Open Access Article
Open Access ArticleExhaustive exploration of the conformational landscape of mono- and disubstituted five-membered rings by DFT and MP2 calculations†
Carlos A. Stortz *a and
Ariel M. Sarotti
*a and
Ariel M. Sarotti *b
*b
aUniversidad de Buenos Aires, Facultad de Ciencias Exactas, Departamento de Química Orgánica, Centro de Investigaciones en Hidratos de Carbono (CIHIDECAR), Ciudad Universitaria, Pab. 2, 1428 Buenos Aires, Argentina. E-mail: stortz@qo.fcen.uba.ar
bInstituto de Química Rosario (CONICET), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Suipacha 531, Rosario 2000, Argentina. E-mail: sarotti@iquir-conicet.gov.ar
First published on 5th August 2019
Abstract
The conformational landscape of 22 different non, mono-, and disubstituted compounds with a five-membered ring was thoroughly explored by ab initio (MP2) and DFT (B3LYP and M06-2X) methods with the 6-311+G** basis set. Our results showed that the conformational preference of these compounds was governed mainly by the specific characteristics of the substituents, with a minor influence of the level of theory employed. After a detailed analysis of the computational data, we found an interesting preference of the electronegative substituents to take pseudo-axial positions, whereas alkyl groups preferred adopting the pseudo-equatorial locations. Such preferences were pronounced with MP2 and M06-2X and underestimated by B3LYP. Despite each level of theory affording different landscapes in many cases, as a general trend, we noticed that M06-2X afforded much higher correlation with the MP2 results than B3LYP.
Introduction
Conformational analysis is of central relevance to understand the chemistry and spectroscopy of organic molecules,1 and understanding the factors controlling the conformational landscape of flexible systems has attracted the interest of generations of chemists. In a certain way, it can be considered that the history of conformational analysis is the history of organic chemistry itself. In the case of cyclic compounds, it has long been known that the molecules pucker to release the strain arising from the (hypothetical) planar structure. This was first recognized by Sachse in 1890 by postulating the chair and boat forms of cyclohexane.2 This brilliant idea, eagerly confronted at that time, had to wait half a century to be finally consolidated, initially with the work of Beckett, Pitzer, and Spitzer in 1947,3 and then with the seminal publication of Barton in 1950.4 Ever since, extensive experimental and computational works have been carried out to understand the conformational behavior of substituted cyclohexanes or the related six-membered rings.5 Despite the number of different conformations being high (involving chairs, boats, skews, half-chairs, and envelopes), the conformational preference of simple systems is usually relegated to the analysis of the relative stability of the two possible chair-like conformations.However, the situation becomes much more challenging when dealing with cyclopentanes or heterocyclic analogues due to their inherent conformational flexibility. These rings are known to exist as a combination of different puckered conformations, where each atom can assume many relative positions.6–8 There are two extreme representative conformations, namely, envelope (E) and twist (T). In the E shape, the torsional strain is reduced dramatically by avoiding the strain that would result from the eclipsing of the hydrogens positioned in a perfect planar conformation. In this arrangement, one atom sticks out from the plane containing the other four ring atoms though this position is not fixed but rotates around the ring through the up and down motions of the methylene groups or heteroatoms. As a result of this pseudorotation, an infinite number of intermediate conformations can be found with similar energies. In the twist conformation, three adjacent atoms lie on the main plane, and the other two contiguous atoms are twisted, lying above and below the main plane. The total torsional strain of T is about 40% of the total torsional strain of the unpuckered structure.1
The puckering of cyclopentanes has been confirmed experimentally by several methods, including entropy measurements9 and electron scattering.10 It is accepted that the E and T conformations show very low pseudorotational barriers (about the value of RT at room temperature or even lower), providing fast interconversion between conformers. Naturally, this idealized conformational landscape can be changed in substituted rings, depending both on the nature of the substituent and the substitution pattern.11 In addition, the presence of heteroatoms at the five-membered ring also affects the conformational preference of the system.12,13 As a result, the conformational landscape of five-membered rings cannot be easily predicted as in the case of their six-membered analogues. For this reason, this field has received some attention with respect to both theoretical14 and spectroscopical methods.15 Given the biological importance of furanoses and pyrrolidines, most of the studies have been carried out on these compounds.13,16 The number of computational studies dealing with cyclopentane motifs are much more scarce.17 To the best of our knowledge, no quantum-based study has been carried out to systematically explore the conformational landscape of mono- and disubstituted five-membered rings. The stability of a given conformation is the result of a fine balance between different types of interactions, which can be estimated differently depending on the level of theory employed during the calculations. Among the plethora of current DFT functionals, B3LYP is undoubtedly one of the most popular and widely employed ones. However, there are many reports suggesting that it is not optimal to describe the conformational behavior of carbohydrates, indicating that the emerging M06-2X provides a better performance.18 For this reason, herein, we reported the potential energy surfaces (PESs) of a wide variety of five-membered rings computed with these two functionals. A comparison of the results with those obtained with the more computationally demanding MP2 ab initio method was also provided.
Results
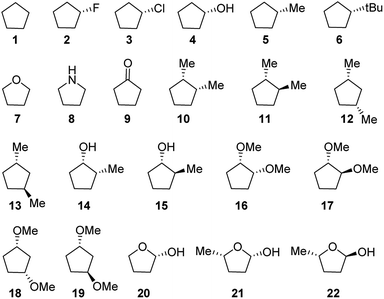
To achieve our goals, we selected a complete set of 22 compounds (Fig. 1) with different types of five-membered rings and substituents.The use of puckering parameters provided a substantial help to understand the pseudorotational pathways. In a pioneering work, Altona and co-workers developed a quantitative description of puckering and conformation in terms of the maximum angle of torsion (θm) and the “phase angle” of pseudorotation (Δ) computed from the five torsion angles in the five-membered ring; their study mainly focused on the D ring of steroids and analogues.19 Later, in 1972, Altona and Sundaralingam refined this concept for nucleosides.20 Two years later, Cremer and Pople generalized the puckering calculation to any type of cyclic compound.21
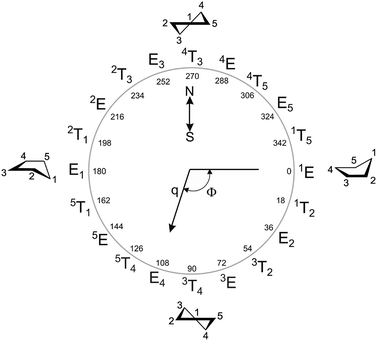
In the particular case of five-membered rings, both models (AS and CP) describe the puckering in terms of amplitude (q) and phase coordinate (Φ). The only difference between the two models is that the phase coordinates shift by a constant angle (90°). In this work, we used the CP definition, in which perfect envelope conformations were defined by Φ values as multiples of 36° (including 0°), whereas the corresponding twist conformations shifted by 18°. Other Φ values gave rise to intermediate geometries between E/T closer to one or the other depending on the specific phase shifting (Fig. 2).
Energies and stationary points
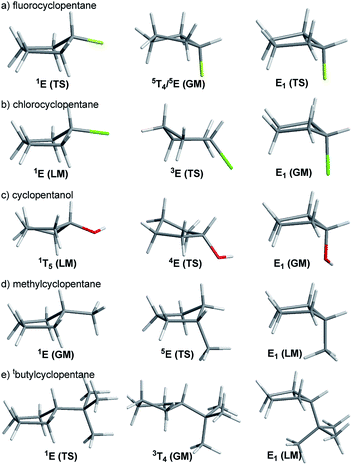
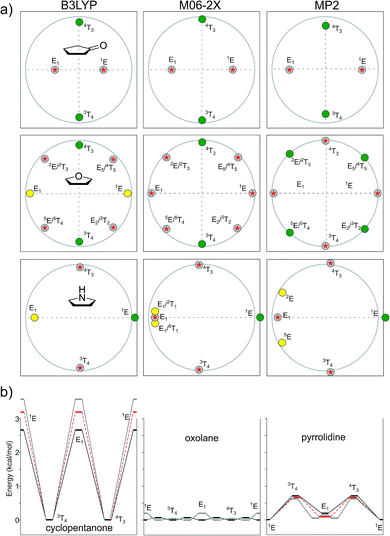
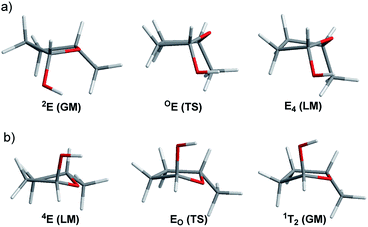
In this study, we evaluated the performance of two well-known DFT methods, namely, B3LYP and M06-2X along with that of MP2. All the stationary points were found using the 6-311+G** basis set except for larger compounds at the MP2 level. In these cases, the geometry optimizations were carried out using the 6-31+G** basis set, and the energies were recomputed at the higher level. We set tight convergence criteria and a pruned (99,590) integration grid, which were crucial for the correct exploration of the potential energy surface (PES). After the exhaustive exploration of PES for all the systems under study, we noticed three different patterns regarding the conformational landscape: a two-conformer (one minimum, one TS), a four-conformer (two minima, two TSs), and a six-conformer pattern (three minima, three TSs). It is important to point out that some molecules under study were symmetrical, making different conformations equivalent (this is indicated in Fig. 3–10 by the symmetry dotted line or lines).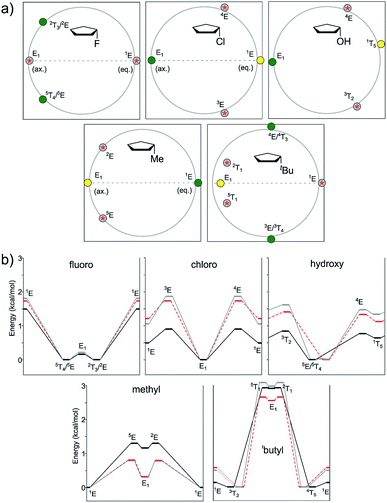 | ||
| Fig. 3 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 2–6 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 2–6 calculated at the B3LYP (black), M06-2X (red), and MP2 (grey) levels. | ||
With M06-2X, the global minimum was found around E1, with a pseudo-axial orientation of the OH group; the other minimum featured the switched 1T5 conformation, with the OH group being pseudo-equatorial (Fig. 3 and 4). Previous vibrational studies indicated that the conformational isomerism of cyclopentanol is dictated by two representative shapes featuring equatorial and axial OH groups,25b which is in agreement with our findings. According to B3LYP and MP2, the global minimum shifted south (around 5E/5T4). The preference towards envelope shapes with the hydroxyl group located at an axial position at the fold of the envelope (5E) was previously found by Abraham et al.26 The B3LYP energy difference between both conformations (0.64 kcal mol−1) was lower than that observed with the other methods (1.12 kcal mol−1 for M06-2X and 1.33 kcal mol−1 for MP2). The transition geometries were located at 4E and 3T2 (closer to 3E), with the relative energies of 0.76 kcal mol−1 (B3LYP) and 1.33–1.47 (M06-2X and MP2, respectively) for the former and 0.84 kcal mol−1 (B3LYP) and 1.41–1.62 kcal mol−1 (M06-2X and MP2, respectively) for the latter. The tendency of B3LYP to underestimate the pseudo-axial stabilization of electronegative atoms was also reflected in these calculations. When using MP2, an additional minimum (at E1) and the corresponding TS to reach it (around 5T1) also appear. Nevertheless, PES was flat in the studied region, showing negligible energy difference (0.02 kcal mol−1 between the minimum and TS, Fig. 3). All attempts to locate such conformations using B3LYP were met with no success as was observed with other common DFT functionals such as B3PW91 and PBE1PBE.
 | ||
| Fig. 6 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 10–13 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 10–13 calculated at the B3LYP (black), M06-2X (red) and MP2 (grey) levels. | ||
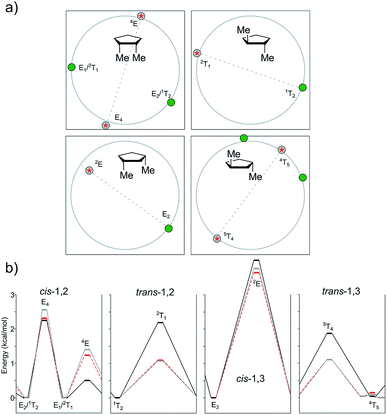
As stated above, the cis/trans energy difference was estimated experimentally as 0.53 kcal mol−1 for 1,3-dimethylcyclopentane.28 According to our computational results, the three levels of theory agreed with the higher stability of the cis isomer, though with lower energy gaps (0.28 kcal mol−1 for B3LYP, 0.19 kcal mol−1 for M06-2X, and 0.14 kcal mol−1 for MP2).
 | ||
| Fig. 7 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 14–15 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 14–15 calculated at the B3LYP (black), M06-2X (red), and MP2 (grey) levels. | ||
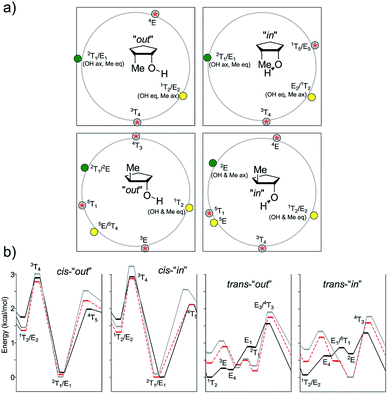
In the case of the cis isomer, several minima and TSs shifted slightly depending on the method used. In all cases, the global minimum was found near 2T1, with pseudo-equatorial and pseudo-axial methyl and OH groups, respectively. Although the energy of the “out” conformer was lower using MP2, it was slightly higher using the DFT methods. In any case, the two-minima, two-TS pattern was observed for all the methods and OH conformers. The other minimum was located near E2, with the exact opposite orientations of the substituents (OH pseudo-equatorial, Me pseudo-axial). The energy difference with the global minimum was quite high (1.31–1.69 kcal mol−1), reinforcing our previous observations. The transition structures were located near 3T4 and 1T5, the second being the most stable one. The barriers changed with the level of theory and orientation of the OH group (in vs. out). In general, the corresponding “in” TS was more stable than the analogous “out” one, with energy differences ranging up to 0.22 kcal mol−1. The differences between 3T4 and 1T5 were in the range of 0.67–0.81 kcal mol−1 for all levels of theory.
In the case of the trans isomer, the pattern was more complicated, with three minima and three TSs, and the shapes depended on the orientation of the OH group as well. In the case of the “out” conformations, according to B3LYP, the global minima were located near 1T2, featuring a pseudo-equatorial arrangement of both groups. The flipped 2T1 conformation (with pseudo-axial substituents) was 0.87 kcal mol−1 higher in energy, whereas the E4 shape with staggered groups was slightly more energetic than 1T2 (0.22 kcal mol−1). The three transition structures connecting these minima had envelope structures (3E, E1 and E3) with energy gaps of 0.26 kcal mol−1, 0.89 kcal mol−1 and 1.56 kcal mol−1, respectively. The preferred pseudo-equatorial orientation of the methyl group was also observed in TSs, with 3E (with pseudo-equatorial methyl group) being the most stable one. However, M06-2X and MP2 depicted a different scenario. In these cases, the most stable conformation (with the “out” OH group) was 2T1 (with a trans-diaxial arrangement of the substituents), and it was 0.23 kcal mol−1 (M06-2X) and 0.38 kcal mol−1 (MP2) lower in energy than 1T2 (the global minimum according to B3LYP). In fact, this last shape was the least stable local minimum according to M06-2X and MP2. The third conformation, with the energy lying between those calculated for 1T2 and 2T1, had a 5E/5T4 shape. The lowest energy TS had the 5E shape, with pseudo-axial and staggered OH and methyl groups, respectively. Here, again, these results suggest that B3LYP underestimates the relative stabilization exerted by pseudo-axial electronegative substituents. The analysis of the “in” conformations allowed a similar approach. The B3LYP minimum was again 1T2, whereas the other two local minima slightly shifted to north (E4 moved to 5T4, and 2T1 moved to 2E), with relative energies of 0.62 and 0.69 kcal mol−1, respectively. M06-2X and MP2 showed that 2E was the global minimum, with a trans-diaxial arrangement of the substituents. The 2E/2T1 gap was larger for MP2 (0.81 kcal mol−1) than that for M06-2X (0.46 kcal mol−1). TSs also slightly shifted than those computed for the “out” conformations.
 | ||
| Fig. 8 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 16–17 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 16–17 calculated at the B3LYP (black), M06-2X (red) and MP2 (grey) levels. | ||
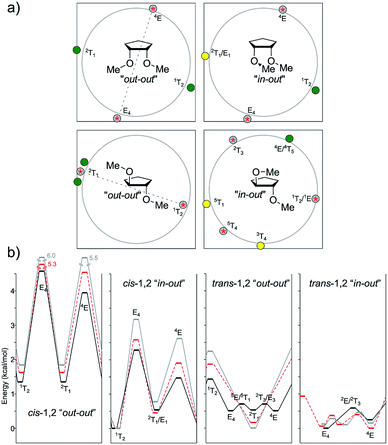
As in the case of cis-2-methylcyclopentanol, the global minimum of 1,2-cis-dimethoxycyclopentane was located at 1T2 (featuring one pseudo-axial methoxy group and the other pseudo-equatorial methoxy group). The other minima were found with the 2T1 shape at 0.53–0.77 kcal mol−1 at all levels of theory under study. The two transition structures connecting these minima had 4E and E4 geometries (both with staggered OMe groups), with the barriers significantly increasing when passing from B3LYP to M06-2X to MP2 (1.46 kcal mol−1, 1.93 kcal mol−1, and 2.62 kcal mol−1 for 4E; 2.27 kcal mol−1, 2.58 kcal mol−1 and 3.17 kcal mol−1 for E4). This trend could be explained on the basis of the gradual extra stabilization of the 1T2 shape.
The 1,2-trans isomer afforded more complex results. The symmetric conformers (“out–out”) exhibited by B3LYP afforded a six-conformation cycle, with three minima and three TSs, whereas M06-2X gave a simpler perspective (two maxima and two minima, very close in geometry). In the case of MP2, both related geometries converged into a single minimum (2T1) and maximum in the antipodes (1T2). Interestingly, although sym-2T1 was the global minimum for the MP2 calculations (with the two pseudo-axial methoxy groups), the B3LYP global minimum was around asym-E4, with the two methoxy groups staggered. This reinforced the previous analysis regarding the underestimation of B3LYP to effectively account for the pseudo-axial stabilization exerted by electronegative groups. For the asymmetric conformations (“in–out”), the behavior was even more complex, with both B3LYP and MP2 showing a three minima-two maxima pattern (i.e., missing one transition state) and M06-2X correctly locating this TS around 1T2/1E. In the case of B3LYP, the three minima were located at E4 (global minima), 5T1 (ΔE = 0.26 kcal mol−1), and 4E (ΔE = 0.25 kcal mol−1). M06-2X and MP2 showed similar patterns but with the envelope conformations displaced towards twisted ones (3T4 and 4E/4T5).
 | ||
| Fig. 9 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 18–19 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 18–19 calculated at the B3LYP (black), M06-2X (red), and MP2 (grey) levels. | ||
 | ||
| Fig. 10 (a) Geometry representations of the Cremer–Pople puckering parameters of the minima (green, global minima; yellow, local minima) and transition states (grey circle with red star) computed for compounds 20–22 at the M06-2X level. Qualitatively similar graphs were observed for the other levels of theory (see the ESI†). (b) Energies of the conformational pathways for compounds 20–22 calculated at the B3LYP (black), M06-2X (red), and MP2 (grey) levels. | ||
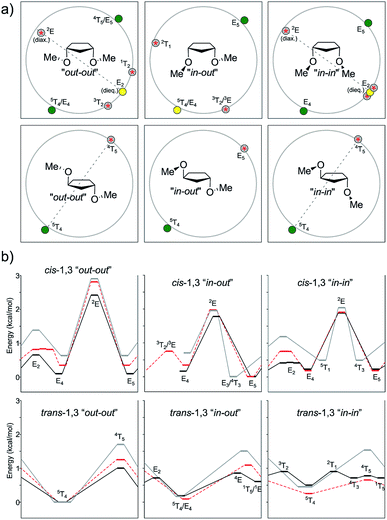
In the case of the 1,3-cis isomer, B3LYP and MP2 exhibited a classical four-conformer pattern for the “out–out” system, whereas according to M06-2X, one of the TSs became a minimum, and two close-by TSs appeared, affording a six-conformer pattern. A four-conformer pattern was also observed with B3LYP, M06-2X, and MP2 for the “in–out” conformations though one of the TSs was missing with B3LYP and MP2 (hence, it was actually a three-conformer pattern), whereas M06-2X succeeded in finding the left-out TS around 3T2. Regarding the “in–in” type of conformations, the DFT methods encountered a six-conformer pattern, but MP2 converted the 1T2 minimum and its surrounding TSs into just one TS, thus conforming a four-conformer pattern. The E5-“in–out” is usually the global minimum and it was shifted by the MP2 calculation toward E3. In such conformations, one methoxyl group is pseudo-equatorial and the other one is staggered. The 2E conformation, featuring the two methoxy groups in a pseudo-axial position, was located as TS at a relatively high energy content for all levels of theory (2.41–2.89 kcal mol−1 for “out–out” conformations, 1.77–1.97 kcal mol−1 for “in–out” conformations, and 1.89–2.04 kcal mol−1 for “in–in” conformations). The corresponding flipped structure (E2) with the two pseudo-equatorial OMe groups was also found as a TS or as a relative minimum depending on the level of theory and conformation of the methoxy groups. For instance, B3LYP predicted E2 as a TS for the “out–out” conformations and a relative minimum for the “in–in” conformations. M06-2X described a relative minimum in both cases, whereas MP2 anticipated a TS. When comparing the relative energies of 2E vs. E2, the last ones were considerably lower (ΔE = 0.86–1.99 kcal mol−1). Here, again, the ΔE values were lower for MP2 than that for B3LYP, which was consistent with our previous findings.
In the case of the trans-1,3 compound, when dealing with the “out–out” system, B3LYP gave just one minimum at 5T4 and one TS at the inverted 4T5 (1.00 kcal mol−1 higher in energy). M06-2X afforded a similar behavior, whereas MP2 turned it into a four-conformer pattern; however, one of the TSs was very close to its surrounding minima, and they all had very low frequencies (<10 cm−1). In all levels of theory, the global minima were found near 5T4, with one pseudo-axial and one staggered OMe group. For “in–out”, B3LYP gave a four-conformer pattern, with the most stable conformation again near 5T4 (Erel = 0.19 kcal mol−1) and the other one close in energy at 1T5 (Erel = 0.60 kcal mol−1). The two transition structures connecting the minima were computed at 4E (ΔE = 0.86 kcal mol−1) and E2 (ΔE = 0.71 kcal mol−1). M06-2X and MP2 afforded a simpler two-conformer pattern, with geometries depending on the level of theory. The minimum and TS were near 5T4 and E5 according to M06-2X and shifted toward E4 and 4T5 according to MP2. Finally, for the “in–in” type of conformations, a four-conformer pattern was observed for B3LYP, with two local minima (5T4 and 1T5) closer in energy (0.50 and 0.71 kcal mol−1, respectively) and two transition structures connecting both minima at 2T1 and 4T5. MP2 also showed a four-conformer pattern, but it was completely different to that of B3LYP, with different conformers at hand (5T4 and E4/3T4 as the local minima and E4 and 4T5 as TS). M06-2X afforded a cleaner scenario, with one minimum at 5T4 and one maximum at 4T5.
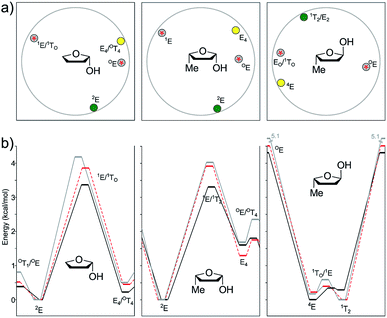
In the case of the cis isomer, the conformational landscape was quite similar to that observed for 2-hydroxy-THF disclosed above. The global minimum was located at 2E in all cases, which was consistent with the stabilization exerted by the pseudo-axial OH group as a consequence of the anomeric effect. The other minima were computed near E4, with pseudo-axial and isoclinal methyl and hydroxyl groups, respectively. The energy difference between both minima was significant (1.29–1.69 kcal mol−1), suggesting that the equilibrium should be cleanly displaced towards 2E at room temperature. The transition structures were found near those observed for 2-OH-THF, with the most stable one at OE and the other one at 1E. The first TS (OE) featured a pseudo-diaxial arrangement of the two substituents, with barriers higher than those computed for the previous related system and underestimated by the DFT methods regarding MP2 (1.77–1.80 vs. 2.36 kcal mol−1). The most unstable transition structure showed 1E geometry, with a pseudo-equatorial OH group and an isoclinal methyl group. Here, again, higher energy barriers were estimated with the three methods under study (3.30 kcal mol−1 for B3LYP, 3.91 kcal mol−1 for M06-2X, and 4.02 kcal mol−1 for MP2).
The trans isomer reflected an excellent model study to illustrate the dependence of the conformational landscape with the relative orientation of the substituents and the level of theory employed during the calculations. The B3LYP global minimum was found at 4E, with a pseudo-equatorial methyl group and an isoclinal OH group. However, according to M06-2X and MP2, the global minima shifted towards 1T2 with a pseudo-axial OH orientation, showing once again that the stabilization exerted by pseudo-axial electronegative groups is underestimated by B3LYP. Nevertheless, the 4E/1T2 energy difference was quite low for the three levels of theory (0.17–0.29 kcal mol−1), indicating that the two type of conformations should be expected to occur in real systems. Interestingly, none of the theoretical models agreed on the structure of the most stable TS connecting both minima (1E for B3LYP, 1TO for M06-2X, and EO for MP2), with similar energy barriers in all cases (0.34–0.59 kcal mol−1). The relative stabilization of these structures was due to the pseudo-axial orientation of the OH group. The other TS, much higher in energy, was found at OE, with a pseudo-OH group. The selected stationary points of 21 and 22 found at the B3LYP/6-311+G** level of theory are shown in Fig. 11.
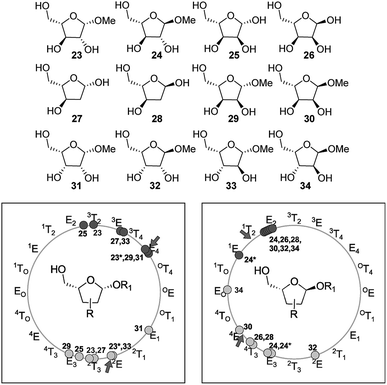
The conformational preferences calculated for these two simplified models for pentofuranoses showed good correlation with the landscape found for more complex furanosic compounds shown in Fig. 12.38 For instance, Cros et al. explored methyl-β-D- and -α-L-arabinofuranosides (ent-23 and 24, respectively) with the MM3 force field.39 They observed that the conformations of the two compounds were distributed in two deep low-energy wells (consistent with the two minima-two maxima reported herein). In the case of 23, the two minima were found near 3T2 and 2T3, whereas the stationary points of 24 were located at 1T2/E2 and E3. Later, Dowd et al. employed MM3 to build the conformational maps of the furanosyl rings of β- and α-D-ribose (ent-25 and ent-26, respectively) and their 2-deoxy counterparts (ent-27 and ent-28, respectively).40 In the case of ribose (25 and 26), the northern (3T2 and E2, respectively) and southern (E3/2T3 and 4T3, respectively) minima were located closer to the proposal made by Cros et al. for arabinofuranosides. The same trend was also noticed for the 2-deoxyribose analogues 27 (3E and 2T3) and 28 (E2 and 4T3). More recently, Lowary and co-workers carried out a detailed examination of the stable conformations of twelve β- and α-methyl furanosides of D-arabinose (ent-23 and ent-24), D-lyxose (ent-29 and ent-30), D-ribose (ent-31 and ent-32), and D-xylose (ent-33 and ent-34) at the B3LYP/6-31+G**//B3LYP/6-31G* level of theory.41 In the cases of β-arabinose and β-ribose (23 and 31, respectively), the northern minima located at E4 shifted by ∼50° southwest from the MM3 calculations by Cros et al. and Dowd et al. The southern minima found at 2E and E3 slightly shifted from the MM3 results. In the case of α-series, the minima of 24 and 32 occurred at 1E and E3 and E2 and 4E, respectively, which were again closer to the MM3 results.
The remaining compounds (31–34) exhibited similar trends but with some disturbances in the southern region. Taking collectively the results reported by Cros et al., Dowd et al., Lowary and coworkers, and us (Fig. 12), insightful relationships can be drawn. The conformational landscape of furanoses is governed by a 2-minima system, with one stationary point at the north and the other at the south of the rotational map. The northern region is mainly populated between 3T2 and E4 for the 1,4-cis isomers and between 1E and E2 for the 1,4-trans counterparts. In the same way, in the southern region, these conformations between E3/2E (1,4-cis) and 4E/E3 (trans) are favored. The results of our calculations on a simplified model correlated well with these interesting trends, suggesting that the conformational preference of furanoses is strongly dictated by the C-1/C-4 relative configuration. Naturally, the presence of other substituents at the ring might introduce additional steric and electronic interactions, hence affecting the location of minima. Including the solvent effect in the calculations might also generate a different outcome provided by gas phase calculations. For instance, the southern minima of 31, 32, and 34 were found at E1, 2E, and EO, respectively, which were slightly different from the above-mentioned range. In these cases, the formation of intramolecular H-bonds accounted for the shifts in the southern region.
Conclusions
The conformational pathways of several mono- and disubstituted five-membered rings were thoroughly explored with three different quantum methods (B3LYP, M06-2X, and MP2). The results of these calculations showed that the conformational preference of a given compound is intimately linked with its own structural characteristics (type and number of substituents, substitution pattern, type of the heteroatom in the ring, etc.). Despite the case that in some systems the relative energies of different conformations fell within the limit provided by the levels of theory under study, interesting trends could be drawn. We found an interesting preference of electronegative substituents (chlorine, OH, etc.) and alkyl groups to take pseudo-axial and pseudo-equatorial positions, respectively. In addition, the conformational landscape computed for many systems showed dependence on the level of theory employed for the calculations. In this regard, we observed a higher correlation between the results provided by MP2 with M06-2X than that with B3LYP, which clearly underestimated the preferences for the pseudo-axial position of electronegative groups. In most cases, free energy calculations confirmed the results obtained using electronic energies. However, in a few cases, such as that for pyrrolidine, PES changed drastically by taking entropic effects into account.Experimental section
Computational methods
Quantum mechanical calculations were performed using Gaussian 09W (rev. C.01)42 with the standard basis sets and default minimization methods and termination conditions, with the exception of the Tight keyword and the use of an Ultrafine grid for the optimizations. All the minima and transition states had zero or one imaginary frequency, respectively, as appropriate. For the transition states, it was verified that this frequency corresponded to the conformational pathway searched for. The transition states were obtained either by the traditional Berny algorithm or by using the QST2 procedure. Free energies were calculated at 298 K, as reported in the Gaussian output file, without scaling. For substituted cyclopentanes, the IUPAC numbering scheme was used, with the substituent facing down when numbering clockwise observed from above. The heterocycles were numbered following the usual carbohydrate convention, with C1 clockwise to the heteroatom (which is equivalent to atom 5).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are Research Members of the National Research Council of Argentina (CONICET). This work was supported by grants from ANPCyT (PICT-2016-0116), CONICET (PIP 298/14), UNR (BIO 316 and 500) and UBA (Q-203 and 0363).References
- E. Juaristi, Introduction to stereochemistry and conformational analysis, John Wiley and Sons, New York, 1991 Search PubMed.
- H. Sachse, Ber. Dtsch. Chem. Ges., 1890, 23, 1363–1370 CrossRef.
- C. W. Beckett, K. S. Pitzer and R. Spitzer, J. Am. Chem. Soc., 1947, 69, 2488–2495 CrossRef CAS PubMed.
- D. Barton, Experientia, 1950, 6, 316–320 CrossRef CAS PubMed.
- C. A. Stortz, J. Phys. Org. Chem., 2010, 23, 1173–1186 CrossRef CAS.
- J. E. Kilpatrick, K. S. Pitzer and R. Spitzer, J. Am. Chem. Soc., 1947, 69, 2483–2488 CrossRef CAS.
- A. Wu, D. Cremer, A. A. Auer and J. Gauss, J. Phys. Chem. A, 2002, 106, 657–667 CrossRef CAS.
- K. S. Pitzer and W. E. Donath, J. Am. Chem. Soc., 1959, 81, 3213–3218 CrossRef CAS.
- (a) J. Aston, H. L. Fink and S. Schumann, J. Am. Chem. Soc., 1943, 65, 341–346 CrossRef CAS; (b) J. McCullough, R. Pennington, J. Smith, I. Hossenlopp and G. Waddington, J. Am. Chem. Soc., 1959, 81, 5880–5883 CrossRef CAS; (c) J. P. McCullough, J. Chem. Phys., 1958, 29, 966–967 CrossRef CAS.
- A. Almenningen, O. Bastiansen and P. Skancke, Acta Chem. Scand., 1958, 12, 5 Search PubMed.
- (a) D. Scott, W. Berg and J. McCullough, J. Phys. Chem., 1960, 64, 906–908 CrossRef CAS; (b) D. Wertz and W. Shasky, J. Chem. Phys., 1971, 55, 2422–2425 CrossRef CAS.
- J. McCullough, D. Douslin, W. Hubbard, S. Todd, J. Messerly, I. Hossenlopp, F. Frow, J. Dawson and G. Waddington, J. Am. Chem. Soc., 1959, 81, 5884–5890 CrossRef CAS.
- H. A. Taha, M. R. Richards and T. L. Lowary, Chem. Rev., 2012, 113, 1851–1876 CrossRef PubMed.
- (a) A. Golebiewski and A. Parczewski, Chem. Rev., 1974, 74, 519–530 CrossRef CAS; (b) W. Cui, F. Li and N. L. Allinger, J. Am. Chem. Soc., 1993, 115, 2943–2951 CrossRef CAS; (c) D. Cremer, Isr. J. Chem., 1983, 23, 72–84 CrossRef CAS; (d) D. Cremer and J. A. Pople, J. Am. Chem. Soc., 1975, 97, 1358–1367 CrossRef CAS.
- (a) Z. Dzakula, M. L. Derider and J. L. Markley, J. Am. Chem. Soc., 1996, 118, 12796–12803 CrossRef CAS; (b) T. J. Church, I. Carmichael and A. S. Serianni, J. Am. Chem. Soc., 1997, 119, 8946–8964 CrossRef CAS; (c) J. B. Houseknecht, T. L. Lowary and C. M. Hadad, J. Phys. Chem. A, 2003, 107, 372–378 CrossRef CAS; (d) J. G. Napolitano, J. A. Gavín, C. García, M. Norte, J. J. Fernández and A. Hernández Daranas, Chem.–Eur. J., 2011, 17, 6338–6347 CrossRef CAS PubMed.
- S. J. Han and Y. K. Kang, J. Mol. Struct.: THEOCHEM, 1996, 369, 157–165 CrossRef CAS.
- N. Sakhaee, S. Jalili and F. Darvish, Comput. Theor. Chem., 2016, 1090, 193–202 CrossRef CAS.
- (a) W. Sameera and D. A. Pantazis, J. Chem. Theory Comput., 2012, 8, 2630–2645 CrossRef CAS PubMed; (b) M. Marianski, A. Supady, T. Ingram, M. Schneider and C. Baldauf, J. Chem. Theory Comput., 2016, 12, 6157–6168 CrossRef CAS PubMed; (c) V. A. Cosenza, D. A. Navarro and C. A. Stortz, Carbohydr. Res., 2016, 426, 15–25 CrossRef CAS PubMed; (d) E. A. Del Vigo, C. Marino and C. A. Stortz, Carbohydr. Res., 2017, 448, 136–147 CrossRef CAS PubMed.
- C. Altona, H. t. Geise and C. Romers, Tetrahedron, 1968, 24, 13–32 CrossRef CAS.
- C. t. Altona and M. Sundaralingam, J. Am. Chem. Soc., 1972, 94, 8205–8212 CrossRef CAS PubMed.
- D. Cremer and J. A. Pople, J. Am. Chem. Soc., 1975, 97, 1354–1358 CrossRef CAS.
- E. J. Ocola, L. E. Bauman and J. Laane, J. Phys. Chem. A, 2011, 115, 6531–6542 CrossRef CAS PubMed.
- L. Carreira, J. Chem. Phys., 1971, 55, 181–185 CrossRef CAS.
- J. R. Durig, A. M. E. Defrawy, A. Ganguly, T. K. Gounev and G. A. Guirgis, J. Phys. Chem. A, 2009, 113, 9675–9683 CrossRef CAS PubMed.
- (a) I. Ekejiuba and H. J. Hallam, J. Mol. Struct., 1970, 6, 341–357 CrossRef CAS; (b) I. Ekejiuba and H. Hallam, J. Chem. Soc. B, 1970, 209–211 RSC; (c) W. Harris, J. Karriker and J. Durig, J. Mol. Struct., 1971, 9, 139–149 CrossRef CAS; (d) H. M. Badawi, W. A. Herrebout, C. Zheng, T. A. Mohamed, B. Van der Veken and J. R. Durig, Struct. Chem., 2003, 14, 617–635 CrossRef CAS.
- R. J. Abraham, R. Koniotou and F. Sancassan, J. Chem. Soc., Perkin Trans. 2, 2002, 2025–2030 RSC.
- R. L. Lipnick, J. Am. Chem. Soc., 1974, 96, 2941–2948 CrossRef CAS.
- J. D. Roberts, M. Christl and H. J. Reich, J. Am. Chem. Soc., 1971, 93, 3463–3468 CrossRef CAS.
- H. Kim and W. D. Gwinn, J. Chem. Phys., 1969, 51, 1815–1819 CrossRef CAS.
- V. M. Rayón and J. A. Sordo, J. Chem. Phys., 2005, 122, 204303 CrossRef PubMed.
- W. Caminati, H. Oberhammer, G. Pfafferott, R. Filgueira and C. Gomez, J. Mol. Spectrosc., 1984, 106, 217–226 CrossRef CAS.
- G. Pfafferott, H. Oberhammer, J. E. Boggs and W. Caminati, J. Am. Chem. Soc., 1985, 107, 2305–2309 CrossRef CAS.
- W. Caminati, A. Dell'Erba, G. Maccaferri and P. G. Favero, J. Mol. Spectrosc., 1998, 191, 45–48 CrossRef CAS PubMed.
- (a) L. Carballeira and I. Pérez-Juste, J. Chem. Soc., Perkin Trans. 2, 1998, 1339–1346 RSC; (b) L. Carballeira, I. Pérez-Juste and C. Van Alsenoy, J. Phys. Chem. A, 2002, 106, 3873–3884 CrossRef CAS.
- M. Kunitski, C. Riehn, V. V. Matylitsky, P. Tarakeshwar and B. Brutschy, Phys. Chem. Chem. Phys., 2010, 12, 72–81 RSC.
- H. B. Mayes, L. J. Broadbelt and G. T. Beckham, J. Am. Chem. Soc., 2014, 136, 1008–1022 CrossRef CAS PubMed.
- The naming of the different conformers for 2-hydroxyTHF and its 5-methylated counterparts follows the nomenclature used in sugar chemistry, i.e., C-1 for the anomeric atom, C-4 for the last carbon in the cycle, and O for the ring oxygen..
- Most of the studies have been carried out with furanoses of D-series. However, the configurations exhibited by 21 and 22 are consistent with furanoses of L-series. Hence, to facilitate the comparison we have inverted (rotated by 180°) the results obtained for furanoses of D-series (i.e. the E1 in D-series is identical to 1E for L-series)..
- S. Cros, C. H. du Penhoat, S. Pérez and A. Imberty, Carbohydr. Res., 1993, 248, 81–93 CrossRef CAS.
- M. K. Dowd, A. D. French and P. J. Reilly, J. Carbohydr. Chem., 2000, 19, 1091–1114 CrossRef CAS.
- J. B. Houseknecht, T. L. Lowary and C. M. Hadad, J. Phys. Chem. A, 2003, 107, 5763–5777 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
Footnote |
| † Electronic supplementary information (ESI) available: Computational data associated with this paper. See DOI: 10.1039/c9ra03524a |
| This journal is © The Royal Society of Chemistry 2019 |