Open Access Article
Open Access ArticleSynthesis of tetracyclic indolin-3-ones through Pd-catalyzed intramolecular deacetylative dearomatization of 3-acetoxy-indoles†
Ren-Xiao Lianga,
Ke Wanga,
Ling-Jie Songa,
Wei-Jian Shenga and
Yi-Xia Jia *ab
*ab
aCollege of Chemical Engineering, State Key Laboratory Breeding Base of Green-Chemical Synthesis Technology, Zhejiang University of Technology, Chaowang Road 18#, Hangzhou 310014, China. E-mail: yxjia@zjut.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, PR China
First published on 7th May 2019
Abstract
An efficient palladium-catalyzed intramolecular deacetylative dearomatization reaction of 3-acetoxyindoles has been developed. A range of tetracyclic indolin-3-ones bearing C2-quaternary stereocenters are achieved in good yields, showing a wide substrate scope for this reaction. A preliminary enantioselective reaction is established to furnish the product in 63% ee by using (R,R,R)-phosphoramide-PE as a chiral ligand.
Introduction
The indolin-3-one containing C2-quaternary stereocenter is an intriguing structural motif that widely appears in natural products and synthetic pharmaceuticals. Representative biologically active molecules include (−)-isatisine A, (−)-isatisine A acetonide, strobilanthoside A, (+)-austamide, and halichrome A (Fig. 1).1 Considerable attention has been directed to the construction of such a unique framework. A number of reliable synthetic methods have been established, which mainly rely on the transformations of the pre-existing 3-indolinone ring system.2 The development of a new strategy for the synthesis of structurally diverse C2-disubstituted indolin-3-ones by using readily available starting materials is still highly desirable.Transition-metal-catalyzed dearomatization of heteroarenes has become a reliable strategy to access heteroalicyclic compounds having carbon-based stereocenters on the ring.3 In this context, a number of dearomatizing transformations of indoles, benzofurans, pyrroles, and furans have been intensely established over the past few years.3,4 Amongst them, the palladium-catalyzed intramolecular dearomatizing arylation of indoles through a Heck reaction pathway has turned out to be very efficient in approaching fused- or spiro-indoline derivatives containing carbon stereocenters at C2 and/or C3 position of indoline ring. Documented examples included the intramolecular dearomatizing Heck reactions,5 reductive-Heck reactions,6 and domino dearomatizing Heck/anionic capture sequences involving the capture of benzyl-Pd species with a range of external nucleophiles,7 such as cyanide,7a organoboron reagents,7b–d alkynes,7e–h and azoles. Nevertheless, the aforementioned methods have not been applied to the construction of C2-disubstituted indolin-3-one substructure, which possesses a cyclic ketone unit at C3. On the other hand, the palladium-catalyzed dearomatizing cross-coupling of phenolates has recently been established as an powerful access to cyclohexenone molecules, in which keto–enol tautomerism of phenols under the basic conditions facilitates the dearomatization, rendering the formation of cyclic ketone motif reliable (Scheme 1a).8,9 Inspired by these transformations, as well as the Pd-catalyzed deacetylative arylation reactions of acetoxy-dihydronaphthalene derivatives developed by Zhou's group,10 we envisioned that a similar dearomatization of 3-acetoxyindole derivatives would provide a straightforward approach to C2-disubstituted indolin-3-ones. Herein, we report the palladium-catalyzed intramolecular deacetylative dearomatization of 3-acetoxyindoles, which results in tetracyclic indolin-3-one derivatives in good yields (Scheme 1b). It represents a new dearomatizing transformation of indoles.
Results and discussion
We commenced the investigation by exploring the conditions for the intramolecular deacetylative dearomatization reaction with N-(2-iodobenzoyl)-3-acetoxyindole 1a as the model substrate. Initial test using 5 mol% Pd(OAc)2 as a catalyst, PPh3 as a ligand, and K2CO3 as a base in the presence of two equivalents of water led to the target tetracyclic indolin-3-one 2a in 15% yield in THF at 100 °C for 12 h (Table 1, entry 1). Solvent effect was then investigated to improve the yield. No desired product 2a was detected in protonic solvent MeOH, while comparable yields were observed for the reactions occurred in 1,4-dioxane and toluene (Table 1, entries 2–4). To our delight, the yield was improved to 45% and 40% in DMF and NMP solvent, respectively (Table 1, entries 5 and 6). The influence of the base was then examined in DMF solvent. Na2CO3 proved to be better than K2CO3, which led to 2a in 54% yield (Table 1, entry 7). In comparison, NaHCO3 and organic bases (NEt3 and TMEDA) resulted in lower yields (Table 1, entries 8–10). Moreover, ligand effect was investigated. Although (o-Tol)3P and tBu3P turned out to be unsuitable ligands (Table 1, entries 11 and 12), the yield of 2a was markedly improved to 72% for JohnPhos and to 84% for tBu-XPhos, respectively (Table 1, entries 13 and 14). This observation might imply that JohnPhos and tBu-XPhos matched the electronically and sterically demanding of the oxidative addition and reductive elimination processes. In addition, RuPhos and DavePhos also furnished product 2a in satisfactory yields (Table 1, entries 15 and 16). Of note, bidentate phosphine ligands, such as dppe and XantPhos, fully suppressed the reaction. Moreover, water proved to be critical to the reaction as the reaction yield was dramatically decreased to 16% when adding 4 Å molecular sieves to the mixture in the absence of water (Table 1, entry 17). Other tests showed that no reaction occurred in pure water and a diminished yield (58%) in a mixed solvent of DMF/H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Moreover, lowering the temperature remarkably decreased the yields even prolonging the reaction time to 48 h (62% for 80 °C and 30% for 60 °C, respectively).
1). Moreover, lowering the temperature remarkably decreased the yields even prolonging the reaction time to 48 h (62% for 80 °C and 30% for 60 °C, respectively).
| Entry | L | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 5 mol% Pd(OAc)2, L (10 mol%), base (2.0 equiv.), H2O (2.0 equiv.), and solvent (2.0 mL) at 100 °C for 12 h.b Isolated yield.c No additional water and 100 mg 4 Å molecular sieves was added. | ||||
| 1 | PPh3 | K2CO3 | THF | 15 |
| 2 | PPh3 | K2CO3 | MeOH | ND |
| 3 | PPh3 | K2CO3 | 1,4-Dioxane | 20 |
| 4 | PPh3 | K2CO3 | Toluene | 10 |
| 5 | PPh3 | K2CO3 | DMF | 45 |
| 6 | PPh3 | K2CO3 | NMP | 40 |
| 7 | PPh3 | Na2CO3 | DMF | 54 |
| 8 | PPh3 | NaHCO3 | DMF | 24 |
| 9 | PPh3 | NEt3 | DMF | 46 |
| 10 | PPh3 | TMEDA | DMF | 20 |
| 11 | (o-Tol)3P | Na2CO3 | DMF | 50 |
| 12 | tBu3P·HBF4 | Na2CO3 | DMF | 30 |
| 13 | JohnPhos | Na2CO3 | DMF | 72 |
| 14 | tBu-XPhos | Na2CO3 | DMF | 84 |
| 15 | RuPhos | Na2CO3 | DMF | 75 |
| 16 | DavePhos | Na2CO3 | DMF | 65 |
| 17c | tBu-XPhos | Na2CO3 | DMF | 16 |
With the optimal reaction conditions in hand, we then investigated the scope of the intramolecular deacetylative dearomatization reaction. As shown in Scheme 2, a range of N-(2-iodobenzoyl)-3-acetoxyindoles were tested and all the reactions proceeded smoothly to afford the desired tetracyclic indolin-3-one products 2 in moderate to excellent yields. The analogous bromo-substrate led to a relatively lower yield (74%) of product 2a. Either electron-withdrawing or electron-donating substituents on the benzene ring of 2-iodobenzoyl moiety were well tolerated, furnishing indolin-3-ones 2b–2g in the yields ranging from 72% to 92%. The electron-donating substituents resulted in relatively higher yields. Note that a longer reaction time (18 h) was needed for the ortho-methyl product 2g. The effect of substituted groups attached on the indole ring was then examined. A range of functionalities at C5 of indole, including methyl, halide, methoxyl, –CF3, and –CO2Me, were compatible to this deacetylative dearomatization reaction, which smoothly delivered products 2h–2n in moderate to good yields, albeit relatively lower yields were observed for those substrates bearing electron-withdrawing substituents. It's worthy to note that a bromine atom of product 2l survived under the reaction conditions, which kept the potential for further functionalization. In addition, substrates having substituents at C6 also led to the desired products 2o–2q in good yields.
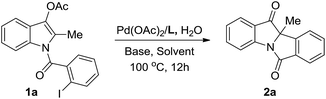
To demonstrate the synthetic utility of the reaction, several transformations of the indolin-3-one products were then conducted. A reduction of indolin-3-one 2a with LiAlH4 gave 3-hydroxylindoline 3 in 87% yield as a single isomer, which provides an efficient method to access tetracyclic indolin-3-ol in high diastereoselectivity (Scheme 3, eqn (1)). The Suzuki and Sonogashira reactions of product 2l having a bromide group were established, which furnished the coupling products 4 and 5 in 86% and 92% yields, respectively (Scheme 3, eqn (2) and (3)). The extended conjugate structure might find potential application as material molecules. In addition, a preliminary enantioselective reaction was also studied. As elucidated in Scheme 4, (R,R,R)-MONOPHOS-PE proved to be a potential ligand in the reaction of 1a′, leading to optically active indolin-3-one 2a in 63% ee albeit with a lower yield.
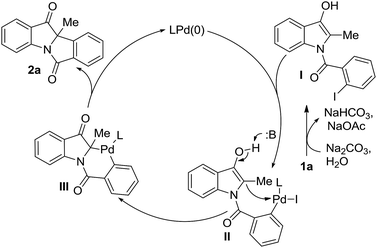
A tentative mechanism is depicted in Scheme 5. Considering the key role of water in this reaction (Table 1, entry 17), an initial hydrolysis of 3-acetoxy indole 1a in the presence of base is proposed to deliver 3-hydroxyindole I. An adduct II was then achieved through oxidative addition of Pd(0) to compound I. Facilitated by a base-mediated keto–enol tautomerism, nucleophilic attack of indole at C2 to Pd-center furnishes organopalladium III. Subsequent reductive elimination affords product 2a and fulfils the catalytic cycle.
Conclusions
In summary, we have developed an efficient intramolecular deacetylative dearomatization reaction of 3-acetoxyindoles by employing the complex of Pd(OAc)2/tBu-XPhos as a catalyst. This protocol provides a rapid access to a range of tetracyclic indolin-3-ones in moderate to good yields. Meanwhile, a preliminary enantioselective reaction is developed to afford the product in 63% ee by using (R,R,R)-phosphoramide-PE as a chiral ligand.Experimental section
General information
Reactions and manipulations involving organometallic or moisture sensitive compounds were carried out under dry nitrogen and glassware heated under oven for two hours prior to use. 1H NMR spectra were recorded on 500 MHz in CDCl3, 13C NMR spectra were recorded on 125 MHz in CDCl3. Melting points were determined on a microscopic apparatus and were uncorrected. Commercial reagents were used as received without further purification unless otherwise noticed. HRMS were recorded on a TOF LC/MS mass spectrometer equipped with an ESI source. Column chromatography was carried out using silica gel (200–300 mesh). Anhydrous THF, 1,4-dioxane, and toluene were freshly distilled over Na and benzophenone. Anhydrous methanol was freshly distilled over Mg. Anhydrous DMF and NMP were freshly distilled over calcium hydride under reduced pressure. 3-Acetoxyindoles 1 was prepared (5.0 mmol scale) according to the known method.11Synthesis of 3-acetoxyindole derivatives 1
According to the known methods,11 to a stirred solution of N-2-iodobenzoyl indole derivatives6a (5.0 mmol) in HOAc (20 mL) was added PhI(OAc)2 (3.22 g, 10.0 mmol). The mixture was then stirred in oil-bath at 70 °C for 12 h. After the reaction was completed, HOAc was removed under vacuum. H2O (5 mL) was added and the solution was extracted with EtOAc (3 × 20 mL). The combined organic layer was dried over Na2SO4 and concentrated under reduced pressure. The crude was purified by column chromatography on silica gel (ethyl acetate/petroleum ether, v/v = 1/20–1/5) to give 1.1-(2-Iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1a)
628 mg, 30% yield, yellow solid, mp 107–109 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.4 Hz, 1H), 7.44 (m, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.29 (d, J = 7.7 Hz, 1H), 7.25–7.19 (m, 2H), 7.14 (t, J = 7.6 Hz, 1H), 2.40 (s, 3H), 2.09 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.8, 168.5, 142.0, 140.0, 134.0, 133.8, 132.1, 129.1, 128.6, 126.2, 124.7, 124.0, 123.8, 116.9, 115.3, 93.1, 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H15INO3 ([M + H]+): 441.9911, found 441.9900.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.4 Hz, 1H), 7.44 (m, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.29 (d, J = 7.7 Hz, 1H), 7.25–7.19 (m, 2H), 7.14 (t, J = 7.6 Hz, 1H), 2.40 (s, 3H), 2.09 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.8, 168.5, 142.0, 140.0, 134.0, 133.8, 132.1, 129.1, 128.6, 126.2, 124.7, 124.0, 123.8, 116.9, 115.3, 93.1, 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H15INO3 ([M + H]+): 441.9911, found 441.9900.
1-(2-Iodo-5-methylbenzoyl)-2-methyl-1H-indol-3-yl acetate (1b)
650 mg, 30% yield, yellow solid, mp 112–114 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.28 (m, 2H), 7.25–7.19 (m, 1H), 7.17–7.12 (m, 1H), 7.06 (dd, J = 8.0, 1.7 Hz, 1H), 2.40 (s, 3H), 2.35 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.9, 168.5, 141.8, 139.7, 139.0, 134.0, 133.7, 133.1, 129.7, 126.2, 124.7, 124.0, 123.7, 116.9, 115.3, 89.0, 20.9, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0058.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.28 (m, 2H), 7.25–7.19 (m, 1H), 7.17–7.12 (m, 1H), 7.06 (dd, J = 8.0, 1.7 Hz, 1H), 2.40 (s, 3H), 2.35 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.9, 168.5, 141.8, 139.7, 139.0, 134.0, 133.7, 133.1, 129.7, 126.2, 124.7, 124.0, 123.7, 116.9, 115.3, 89.0, 20.9, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0058.
1-(2-Iodo-5-methoxybenzoyl)-2-methyl-1H-indol-3-yl ace-tate (1c)
562 mg, 25% yield, yellow solid, mp 104–106 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.8 Hz, 1H), 7.36 (m, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.23 (m, 1H), 7.19–7.12 (m, 1H), 6.99 (d, J = 3.0 Hz, 1H), 6.83 (dd, J = 8.8, 3.1 Hz, 1H), 3.80 (s, 3H), 2.40 (s, 3H), 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 168.6, 168.5, 160.1, 142.7, 140.7, 134.0, 133.9, 126.2, 124.8, 124.0, 123.8, 118.9, 116.9, 115.3, 114.3, 81.4, 55.7, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO4Na ([M + Na]+): 472.0016, found 472.0013.
10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.8 Hz, 1H), 7.36 (m, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.23 (m, 1H), 7.19–7.12 (m, 1H), 6.99 (d, J = 3.0 Hz, 1H), 6.83 (dd, J = 8.8, 3.1 Hz, 1H), 3.80 (s, 3H), 2.40 (s, 3H), 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 168.6, 168.5, 160.1, 142.7, 140.7, 134.0, 133.9, 126.2, 124.8, 124.0, 123.8, 118.9, 116.9, 115.3, 114.3, 81.4, 55.7, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO4Na ([M + Na]+): 472.0016, found 472.0013.
1-(5-Fluoro-2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1d)
590 mg, 27% yield, yellow solid, mp 128–130 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.87 (m, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 7.5 Hz, 1H), 7.27–7.24 (m, 1H), 7.19 (m, 2H), 7.03–6.97 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 167.4, 162.8 (d, J = 250.0 Hz), 143.5 (d, J = 6.3 Hz), 141.6 (d, J = 7.5 Hz), 134.1, 133.9, 125.9, 124.9, 124.1, 124.0, 119.7 (d, J = 21.3 Hz), 117.1, 116.6 (d, J = 23.8 Hz), 115.2, 86.2 (d, J = 3.8 Hz), 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9805.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.87 (m, 1H), 7.38 (d, J = 8.0 Hz, 1H), 7.30 (d, J = 7.5 Hz, 1H), 7.27–7.24 (m, 1H), 7.19 (m, 2H), 7.03–6.97 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 167.4, 162.8 (d, J = 250.0 Hz), 143.5 (d, J = 6.3 Hz), 141.6 (d, J = 7.5 Hz), 134.1, 133.9, 125.9, 124.9, 124.1, 124.0, 119.7 (d, J = 21.3 Hz), 117.1, 116.6 (d, J = 23.8 Hz), 115.2, 86.2 (d, J = 3.8 Hz), 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9805.
1-(4-Chloro-2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1e)
680 mg, 30% yield, yellow solid, mp 52–53 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 1.9 Hz, 1H), 7.49 (m, 1H), 7.36 (m, 2H), 7.30 (d, J = 7.6 Hz, 1H), 7.25–7.21 (m, 1H), 7.19–7.14 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 168.0, 140.4, 139.6, 137.3, 134.0, 133.9, 129.9, 129.0, 126.0, 124.8, 124.1, 123.9, 117.1, 115.1, 93.5, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9512.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 1.9 Hz, 1H), 7.49 (m, 1H), 7.36 (m, 2H), 7.30 (d, J = 7.6 Hz, 1H), 7.25–7.21 (m, 1H), 7.19–7.14 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 168.0, 140.4, 139.6, 137.3, 134.0, 133.9, 129.9, 129.0, 126.0, 124.8, 124.1, 123.9, 117.1, 115.1, 93.5, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9512.
1-(5-Chloro-2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1f)
680 mg, 30% yield, yellow solid, mp 112–113 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 7.40 (d, J = 6.6 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.26–7.21 (m, 2H), 7.19 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 167.4, 143.4, 141.1, 135.3, 134.1, 133.9, 132.2, 129.0, 125.9, 124.9, 124.1, 124.0, 117.1, 115.2, 90.2, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9513.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 8.4 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 7.40 (d, J = 6.6 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.26–7.21 (m, 2H), 7.19 (m, 1H), 2.40 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.4, 167.4, 143.4, 141.1, 135.3, 134.1, 133.9, 132.2, 129.0, 125.9, 124.9, 124.1, 124.0, 117.1, 115.2, 90.2, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9513.
1-(2-Iodo-3-methylbenzoyl)-2-methyl-1H-indol-3-yl acetate (1g)
650 mg, 30% yield, yellow solid, mp 119–120 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.39 (s, 2H), 7.37 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 7.3 Hz, 1H), 7.22–7.19 (m, 1H), 7.15 (t, J = 7.5 Hz, 1H), 2.52 (s, 3H), 2.40 (s, 3H), 2.08 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.4, 168.5, 143.4, 143.3, 134.1, 133.8, 131.4, 128.7, 126.3, 126.1, 124.7, 124.0, 123.8, 116.9, 115.5, 99.6, 28.8, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0069.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.39 (s, 2H), 7.37 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 7.3 Hz, 1H), 7.22–7.19 (m, 1H), 7.15 (t, J = 7.5 Hz, 1H), 2.52 (s, 3H), 2.40 (s, 3H), 2.08 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 169.4, 168.5, 143.4, 143.3, 134.1, 133.8, 131.4, 128.7, 126.3, 126.1, 124.7, 124.0, 123.8, 116.9, 115.5, 99.6, 28.8, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0069.
1-(2-Iodobenzoyl)-2,5-dimethyl-1H-indol-3-yl acetate (1h)
606 mg, 28% yield, yellow solid, mp 116–118 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.7 Hz, 1H), 7.49 (m, 1H), 7.44–7.41 (m, 1H), 7.25–7.15 (m, 2H), 7.09–6.90 (m, 2H), 2.40 (s, 3H), 2.39 (s, 3H), 2.08 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.7, 168.6, 142.1, 141.9, 139.9, 133.5, 132.3, 131.9, 129.0, 128.6, 128.0, 126.0, 124.2, 116.8, 115.0, 93.1, 21.3, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0060.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.7 Hz, 1H), 7.49 (m, 1H), 7.44–7.41 (m, 1H), 7.25–7.15 (m, 2H), 7.09–6.90 (m, 2H), 2.40 (s, 3H), 2.39 (s, 3H), 2.08 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.7, 168.6, 142.1, 141.9, 139.9, 133.5, 132.3, 131.9, 129.0, 128.6, 128.0, 126.0, 124.2, 116.8, 115.0, 93.1, 21.3, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0060.
1-(2-Iodobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl ace-tate (1i)
404 mg, 18% yield, yellow solid, mp 132–134 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.9 Hz, 1H), 7.49 (t, J = 7.5 Hz, 1H), 7.43 (m, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.23 (m, 1H), 6.77–6.70 (m, 2H), 3.82 (s, 3H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.5, 168.4, 156.6, 142.1, 139.9, 133.8, 131.9, 128.9, 128.59, 128.55, 126.8, 125.0, 116.4, 113.0, 99.9, 93.1, 55.6, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H16INO4Na ([M + Na]+): 472.0016, found 472.0006.
10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.9 Hz, 1H), 7.49 (t, J = 7.5 Hz, 1H), 7.43 (m, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.23 (m, 1H), 6.77–6.70 (m, 2H), 3.82 (s, 3H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.5, 168.4, 156.6, 142.1, 139.9, 133.8, 131.9, 128.9, 128.59, 128.55, 126.8, 125.0, 116.4, 113.0, 99.9, 93.1, 55.6, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H16INO4Na ([M + Na]+): 472.0016, found 472.0006.
5-Chloro-1-(2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1j)
454 mg, 20% yield, yellow solid, mp 117–119 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 7.6 Hz, 1H), 7.51 (td, J = 7.6, 0.8 Hz, 1H), 7.44 (dd, J = 7.6, 1.6 Hz, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.27 (d, J = 1.9 Hz, 1H), 7.26–7.24 (m, 1H), 7.11 (dd, J = 8.9, 2.1 Hz, 1H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.4, 141.6, 140.0, 132.9, 132.4, 132.3, 129.6, 129.1, 128.7, 127.7, 125.3, 124.8, 116.8, 116.4, 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9515.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 7.6 Hz, 1H), 7.51 (td, J = 7.6, 0.8 Hz, 1H), 7.44 (dd, J = 7.6, 1.6 Hz, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.27 (d, J = 1.9 Hz, 1H), 7.26–7.24 (m, 1H), 7.11 (dd, J = 8.9, 2.1 Hz, 1H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.4, 141.6, 140.0, 132.9, 132.4, 132.3, 129.6, 129.1, 128.7, 127.7, 125.3, 124.8, 116.8, 116.4, 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9515.
5-Fluoro-1-(2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1k)
437 mg, 20% yield, yellow solid, mp 114–116 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.95–7.91 (m, 1H), 7.52–7.49 (m, 1H), 7.44 (m, J = 7.6, 1.6 Hz, 2H), 7.25 (m, J = 7.6, 6.0 Hz, 1H), 6.96 (dd, J = 8.2, 2.6 Hz, 1H), 6.89 (td, J = 9.1, 2.6 Hz, 1H), 2.39 (s, 3H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 159.7 (d, J = 241.3 Hz), 141.8, 140.0, 133.4 (d, J = 3.8 Hz), 132.2, 130.3, 129.0, 128.6, 127.8, 125.2 (d, J = 8.8 Hz), 116.7 (d, J = 8.8 Hz), 112.4 (d, J = 23.8 Hz), 103.0 (d, J = 25.0 Hz), 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9813.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.95–7.91 (m, 1H), 7.52–7.49 (m, 1H), 7.44 (m, J = 7.6, 1.6 Hz, 2H), 7.25 (m, J = 7.6, 6.0 Hz, 1H), 6.96 (dd, J = 8.2, 2.6 Hz, 1H), 6.89 (td, J = 9.1, 2.6 Hz, 1H), 2.39 (s, 3H), 2.03 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 159.7 (d, J = 241.3 Hz), 141.8, 140.0, 133.4 (d, J = 3.8 Hz), 132.2, 130.3, 129.0, 128.6, 127.8, 125.2 (d, J = 8.8 Hz), 116.7 (d, J = 8.8 Hz), 112.4 (d, J = 23.8 Hz), 103.0 (d, J = 25.0 Hz), 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9813.
5-Bromo-1-(2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1l)
697 mg, 28% yield, yellow solid, mp 143–145 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 7.7 Hz, 1H), 7.51 (td, J = 7.5, 0.7 Hz, 1H), 7.46–7.41 (m, 2H), 7.28–7.23 (m, 3H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 141.6, 140.0, 132.7, 132.7, 132.3, 129.1, 128.7, 127.6, 127.5, 125.7, 119.8, 117.2, 116.7, 93.0, 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H13BrINO3Na ([M + Na]+): 519.9016, found 519.9003.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 7.7 Hz, 1H), 7.51 (td, J = 7.5, 0.7 Hz, 1H), 7.46–7.41 (m, 2H), 7.28–7.23 (m, 3H), 2.40 (s, 3H), 2.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 141.6, 140.0, 132.7, 132.7, 132.3, 129.1, 128.7, 127.6, 127.5, 125.7, 119.8, 117.2, 116.7, 93.0, 20.5, 12.1. HRMS m/z (ESI+): calculated for C18H13BrINO3Na ([M + Na]+): 519.9016, found 519.9003.
1-(2-Iodobenzoyl)-2-methyl-5-(trifluoromethyl)-1H-indol-3-yl acetate (1m)
487 mg, 20% yield, white solid, mp 139–141 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.97–7.92 (m, 1H), 7.57 (s, 1H), 7.55–7.50 (m, 1H), 7.46 (dd, J = 7.8, 1.5 Hz, 2H), 7.42–7.38 (m, 1H), 7.30–7.26 (m, 1H), 2.43 (s, 3H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.8, 168.4, 141.4, 140.2, 135.5, 133.4, 132.5, 129.3, 128.8, 128.3, 126.1 (q, J = 32.5 Hz), 124.4 (q, J = 270.0 Hz), 123.9, 121.5 (q, J = 3.8 Hz), 115.5, 114.6 (q, J = 5.0 Hz), 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H13F3INO3Na ([M + Na]+): 509.9784, found 509.9776.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.97–7.92 (m, 1H), 7.57 (s, 1H), 7.55–7.50 (m, 1H), 7.46 (dd, J = 7.8, 1.5 Hz, 2H), 7.42–7.38 (m, 1H), 7.30–7.26 (m, 1H), 2.43 (s, 3H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.8, 168.4, 141.4, 140.2, 135.5, 133.4, 132.5, 129.3, 128.8, 128.3, 126.1 (q, J = 32.5 Hz), 124.4 (q, J = 270.0 Hz), 123.9, 121.5 (q, J = 3.8 Hz), 115.5, 114.6 (q, J = 5.0 Hz), 93.0, 20.5, 12.2. HRMS m/z (ESI+): calculated for C19H13F3INO3Na ([M + Na]+): 509.9784, found 509.9776.
Methyl 3-acetoxy-1-(2-iodobenzoyl)-2-methyl-1H-indole-5-carboxylate (1n)
597 mg, 25% yield, white solid, mp 148–150 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 1.2 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.85 (dd, J = 8.8, 1.6 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.47 (m, 1H), 7.35 (d, J = 8.8 Hz, 1H), 7.27 (m, 1H), 3.92 (s, 3H), 2.43 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.7, 168.4, 166.9, 141.4, 140.1, 136.5, 133.6, 132.4, 129.2, 128.7, 127.8, 125.9, 125.6, 123.9, 119.1, 114.8, 93.0, 52.1, 20.5, 12.1. HRMS m/z (ESI+): calculated for C20H16INO5Na ([M + Na]+): 499.9965, found 499.9953.
5 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 8.02 (d, J = 1.2 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.85 (dd, J = 8.8, 1.6 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.47 (m, 1H), 7.35 (d, J = 8.8 Hz, 1H), 7.27 (m, 1H), 3.92 (s, 3H), 2.43 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.7, 168.4, 166.9, 141.4, 140.1, 136.5, 133.6, 132.4, 129.2, 128.7, 127.8, 125.9, 125.6, 123.9, 119.1, 114.8, 93.0, 52.1, 20.5, 12.1. HRMS m/z (ESI+): calculated for C20H16INO5Na ([M + Na]+): 499.9965, found 499.9953.
1-(2-Iodobenzoyl)-2,6-dimethyl-1H-indol-3-yl acetate (1o)
433 mg, 20% yield, yellow solid, mp 57–58 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.94–7.91 (m, 1H), 7.50–7.41 (m, 3H), 7.23 (m, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.07 (d, J = 8.0 Hz, 1H), 2.38 (s, 3H), 2.35 (s, 3H), 1.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.9, 168.4, 142.1, 139.8, 134.9, 134.5, 134.0, 131.9, 129.0, 128.5, 125.2, 124.9, 121.7, 116.5, 116.0, 93.2, 22.0, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0059.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.94–7.91 (m, 1H), 7.50–7.41 (m, 3H), 7.23 (m, 1H), 7.17 (d, J = 7.9 Hz, 1H), 7.07 (d, J = 8.0 Hz, 1H), 2.38 (s, 3H), 2.35 (s, 3H), 1.96 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.9, 168.4, 142.1, 139.8, 134.9, 134.5, 134.0, 131.9, 129.0, 128.5, 125.2, 124.9, 121.7, 116.5, 116.0, 93.2, 22.0, 20.5, 12.1. HRMS m/z (ESI+): calculated for C19H16INO3Na ([M + Na]+): 456.0067, found 456.0059.
6-Chloro-1-(2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1p)
408 mg, 18% yield, yellow solid, mp 110–112 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.7 Hz, 1H), 7.65 (s, 1H), 7.50 (m, 1H), 7.43 (m, 1H), 7.27–7.19 (m, 3H), 2.37 (s, 3H), 1.95 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 141.5, 139.9, 134.3, 133.4, 132.2, 130.8, 129.0, 128.6, 126.5, 124.4, 122.5, 117.7, 115.9, 93.0, 20.4, 12.1. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9512.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 7.7 Hz, 1H), 7.65 (s, 1H), 7.50 (m, 1H), 7.43 (m, 1H), 7.27–7.19 (m, 3H), 2.37 (s, 3H), 1.95 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 141.5, 139.9, 134.3, 133.4, 132.2, 130.8, 129.0, 128.6, 126.5, 124.4, 122.5, 117.7, 115.9, 93.0, 20.4, 12.1. HRMS m/z (ESI+): calculated for C18H13ClINO3Na ([M + Na]+): 475.9521, found 475.9512.
6-Fluoro-1-(2-iodobenzoyl)-2-methyl-1H-indol-3-yl acetate (1q)
437 mg, 20% yield, yellow solid, mp 96–98 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 7.9 Hz, 1H), 7.52 (m, 1H), 7.44 (m, 1H), 7.28 (d, J = 1.5 Hz, 1H), 7.26–7.19 (m, 2H), 7.00 (td, J = 8.8, 2.2 Hz, 1H), 2.39 (s, 3H), 2.00 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 160.9 (d, J = 240.0 Hz), 141.5, 139.9, 134.1 (d, J = 11.3 Hz), 133.3, 132.2, 128.7 (d, J = 40.0 Hz), 126.1, 126.0, 120.3, 117.6 (d, J = 10.0 Hz), 111.9 (d, J = 23.8 Hz), 103.2 (d, J = 28.8 Hz), 92.9, 20.4, 12.0. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9809.
20 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 7.9 Hz, 1H), 7.52 (m, 1H), 7.44 (m, 1H), 7.28 (d, J = 1.5 Hz, 1H), 7.26–7.19 (m, 2H), 7.00 (td, J = 8.8, 2.2 Hz, 1H), 2.39 (s, 3H), 2.00 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 168.6, 168.3, 160.9 (d, J = 240.0 Hz), 141.5, 139.9, 134.1 (d, J = 11.3 Hz), 133.3, 132.2, 128.7 (d, J = 40.0 Hz), 126.1, 126.0, 120.3, 117.6 (d, J = 10.0 Hz), 111.9 (d, J = 23.8 Hz), 103.2 (d, J = 28.8 Hz), 92.9, 20.4, 12.0. HRMS m/z (ESI+): calculated for C18H13FINO3Na ([M + Na]+): 459.9816, found 459.9809.
General procedure for the deacetylative dearomatization of 3-acetoxyindoles
To a Schlenk tube were added Pd(OAc)2 (5 mol%), tBu-XPhos (10 mol%), Na2CO3 (2.0 equiv., 0.4 mmol), indole 1 (0.2 mmol), and 2.0 mL anhydrous DMF under N2, after which H2O (2.0 equiv., 0.4 mmol) was added to the reaction mixture by a syringe. The mixture was then stirred in oil-bath at 100 °C for 12–18 h. When the reaction was completed, the mixture was extracted with EtOAc (3 × 10 mL) and the combined organic layer was concentrated under reduced pressure. The residue was then purified by column chromatography on silica gel (ethyl acetate/petroleum ether, v/v = 1/20–1/10) to give products 2.10b-Methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2a)
42.0 mg, 84% yield, white solid, mp 135–136 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz, 1H), 7.83 (m, 2H), 7.78–7.73 (m, 2H), 7.73–7.68 (m, 1H), 7.53 (m, 1H), 7.29 (t, J = 7.5 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.4, 169.6, 151.7, 145.6, 137.4, 134.4, 131.1, 129.5, 127.5, 125.3, 125.24, 125.16, 122.8, 118.6, 73.6, 25.9. HRMS m/z (ESI+): calculated for C16H11NO2Na ([M + Na]+): 272.0682, found 272.0672. For the reaction with (R,R,R)-phosphoramide-PE as a chiral ligand: 30% yield, 63% ee [Daicel Chiralpak AD-H column (25 cm × 0.46 cm ID), nhexane/iPrOH = 95/5, 0.7 mL min−1, 254 nm; tmajor = 18.6 min, tminor = 21.4 min].
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz, 1H), 7.83 (m, 2H), 7.78–7.73 (m, 2H), 7.73–7.68 (m, 1H), 7.53 (m, 1H), 7.29 (t, J = 7.5 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.4, 169.6, 151.7, 145.6, 137.4, 134.4, 131.1, 129.5, 127.5, 125.3, 125.24, 125.16, 122.8, 118.6, 73.6, 25.9. HRMS m/z (ESI+): calculated for C16H11NO2Na ([M + Na]+): 272.0682, found 272.0672. For the reaction with (R,R,R)-phosphoramide-PE as a chiral ligand: 30% yield, 63% ee [Daicel Chiralpak AD-H column (25 cm × 0.46 cm ID), nhexane/iPrOH = 95/5, 0.7 mL min−1, 254 nm; tmajor = 18.6 min, tminor = 21.4 min].
8,10b-Dimethyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2b)
44.7 mg, 85% yield, yellow solid, mp 105–107 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.1 Hz, 1H), 7.78–7.72 (m, 2H), 7.69 (d, J = 7.8 Hz, 1H), 7.64 (s, 1H), 7.53–7.50 (m, 1H), 7.29 (d, J = 7.4 Hz, 1H), 2.44 (s, 3H), 1.78 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.8, 169.9, 151.8, 143.1, 139.9, 137.4, 135.6, 131.3, 127.6, 125.4, 125.3, 125.2, 122.5, 118.7, 73.5, 26.0, 21.4. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1016.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.1 Hz, 1H), 7.78–7.72 (m, 2H), 7.69 (d, J = 7.8 Hz, 1H), 7.64 (s, 1H), 7.53–7.50 (m, 1H), 7.29 (d, J = 7.4 Hz, 1H), 2.44 (s, 3H), 1.78 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.8, 169.9, 151.8, 143.1, 139.9, 137.4, 135.6, 131.3, 127.6, 125.4, 125.3, 125.2, 122.5, 118.7, 73.5, 26.0, 21.4. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1016.
8-Methoxy-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2c)
51.4 mg, 92% yield, white solid, mp 110–112 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.0 Hz, 1H), 7.79–7.73 (m, 2H), 7.70 (d, J = 8.4 Hz, 1H), 7.31–7.27 (m, 2H), 7.27–7.25 (m, 1H), 3.86 (s, 3H), 1.77 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.7, 169.8, 161.1, 151.6, 138.2, 137.4, 132.5, 127.8, 125.4, 125.2, 123.7, 122.9, 118.7, 107.6, 73.3, 55.8, 25.9. HRMS m/z (ESI+): calculated for C17H13NO3Na ([M + Na]+): 302.0788, found 302.0788.
10 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.0 Hz, 1H), 7.79–7.73 (m, 2H), 7.70 (d, J = 8.4 Hz, 1H), 7.31–7.27 (m, 2H), 7.27–7.25 (m, 1H), 3.86 (s, 3H), 1.77 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.7, 169.8, 161.1, 151.6, 138.2, 137.4, 132.5, 127.8, 125.4, 125.2, 123.7, 122.9, 118.7, 107.6, 73.3, 55.8, 25.9. HRMS m/z (ESI+): calculated for C17H13NO3Na ([M + Na]+): 302.0788, found 302.0788.
8-Fluoro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2d)
39.0 mg, 73% yield, yellow solid, mp 108–110 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 7.9 Hz, 1H), 7.78 (m, 3H), 7.50 (dd, J = 7.4, 2.4 Hz, 1H), 7.41 (td, J = 8.6, 2.4 Hz, 1H), 7.34–7.28 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.0, 168.43, 168.41, 163.6 (d, J = 248.8 Hz), 151.3, 141.2 (d, J = 1.3 Hz), 137.5, 133.3 (d, J = 7.5 Hz), 127.5, 125.4 (d, J = 6.3 Hz), 124.4 (d, J = 8.8 Hz), 122.1, 121.9, 118.6, 111.7 (d, J = 22.5 Hz), 73.2, 25.9. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 268.0768, found 268.0768.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 7.9 Hz, 1H), 7.78 (m, 3H), 7.50 (dd, J = 7.4, 2.4 Hz, 1H), 7.41 (td, J = 8.6, 2.4 Hz, 1H), 7.34–7.28 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.0, 168.43, 168.41, 163.6 (d, J = 248.8 Hz), 151.3, 141.2 (d, J = 1.3 Hz), 137.5, 133.3 (d, J = 7.5 Hz), 127.5, 125.4 (d, J = 6.3 Hz), 124.4 (d, J = 8.8 Hz), 122.1, 121.9, 118.6, 111.7 (d, J = 22.5 Hz), 73.2, 25.9. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 268.0768, found 268.0768.
9-Chloro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2e)
40.8 mg, 72% yield, yellow solid, mp 159–161 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 1.6 Hz, 1H), 7.76 (m, 3H), 7.50 (dd, J = 8.2, 1.8 Hz, 1H), 7.30 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.7, 168.5, 151.5, 147.0, 141.1, 137.6, 130.3, 129.6, 127.3, 126.4, 125.4, 123.3, 118.6, 73.1, 25.9. HRMS m/z (ESI+): calculated for C16H11ClNO2 ([M + H]+): 284.0473, found 284.0466.
20 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 1.6 Hz, 1H), 7.76 (m, 3H), 7.50 (dd, J = 8.2, 1.8 Hz, 1H), 7.30 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.7, 168.5, 151.5, 147.0, 141.1, 137.6, 130.3, 129.6, 127.3, 126.4, 125.4, 123.3, 118.6, 73.1, 25.9. HRMS m/z (ESI+): calculated for C16H11ClNO2 ([M + H]+): 284.0473, found 284.0466.
8-Chloro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2f)
46.5 mg, 82% yield, yellow solid, mp 125–127 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.79–7.74 (m, 3H), 7.67 (m, 1H), 7.31 (t, J = 7.5 Hz, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 168.2, 151.3, 143.7, 137.6, 136.0, 134.5, 132.9, 127.5, 125.5, 125.4, 125.2, 124.0, 118.6, 73.3, 25.9. HRMS m/z (ESI+): calculated for C16H10ClNO2Na ([M + Na]+): 306.0292, found 306.0286.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 1.8 Hz, 1H), 7.79–7.74 (m, 3H), 7.67 (m, 1H), 7.31 (t, J = 7.5 Hz, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 168.2, 151.3, 143.7, 137.6, 136.0, 134.5, 132.9, 127.5, 125.5, 125.4, 125.2, 124.0, 118.6, 73.3, 25.9. HRMS m/z (ESI+): calculated for C16H10ClNO2Na ([M + Na]+): 306.0292, found 306.0286.
10,10b-Dimethyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2g)
40.0 mg, 76% yield, yellow solid, mp 40–41 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz, 1H), 7.78–7.71 (m, 2H), 7.68 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.28 (t, J = 5.0 Hz, 1H), 2.84 (s, 3H), 1.88 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.5, 169.0, 150.7, 143.5, 137.3, 136.5, 135.0, 132.0, 129.4, 127.8, 125.2, 125.0, 122.6, 118.3, 74.9, 24.4, 19.3. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1011.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz, 1H), 7.78–7.71 (m, 2H), 7.68 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.28 (t, J = 5.0 Hz, 1H), 2.84 (s, 3H), 1.88 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.5, 169.0, 150.7, 143.5, 137.3, 136.5, 135.0, 132.0, 129.4, 127.8, 125.2, 125.0, 122.6, 118.3, 74.9, 24.4, 19.3. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1011.
2,10b-Dimethyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2h)
47.4 mg, 90% yield, yellow solid, mp 97–98 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.89–7.78 (m, 3H), 7.69 (t, J = 7.5 Hz, 1H), 7.56 (d, J = 8.5 Hz, 2H), 7.52 (t, J = 7.5 Hz, 1H), 2.40 (s, 3H), 1.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.4, 169.6, 149.7, 145.6, 138.4, 135.2, 134.2, 131.2, 129.4, 127.6, 125.1, 125.0, 122.7, 118.2, 73.8, 25.9, 20.9. HRMS m/z (ESI+): calculated for C17H13NO2Na ([M + Na]+): 286.0838, found 286.0829.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.89–7.78 (m, 3H), 7.69 (t, J = 7.5 Hz, 1H), 7.56 (d, J = 8.5 Hz, 2H), 7.52 (t, J = 7.5 Hz, 1H), 2.40 (s, 3H), 1.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.4, 169.6, 149.7, 145.6, 138.4, 135.2, 134.2, 131.2, 129.4, 127.6, 125.1, 125.0, 122.7, 118.2, 73.8, 25.9, 20.9. HRMS m/z (ESI+): calculated for C17H13NO2Na ([M + Na]+): 286.0838, found 286.0829.
2-Methoxy-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2i)
44.7 mg, 80% yield, white solid, mp 87–88 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 1H), 7.82 (dd, J = 14.8, 7.7 Hz, 2H), 7.70 (td, J = 7.7, 1.0 Hz, 1H), 7.53 (m, 1H), 7.35 (dd, J = 8.8, 2.7 Hz, 1H), 7.19 (d, J = 2.7 Hz, 1H), 3.83 (s, 3H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.5, 169.9, 157.5, 146.2, 145.5, 134.3, 131.2, 129.5, 128.3, 126.1, 125.2, 122.7, 119.6, 106.5, 74.3, 55.9, 25.9. HRMS m/z (ESI+): calculated for C17H13NO3Na ([M + Na]+): 302.0788, found 302.0778.
10 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 8.8 Hz, 1H), 7.82 (dd, J = 14.8, 7.7 Hz, 2H), 7.70 (td, J = 7.7, 1.0 Hz, 1H), 7.53 (m, 1H), 7.35 (dd, J = 8.8, 2.7 Hz, 1H), 7.19 (d, J = 2.7 Hz, 1H), 3.83 (s, 3H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.5, 169.9, 157.5, 146.2, 145.5, 134.3, 131.2, 129.5, 128.3, 126.1, 125.2, 122.7, 119.6, 106.5, 74.3, 55.9, 25.9. HRMS m/z (ESI+): calculated for C17H13NO3Na ([M + Na]+): 302.0788, found 302.0778.
2-Chloro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2j)
47.6 mg, 84% yield, yellow solid, mp 118–120 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 7.7 Hz, 1H), 7.80 (d, J = 7.7 Hz, 1H), 7.74–7.68 (m, 3H), 7.57–7.52 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.4, 169.5, 150.1, 145.4, 137.3, 134.7, 131.2, 130.9, 129.8, 128.8, 125.5, 125.0, 122.8, 119.8, 74.2, 26.0. HRMS m/z (ESI+): calculated for C16H11ClNO2 ([M + H]+): 325.2420, found 325.2411.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 7.7 Hz, 1H), 7.80 (d, J = 7.7 Hz, 1H), 7.74–7.68 (m, 3H), 7.57–7.52 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.4, 169.5, 150.1, 145.4, 137.3, 134.7, 131.2, 130.9, 129.8, 128.8, 125.5, 125.0, 122.8, 119.8, 74.2, 26.0. HRMS m/z (ESI+): calculated for C16H11ClNO2 ([M + H]+): 325.2420, found 325.2411.
2-Fluoro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2k)
43.8 mg, 82% yield, yellow solid, mp 180–182 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 8.8, 4.1 Hz, 1H), 7.83 (m, 2H), 7.74–7.70 (m, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.48 (td, J = 8.7, 2.7 Hz, 1H), 7.43 (dd, J = 6.9, 2.7 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 169.7, 160.1 (d, J = 246.3 Hz), 148.0, 145.4, 134.6, 131.0, 129.7, 128.7 (d, J = 8.8 Hz), 125.4, 124.8 (d, J = 25.0 Hz), 122.8, 120.0 (d, J = 7.5 Hz), 111.1 (d, J = 23.8 Hz), 74.4, 25.9. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 268.0768, found 268.0762.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 8.8, 4.1 Hz, 1H), 7.83 (m, 2H), 7.74–7.70 (m, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.48 (td, J = 8.7, 2.7 Hz, 1H), 7.43 (dd, J = 6.9, 2.7 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 169.7, 160.1 (d, J = 246.3 Hz), 148.0, 145.4, 134.6, 131.0, 129.7, 128.7 (d, J = 8.8 Hz), 125.4, 124.8 (d, J = 25.0 Hz), 122.8, 120.0 (d, J = 7.5 Hz), 111.1 (d, J = 23.8 Hz), 74.4, 25.9. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 268.0768, found 268.0762.
2-Bromo-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2l)
34.1 mg, 52% yield, white solid, mp 103–105 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.88 (m, 2H), 7.86–7.82 (m, 2H), 7.80 (d, J = 7.7 Hz, 1H), 7.72 (td, J = 7.6, 0.9 Hz, 1H), 7.55 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.2, 169.4, 150.5, 145.3, 140.0, 134.7, 130.9, 129.7, 129.2, 128.0, 125.4, 122.8, 120.1, 118.4, 73.9, 25.9. HRMS m/z (ESI+): calculated for C16H10BrNO2Na ([M + Na]+): 349.9787, found 349.9796.
20 (v/v); Rf = 0.4. 1H NMR (500 MHz, CDCl3) δ 7.88 (m, 2H), 7.86–7.82 (m, 2H), 7.80 (d, J = 7.7 Hz, 1H), 7.72 (td, J = 7.6, 0.9 Hz, 1H), 7.55 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.2, 169.4, 150.5, 145.3, 140.0, 134.7, 130.9, 129.7, 129.2, 128.0, 125.4, 122.8, 120.1, 118.4, 73.9, 25.9. HRMS m/z (ESI+): calculated for C16H10BrNO2Na ([M + Na]+): 349.9787, found 349.9796.
10b-Methyl-2-(trifluoromethyl)-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2m)
40.0 mg, 63% yield, white solid, mp 52–54 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 8.5 Hz, 1H), 8.05 (s, 1H), 8.00 (dd, J = 8.4, 1.5 Hz, 1H), 7.87 (d, J = 7.7 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.75 (m, 1H), 7.59–7.54 (m, 1H), 1.83 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.4, 169.2, 153.7, 145.3, 134.9, 134.1 (q, J = 3.8 Hz), 130.7, 129.9, 127.7, 127.6 (q, J = 33.8 Hz), 125.6, 124.6, 122.88, 122.85, 119.0, 74.1, 26.0. HRMS m/z (ESI+): calculated for C17H10F3NO2Na ([M + Na]+): 347.2224, found 347.2226.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 8.5 Hz, 1H), 8.05 (s, 1H), 8.00 (dd, J = 8.4, 1.5 Hz, 1H), 7.87 (d, J = 7.7 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.75 (m, 1H), 7.59–7.54 (m, 1H), 1.83 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.4, 169.2, 153.7, 145.3, 134.9, 134.1 (q, J = 3.8 Hz), 130.7, 129.9, 127.7, 127.6 (q, J = 33.8 Hz), 125.6, 124.6, 122.88, 122.85, 119.0, 74.1, 26.0. HRMS m/z (ESI+): calculated for C17H10F3NO2Na ([M + Na]+): 347.2224, found 347.2226.
Methyl 10b-methyl-6,11-dioxo-10b,11-dihydro-6H-isoindolo [2,1-a]indole-2-carboxylate (2n)
30.7 mg, 50% yield, white solid, mp 125–127 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 8.47–8.41 (m, 2H), 8.03 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.76–7.71 (m, 1H), 7.56 (m, 1H), 3.95 (s, 3H), 1.82 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.6, 169.1, 165.7, 154.4, 145.5, 138.5, 134.8, 130.8, 129.8, 127.6, 127.3, 127.2, 125.5, 122.8, 118.2, 74.1, 52.5, 26.1. HRMS m/z (ESI+): calculated for C18H14NO4 ([M + H]+): 308.0917, found 308.0903.
10 (v/v); Rf = 0.2. 1H NMR (500 MHz, CDCl3) δ 8.47–8.41 (m, 2H), 8.03 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.76–7.71 (m, 1H), 7.56 (m, 1H), 3.95 (s, 3H), 1.82 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.6, 169.1, 165.7, 154.4, 145.5, 138.5, 134.8, 130.8, 129.8, 127.6, 127.3, 127.2, 125.5, 122.8, 118.2, 74.1, 52.5, 26.1. HRMS m/z (ESI+): calculated for C18H14NO4 ([M + H]+): 308.0917, found 308.0903.
3,10b-Dimethyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2o)
46.3 mg, 88% yield, yellow solid, mp 149–150 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.87–7.78 (m, 3H), 7.70 (td, J = 7.7, 1.0 Hz, 1H), 7.65 (d, J = 7.9 Hz, 1H), 7.53 (m, 1H), 7.10 (d, J = 7.8 Hz, 1H), 2.52 (s, 3H), 1.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 169.7, 152.0, 149.5, 145.8, 134.3, 131.2, 129.4, 126.5, 125.2, 125.1, 125.0, 122.7, 118.8, 73.9, 26.1, 22.6. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1009.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.87–7.78 (m, 3H), 7.70 (td, J = 7.7, 1.0 Hz, 1H), 7.65 (d, J = 7.9 Hz, 1H), 7.53 (m, 1H), 7.10 (d, J = 7.8 Hz, 1H), 2.52 (s, 3H), 1.79 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.8, 169.7, 152.0, 149.5, 145.8, 134.3, 131.2, 129.4, 126.5, 125.2, 125.1, 125.0, 122.7, 118.8, 73.9, 26.1, 22.6. HRMS m/z (ESI+): calculated for C17H14NO2 ([M + H]+): 264.1019, found 264.1009.
3-Chloro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2p)
44.3 mg, 78% yield, white solid, mp 160–162 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.99 (d, J = 1.6 Hz, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.73 (m, 1H), 7.69 (d, J = 8.2 Hz, 1H), 7.56–7.53 (m, 1H), 7.26 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.2, 169.4, 152.3, 145.6, 144.0, 134.7, 130.8, 129.7, 126.2, 125.92, 125.90, 125.5, 122.8, 118.9, 74.0, 26.0. HRMS m/z (ESI+): calculated for C16H10ClNO2Na ([M + Na]+): 306.0292, found 306.0280.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.99 (d, J = 1.6 Hz, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.73 (m, 1H), 7.69 (d, J = 8.2 Hz, 1H), 7.56–7.53 (m, 1H), 7.26 (m, 1H), 1.80 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.2, 169.4, 152.3, 145.6, 144.0, 134.7, 130.8, 129.7, 126.2, 125.92, 125.90, 125.5, 122.8, 118.9, 74.0, 26.0. HRMS m/z (ESI+): calculated for C16H10ClNO2Na ([M + Na]+): 306.0292, found 306.0280.
3-Fluoro-10b-methyl-6H-isoindolo[2,1-a]indole-6,11(10bH)-dione (2q)
39.6 mg, 74% yield, white solid, mp 168–170 °C; purified by chromatography on silica gel, eluting with ethyl acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 7.6 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.77 (dd, J = 8.5, 5.5 Hz, 1H), 7.72 (t, J = 7.5 Hz, 1H), 7.66 (dd, J = 8.9, 2.1 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 6.98 (td, J = 8.6, 2.1 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.7, 169.4, 168.7 (d, J = 256.3 Hz), 153.6 (d, J = 13.8 Hz), 145.7, 134.7, 130.7, 129.6, 127.4 (d, J = 12.5 Hz), 125.4, 123.7, 122.8, 113.4 (d, J = 23.8 Hz), 106.1 (d, J = 26.3 Hz), 74.2, 26.0. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 279.2795, found 279.2784.
20 (v/v); Rf = 0.3. 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 7.6 Hz, 1H), 7.81 (d, J = 7.7 Hz, 1H), 7.77 (dd, J = 8.5, 5.5 Hz, 1H), 7.72 (t, J = 7.5 Hz, 1H), 7.66 (dd, J = 8.9, 2.1 Hz, 1H), 7.54 (t, J = 7.5 Hz, 1H), 6.98 (td, J = 8.6, 2.1 Hz, 1H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 195.7, 169.4, 168.7 (d, J = 256.3 Hz), 153.6 (d, J = 13.8 Hz), 145.7, 134.7, 130.7, 129.6, 127.4 (d, J = 12.5 Hz), 125.4, 123.7, 122.8, 113.4 (d, J = 23.8 Hz), 106.1 (d, J = 26.3 Hz), 74.2, 26.0. HRMS m/z (ESI+): calculated for C16H11FNO2 ([M + H]+): 279.2795, found 279.2784.
Reduction of 2a
To a solution of 2a (1.0 equiv., 0.2 mmol) in THF (2.0 mL) was added LiAlH4 (10.0 equiv., 2.0 mmol) in portions at 0 °C, after which the mixture was stirred at 90 °C for 12 h. The resulting mixture was then quenched with H2O at 0 °C, and extracted with EtOAc. After the solvent EtOAc was removed under vacuum, the residue was purified by flash column chromatography on silica gel (ethyl acetate/petroleum ether, v/v = 1/3) to give compound 3 (41.0 mg, 87%) as a white solid, Rf = 0.4. Mp 85–87 °C; 1H NMR (500 MHz, CDCl3) δ 7.32–7.25 (m, 2H), 7.20 (m, 3H), 7.14 (d, J = 6.7 Hz, 1H), 6.83 (m, 2H), 5.40 (s, 1H), 4.61 (d, J = 15.2 Hz, 1H), 4.41 (d, J = 15.2 Hz, 1H), 2.31 (brs, 1H), 1.52 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 154.4, 147.1, 138.5, 132.0, 130.3, 128.0, 127.4, 125.9, 122.9, 122.3, 121.5, 113.3, 80.4, 78.6, 56.9, 21.0. HRMS m/z (ESI+): calculated for C16H16NO ([M + H]+): 238.1226, found 238.1223.Suzuki reaction of 2l with PhB(OH)2
The mixture of PhB(OH)2 (1.2 equiv., 0.24 mmol), Pd(PPh3)4 (8 mol%), K2CO3 (2.5 equiv., 0.5 mmol), and 2l (1.0 equiv., 0.2 mmol) in THF (2.0 mL) and H2O (1.0 mL) was stirred at 65 °C for 12 h under N2 atmosphere. The resulting mixture was then extracted with EtOAc and the combined organic phases were concentrated under vacuum. The residue was then purified by flash column chromatography on silica gel (ethyl acetate/petroleum ether, v/v = 1/10) to give compound 4 (55.8 mg, 86%) as a white solid, Rf = 0.5. Mp 120–122 °C; 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 8.3 Hz, 1H), 7.99 (m, 2H), 7.85 (dd, J = 14.9, 7.7 Hz, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.59–7.52 (m, 3H), 7.46 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 1.84 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 197.5, 169.7, 150.9, 145.6, 139.4, 138.7, 136.5, 134.5, 131.1, 129.6, 129.0, 128.1, 127.8, 127.0, 125.3, 123.4, 122.8, 118.8, 74.1, 26.0. HRMS m/z (ESI+): calculated for C22H15NO2Na ([M + Na]+): 348.0995, found 348.0997.Sonogashira reaction of 2l with phenylacetylene
The mixture of phenylacetylene (1.5 equiv., 0.3 mmol), CuI (5 mol%), Pd(PPh3)2Cl2 (2 mol%), and 2l (1.0 equiv., 0.2 mmol) in Et3N (2.0 mL) was stirred at 100 °C for 12 h under N2 atmosphere. After the reaction was completed, Et3N was removed under vacuum. The crude was purified by flash column chromatography on silica gel (ethyl acetate/petroleum ether, v/v = 1/10) to give compound 5 (64.4 mg, 92%) as a yellow solid, Rf = 0.2. Mp 145–146 °C; 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.3 Hz, 1H), 7.93–7.83 (m, 3H), 7.81 (d, J = 7.6 Hz, 1H), 7.71 (m, 1H), 7.53 (m, 3H), 7.39–7.30 (m, 3H), 1.81 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 196.6, 169.3, 150.8, 145.5, 140.3, 134.5, 131.6, 131.0, 129.6, 128.6, 128.4, 128.2, 127.7, 125.3, 122.74, 122.66, 120.6, 118.5, 90.4, 87.7, 73.8, 26.0. HRMS m/z (ESI+): calculated for C24H16NO2 ([M + H]+): 350.1176, found 350.1178.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21702184 and 21772175).Notes and references
- (a) R. M. Williams, T. Glinka, E. Kwast, H. Coffman and J. K. Stille, J. Am. Chem. Soc., 1990, 112, 808 CrossRef CAS; (b) J.-F. Liu, Z.-Y. Jiang, R.-R. Wang, Y.-T. Zeng, J.-J. Chen, X.-M. Zhang and Y.-B. Ma, Org. Lett., 2007, 9, 4127 CrossRef CAS PubMed; (c) C. V. S. Kumar, V. G. Puranik and C. V. Ramana, Chem.–Eur. J., 2012, 18, 9601 CrossRef CAS PubMed; (d) T. Abe, A. Kukita, K. Akiyama, T. Naito and D. Uemura, Chem. Lett., 2012, 41, 728 CrossRef CAS; (e) W. Gu, Y. Zhang, X.-J. Hao, F. M. Yang, Q.-Y. Sun, S. L. Morris-Natschke, K.-H. Lee, Y.-H. Wang and C.-L. Long, J. Nat. Prod., 2014, 77, 2590 CrossRef CAS.
- For selected recent examples: (a) Q. Yin and S.-L. You, Chem. Sci., 2011, 2, 1344 RSC; (b) M. Rueping, S. Raja and A. Núñez, Adv. Synth. Catal., 2011, 353, 563 CrossRef CAS; (c) A. Parra, R. Alfaro, L. Marzo, A. Moreno-Carrasco, J. L. Garcia Ruano and J. Aleman, Chem. Commun., 2012, 48, 9759 RSC; (d) Y.-L. Zhao, Y. Wang, J. Cao, Y.-M. Liang and P.-F. Xu, Org. Lett., 2014, 16, 2438 CrossRef CAS PubMed; (e) R.-R. Liu, S.-C. Ye, C.-J. Lu, G.-L. Zhuang, J.-R. Gao and Y.-X. Jia, Angew. Chem., Int. Ed., 2015, 54, 11205 CrossRef CAS PubMed; (f) J.-S. Li, Y.-J. Liu, S. Li and J.-A. Ma, Chem. Commun., 2018, 54, 9151 RSC; (g) P. Li, W. Yong, R. Sheng, W. Rao, X. Zhu and X. Zhang, Adv. Synth. Catal., 2019, 361, 201 CrossRef CAS.
- For recent reviews: (a) Q.-A. Chen, Z.-S. Ye, Y. Duan and Y.-G. Zhou, Chem. Soc. Rev., 2013, 42, 497 RSC; (b) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807 RSC; (c) C.-X. Zhuo, C. Cheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558 CrossRef CAS PubMed; (d) N. Denizot, T. Tomakinian, R. Beaud, C. Kouklovsky and G. Vincent, Tetrahedron Lett., 2015, 56, 4413 CrossRef CAS; (e) W. T. Chen, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (f) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164 RSC.
- For selected recent examples: (a) R. B. Bedford, N. Fey, M. F. Haddow and R. F. Sankey, Chem. Commun., 2011, 47, 3649 RSC; (b) K.-J. Wu, L.-X. Dai and S.-L. You, Org. Lett., 2012, 14, 3772 CrossRef CAS PubMed; (c) B. Yin, C. Cai, G. Zeng, R. Zhang, X. Li and H. Jiang, Org. Lett., 2012, 14, 1098 CrossRef CAS PubMed; (d) K.-J. Wu, L.-X. Dai and S.-L. You, Chem. Commun., 2013, 49, 8620 RSC; (e) K. Hata, Z. He, C. G. Daniliuc, K. Itami and A. Studer, Chem. Commun., 2014, 50, 463 RSC; (f) V. Ramella, Z. He, C. G. Daniliuc and A. Studer, Eur. J. Org. Chem., 2016, 2268 CrossRef CAS; (g) X. Lei, H.-Y. Xie, C. Xu, X. Liu, X. Wen, H. Sun and Q.-L. Xu, Adv. Synth. Catal., 2016, 358, 1892 CrossRef CAS; (h) J. Liu, H. Peng, L. Lu, X. Xu, H. Jiang and B. Yin, Org. Lett., 2016, 18, 6440 CrossRef CAS PubMed; (i) P. Yang and S.-L. You, Org. Lett., 2018, 20, 7684 CrossRef CAS PubMed.
- (a) L. Zhao, Z. Li, L. Chang, J. Xu, H. Yao and X. Wu, Org. Lett., 2012, 14, 2066 CrossRef CAS PubMed; (b) S. Gao, C. Yang, Y. Huang, L. Zhao, X. Wu, H. Yao and A. Lin, Org. Biomol. Chem., 2016, 14, 840 RSC; (c) K. Douki, H. Ono, T. Taniguchi, J. Shimokawa, M. Kitamura and T. Fukuyama, J. Am. Chem. Soc., 2016, 138, 14578 CrossRef CAS PubMed; (d) X. Li, B. Zhou, R.-Z. Yang, F.-M. Yang, R.-X. Liang, R.-R. Liu and Y.-X. Jia, J. Am. Chem. Soc., 2018, 140, 13945 CrossRef CAS PubMed.
- (a) C. Shen, R.-R. Liu, R.-J. Fan, Y.-L. Li, T.-F. Xu, J.-R. Gao and Y.-X. Jia, J. Am. Chem. Soc., 2015, 137, 4936 CrossRef CAS PubMed; (b) F. Wei, L. Wei, L. Zhou, C.-H. Tung, Y. Ma and Z. Xu, Asian J. Org. Chem., 2016, 5, 971 CrossRef CAS; (c) R.-R. Liu, Y. Xu, R.-X. Liang, B. Xiang, H.-J. Xie, J.-R. Gao and Y.-X. Jia, Org. Biomol. Chem., 2017, 15, 2711 RSC.
- (a) D. A. Petrone, A. Yen, N. Zeidan and M. Lautens, Org. Lett., 2015, 17, 4838 CrossRef CAS PubMed; (b) D. A. Petrone, M. Kondo, N. Zeidan and M. Lautens, Chem.–Eur. J., 2016, 22, 5684 CrossRef CAS PubMed; (c) C. Shen, N. Zeidan, Q. Wu, C. B. J. Breuers, R.-R. Liu, Y.-X. Jia and M. Lautens, Chem. Sci., 2019, 10, 3118 RSC; (d) S. Chen, X.-X. Wu, J. Wang, X.-H. Hao, Y. Xia, Y. Shen, H. Jing and Y.-M. Liang, Org. Lett., 2016, 18, 4016 CrossRef CAS PubMed; (e) R.-R. Liu, T.-F. Xu, Y.-G. Wang, B. Xiang, J.-R. Gao and Y.-X. Jia, Chem. Commun., 2016, 52, 13664 RSC; (f) R.-R. Liu, Y.-G. Wang, Y.-L. Li, B.-B. Huang, R.-X. Liang and Y.-X. Jia, Angew. Chem., Int. Ed., 2017, 56, 7475 CrossRef CAS PubMed; (g) Y.-G. Wang, R.-R. Liu, J.-R. Gao and Y.-X. Jia, Chin. J. Chem., 2017, 37, 691 CrossRef CAS; (h) N. Zeidan, T. Beisel, R. Ross and M. Lautens, Org. Lett., 2018, 20, 7332 CrossRef CAS PubMed; (i) .
- For representative examples: (a) S. Rousseaux, J. García-Fortanet, M. A. D. A. Sanchez and S. L. Buchwald, J. Am. Chem. Soc., 2011, 133, 9282 CrossRef CAS PubMed; (b) T. Nemoto, Z. Zhao, T. Yokosaka, Y. Suzuki, R. Wu and Y. Hamada, Angew. Chem., Int. Ed., 2013, 52, 2217 CrossRef CAS PubMed; (c) R.-Q. Xu, Q. Gu, W.-T. Wu, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2014, 136, 15469 CrossRef CAS PubMed; (d) K. Du, P. Guo, Y. Chen, Z. Cao, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 3033 CrossRef CAS PubMed; (e) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876 CrossRef CAS PubMed; (f) R.-Q. Xu, P. Yang, H.-F. Tu, S.-G. Wang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 15137 CrossRef CAS PubMed; (g) L. Bai, Y. Yuan, J. Liu, J. Wu, L. Han, H. Wang, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2016, 55, 6946 CrossRef CAS PubMed; (h) R.-Q. Xu, Q. Gu and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 7252 CrossRef CAS PubMed; (i) Z. Zuo, H. Wang, L. Fan, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2017, 56, 2767 CrossRef CAS PubMed.
- (a) J. García-Fortanet, F. Kessler and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 6676 CrossRef PubMed; (b) R. B. Bedford, C. P. Butts, M. F. Haddow, R. Osborneb and R. F. Sankey, Chem. Commun., 2009, 45, 4832 RSC.
- For Pd-catalyzed transformations of acetoxy-dihydro-naphthalene derivatives: (a) Z. Huang, L. H. Lim, Z. Chen, Y. Li, F. Zhou, H. Su and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 4906 CrossRef CAS PubMed; (b) J. Liu, S. Mishra and A. Aponick, J. Am. Chem. Soc., 2018, 140, 16152 CrossRef CAS PubMed.
- (a) I. Mutule, E. Suna, K. Olofsson and B. Pelcman, J. Org. Chem., 2009, 74, 7195 CrossRef CAS PubMed; (b) K. Liu, P. Wen, J. Liu and G. Huang, Synthesis, 2010, 21, 3623 Search PubMed; (c) V. Soni, U. N. Patel and B. Punji, RSC Adv., 2015, 5, 57472 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02569c |
| This journal is © The Royal Society of Chemistry 2019 |