaller metal particles were well dispersed in the catalyst. With the increasing of Co content, the diffraction peak intensity of the metal gradually increased, accompanied by the narrowing of the half-peak width, which indicating the growth of the metallic particles. It might be the fact that the formation of Ni–Co alloy decreased the Ni species entering the framework. The interaction between metal Ni and molecular sieve was weakened, leading to the metal agglomeration at high temperature. In addition, it was found that the influence on the size of Ni particles was small when the content of Co was less than 1% according to Debye–Scherrer formula.29
Fig. 2C showed the diffraction patterns of the used catalysts. The 0.5Co–Ni–MCM-41 and 1Co–Ni–MCM-41 exhibited high reflection of metallic Ni, while most Ni existed as oxides in the Ni–MCM-41 and 1.5Co–Ni–MCM-41. This result might be due to the formation of Ni–Co alloy inhibited the oxidation of Ni in the Co–Ni–MCM-41 catalysts. In addition, the NiO particle size in 1.5Co–Ni–MCM-41 catalyst was larger that of other catalysts. It might be due to that the addition of a large number of Co ions caused the collapse of the framework of the molecular sieve, Ni particles were more likely to form NiO particles with oxygen without the confinement of the channel and bare NiO particles were easily aggregated into larger particles.30
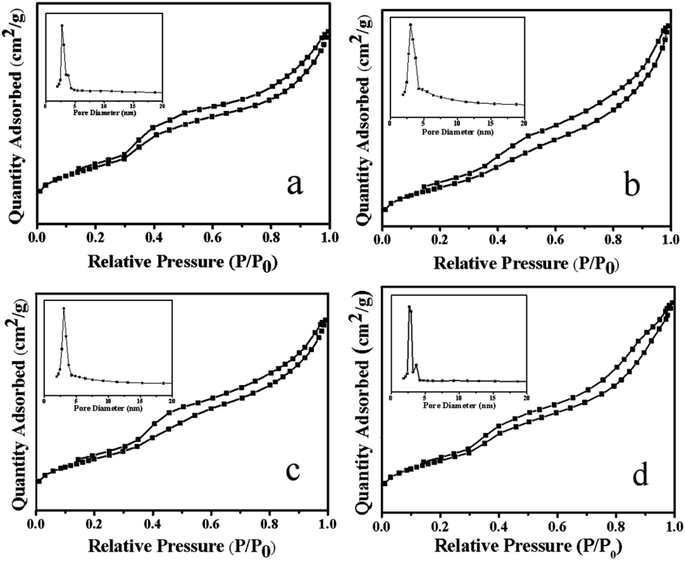 | ||
| Fig. 4 Adsorption–desorption isotherms and pore size distribution of the catalysts: (a) 0.5Co–Ni–MCM-41 (b) 1Co–Ni–MCM-41 (c) 1.5Co–Ni–MCM-41 (d) Ni–MCM-41. | ||
| Catalysts | Nia (wt%) | Ni–NH4ACa (wt%) | Surface areab (m2 g−1) | Pore volumec (cm3 g−1) | Pore sized (nm) |
|---|---|---|---|---|---|
| a Calculated by ICP.b Calculated by the BET.c BJH desorption pore volume.d BJH desorption average pore size. | |||||
| Ni–MCM-41 | 8.92 | 5.74 | 594 | 0.69 | 4.77 |
| 0.5Co–Ni–MCM-41 | 9.01 | 5.48 | 541 | 0.74 | 4.68 |
| 1Co–Ni–MCM-41 | 8.87 | 5.26 | 472 | 0.68 | 4.99 |
| 1.5Co–Ni–MCM-41 | 9.12 | 5.02 | 382 | 0.63 | 5.57 |
3.2 Catalytic performance of the catalysts
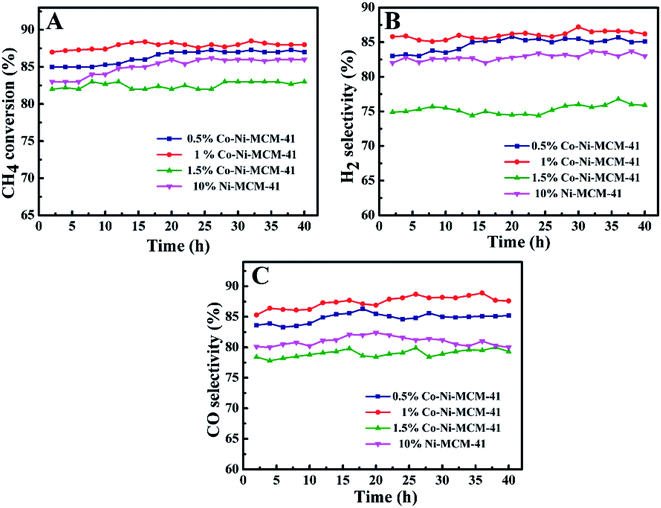 | ||
| Fig. 9 The catalytic ability comparison of the Ni–MCM-41 and Co–MCM-41 catalysts: (A) CH4 conversion, (B) H2 selectivity, (C) CO selectivity, at 750 °C, GHSV = 18 L g−1 h−1, atmospheric pressure. | ||
Ni-based catalysts have been widely studied in methane reforming. Liu et al.48 prepared Ni-based bimetallic catalysts supported on MCM-41 by a direct hydrothermal method, and high metal dispersions were obtained with the incorporation of Zr cations, while remarkably low dispersions were obtained with the incorporation of Ti or Mn. Ni/ZrO2 catalyst prepared by hydrothermal method possessed small metal particles and higher catalytic performance (about 85% of CH4 conversion) in partial oxidation of methane.49 Habimana et al.50 reported Ni-based SBA-15 catalysts with Cu promoter for partial oxidation of methane to syngas, best catalytic performance was obtained in 12.5%Ni/2.5%Cu/SBA-15 catalyst (at 750 °C the conversion of CH4 reached 89%). When the Cu content was 10%, the catalytic performance was significantly affected (about 84% of CH4 conversion). In the present study, the appropriate addition of Co in the Ni–MCM-41 catalyst could increase the dispersion of the metals and prevent the oxidation of Ni which was contributed to the improvement of the catalytic activity. And our catalysts remained high active after reaction of 100 h with little carbon deposition.
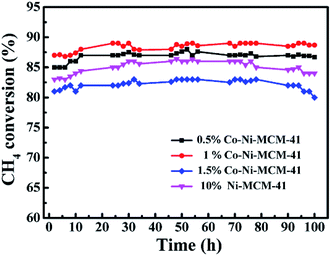 | ||
| Fig. 10 The catalytic stability comparison of the Ni–MCM-41 and Co–MCM-41 catalysts, at 750 °C, GHSV = 18 L g−1 h−1, atmospheric pressure. | ||
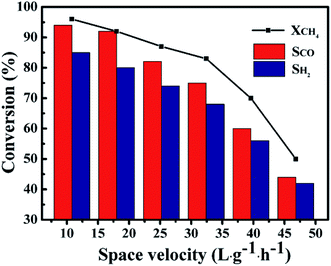
Fig. 11 showed the influence of GHSV on the catalytic performance of the 1Co–Ni–MCM-41 catalyst. It was observed that the CH4 conversion rate and the selectivity of the product presented a slow decline trend with the rise of GHSV from 10.8 L g−1 h−1 to 32.4 L g−1 h−1. The CH4 conversion decreased sharply with continuous increase of GHSV, which might be caused by insufficient contact of the feed gas at the active site on the catalyst. The product selectivity presented similar trend with the CH4 conversion. This result might be explained by combustion reforming mechanism in the POM reaction: CH4 was firstly completely oxidized to CO2 and H2O, and then the remaining CH4 reformed with CO2 and H2O. Under fast flow rates, CH4 had no enough time to react with pregenerated CO2 and H2O, resulting low product selectivity.51
4. Conclusions
In this work, the well-ordered Co–Ni–MCM-41 catalysts with the assistant of Co were synthesized under direct hydrothermal condition for POM reaction. The result showed that the Ni–Co alloy formed in the channel of MCM-41 zeolite, and inhibited the formation of the NiO. With the increase of the Co content, the nickel species in the framework of zeolite decreased significantly, weakening the damage of Ni ions to the framework. The Co–Ni–MCM-41 catalysts displayed superior catalytic performance and stability than Ni–MCM-41 due to the highly dispersed Ni–Co alloy and more exposed active sites. The Co–Ni–MCM-41 catalyst remained high active after reaction of 100 h with little carbon deposition. The confinement effect of the regular pore structure and the formation of Ni–Co alloy improved the sintering and coking resistance of the catalyst. Moreover, such alloy encapsulated catalyst could also be extended to apply in other field.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Natural Science Foundation of China, grant number 21706178; the Natural Science Foundation of Shanxi Province, grant number 201801D221074, 201801D121058; China Postdoctoral Science Foundation 2019M651079. Authors also gratefully acknowledge the support and guidance of the 602 groups of teachers from Institute of Coal Chemistry, Chinese Academy of Sciences.References
- H. Hiblot, I. Ziegler-Devin, R. Fournet and P. A. Glaude, Int. J. Hydrogen Energy, 2016, 41, 18329–18338 CrossRef CAS.
- R. Siriwardanea, H. Tian and J. Fisher, Int. J. Hydrogen Energy, 2015, 40, 1698–1708 CrossRef.
- H. Liu, H. Hao and D. He, Fuel Process. Technol., 2014, 119, 81–86 CrossRef CAS.
- M. Peymani, S. M. Alavi and M. Rezaei, Int. J. Hydrogen Energy, 2016, 41, 6316–6325 CrossRef CAS.
- J. Horiguchi, Y. Kobayashi, S. Kobayashi, Y. Yamazaki, K. Omata, D. Nagao, M. Konno and M. Yamada, Appl. Catal., A, 2011, 392, 86–92 CrossRef CAS.
- M. A. Carreon and V. V. Guliants, Eur. J. Inorg. Chem., 2005, 2005, 27–43 CrossRef.
- W. Yang, H. Liu, Y. Li, J. Zhang, H. Wu and D. He, Catal. Today, 2016, 259, 438–445 CrossRef CAS.
- Y. Zhang, G. Xiong, S. Sheng and W. Yang, Catal. Today, 2000, 63, 517–522 CrossRef CAS.
- T. Xie, X. Zhao, J. Zhang, L. Shi and D. Zhang, Int. J. Hydrogen Energy, 2015, 40, 9685–9695 CrossRef CAS.
- Z. Wu, S. Goel, M. Choi and E. Iglesia, J. Catal., 2014, 311, 458–468 CrossRef CAS.
- S. Goel, Z. Wu, S. I. Zones and E. Iglesia, J. Am. Chem. Soc., 2012, 134, 17688–17695 CrossRef CAS PubMed.
- M. Choi, Z. Wu and E. Iglesia, J. Am. Chem. Soc., 2010, 132, 9129–9137 CrossRef CAS PubMed.
- S. Karnjanakom, G. Guan, B. Asep, X. Du, X. Hao, C. Samart and A. Abudula, Energy Convers. Manage., 2015, 98, 359–368 CrossRef CAS.
- J. Qin, B. Li, W. Zhang, W. Lv, C. Han and J. Liu, Microporous Mesoporous Mater., 2015, 208, 181–187 CrossRef CAS.
- D. Liu, R. Lau, A. Borgna and Y. Yang, Appl. Catal., A, 2009, 358, 110–118 CrossRef CAS.
- C. E. Daza, J. Gallego, F. Mondragón, S. Moreno and R. Molina, Fuel, 2010, 89, 592–603 CrossRef CAS.
- Y. Wei, H. Wang, K. Li, X. Zhu and Y. Du, J. Rare Earths, 2010, 28, 357–361 CrossRef.
- Y. Bang, J. G. Seo and I. K. Song, Int. J. Hydrogen Energy, 2011, 36, 8307–8315 CrossRef CAS.
- L. Li, P. Lu, Y. Yao and W. Ji, Catal. Commun., 2012, 26, 72–77 CrossRef CAS.
- X. K. Li, W. J. Ji, J. Zhao, S. J. Wang and C. T. Au, J. Catal., 2005, 236, 181–189 CrossRef CAS.
- A. Palani, N. Gokulakrishnan, M. Palanichamy and A. Pandurangan, Appl. Catal., A, 2006, 304, 152–158 CrossRef CAS.
- R. Nares, J. Ramírez, A. Gutiérrez-Alejandre and R. Cuevas, Ind. Eng. Chem. Res., 2008, 48, 1154–1162 CrossRef.
- J. Xu, W. Zhou, Z. Li, J. Wang and J. Ma, Int. J. Hydrogen Energy, 2009, 34, 6646–6654 CrossRef CAS.
- H. Arbag, S. Yasyerli, N. Yasyerli, G. Dogu and T. Dogu, Appl. Catal., B, 2016, 198, 254–265 CrossRef CAS.
- K. Takanabe, K. Nagaoka, K. Nariai and K. Aika, J. Catal., 2005, 232, 268–275 CrossRef CAS.
- B. Erdogan, H. Arbag and N. Yasyerli, Int. J. Hydrogen Energy, 2018, 43, 1396–1405 CrossRef CAS.
- J. Zhang, H. Wang and A. K. Dalai, J. Catal., 2007, 249, 300–310 CrossRef CAS.
- S. Sengupta, K. Ray and G. Deo, Int. J. Hydrogen Energy, 2014, 39, 11462–11472 CrossRef CAS.
- U. Holzwarth and N. Gibson, Nat. Nanotechnol., 2011, 6, 534 CrossRef CAS PubMed.
- C. Ding, J. Wang, G. Ai, S. Liu, P. Liu, K. Zhang, Y. Han and X. Ma, Fuel, 2016, 175, 1–12 CrossRef CAS.
- Y. Shu, Y. Shao, X. Wei, X. Wang, Q. Sun, Q. Zhang and L. Li, Microporous Mesoporous Mater., 2015, 214, 88–94 CrossRef CAS.
- A. Derylo-Marczewska, W. Gac, N. Popivnyak, G. Zukocinski and S. Pasieczna, Catal. Today, 2006, 114, 293–306 CrossRef CAS.
- Y. Wang, R. Wu and Y. Zhao, Catal. Today, 2010, 158, 470–474 CrossRef CAS.
- S. J. Park and S. Y. Lee, J. Colloid Interface Sci., 2010, 346, 194–198 CrossRef CAS PubMed.
- A. Sakthivel, S. E. Dapurkar and P. Selvam, Catal. Lett., 2001, 77, 155–158 CrossRef CAS.
- P. A. Barrett, G. Sankar, C. R. A. Catlow and J. Thomas, J. Phys. Chem., 1996, 100, 8977–8985 CrossRef CAS.
- G. Du, S. Lim, Y. Yang, C. Wang, L. Pfefferle and G. L. Haller, J. Catal., 2007, 249, 370–379 CrossRef CAS.
- Y. Yang, S. Lim, G. Du, Y. Chen, D. Ciuparu and G. L. Haller, J. Phys. Chem. B, 2005, 109, 13237–13246 CrossRef CAS PubMed.
- Y. Zhao, H. Li and H. Li, Nano Energy, 2018, 45, 101–108 CrossRef CAS.
- J. Zhang, H. Wang and A. K. Dalai, Appl. Catal., A, 2008, 339, 121–129 CrossRef CAS.
- J. M. Rynkowski, T. Paryjczak, M. Lenik, M. Farbotko and J. Goralski, J. Chem. Soc., Faraday Trans., 1995, 91, 3481–3484 RSC.
- L. Zhao, X. Mu, T. Liu and K. Fang, Catal. Sci. Technol., 2018, 8, 2066–2076 RSC.
- M. M. Nair, F. Kleitz and S. Kaliaguine, ChemCatChem, 2012, 4, 387–394 CrossRef CAS.
- T. Iida, D. Zanchet, K. Ohara, T. Wakihara and Y. Román-Leshkov, Angew. Chem., Int. Ed., 2018, 57, 6454–6458 CrossRef CAS PubMed.
- J. Zelin, S. A. Regenhardt, C. I. Meyer, H. A. Duarte, V. Sebastian and A. J. Marchi, Chem. Eng. Sci., 2019, 206, 315–326 CrossRef CAS.
- Y. Bao, W. J. Lee, T. T. Lim, R. Wang and X. Hu, Appl. Catal., B, 2019, 254, 37–46 CrossRef CAS.
- S. Rabe, M. Nachtegaal and F. Vogel, Phys. Chem. Chem. Phys., 2007, 9, 1461–1468 RSC.
- D. Liu, X. Y. Quek, W. N. E. Cheo, R. Lau, A. Borgna and Y. Yang, J. Catal., 2009, 266, 380–390 CrossRef CAS.
- Y. Q. Song, D. H. He and B. Q. Xu, Appl. Catal., A, 2008, 337, 19–28 CrossRef CAS.
- F. Habimana, X. Li, S. Ji, B. Lang, D. Sun and C. Li, J. Nat. Gas Chem., 2009, 18, 392–398 CrossRef CAS.
- M. Prettre, C. Eichner and M. Perrin, Trans. Faraday Soc., 1946, 42, 335–339 RSC.
| This journal is © The Royal Society of Chemistry 2019 |