Open Access Article
Open Access ArticleCharacterization of the genome from Geobacter anodireducens, a strain with enhanced current production in bioelectrochemical systems†
Dan Sun *a,
Xinyuan Wana,
Wenzong Liub,
Xue Xiab,
Fangliang Huangc,
Aijie Wang*b,
Jessica A. Smith
*a,
Xinyuan Wana,
Wenzong Liub,
Xue Xiab,
Fangliang Huangc,
Aijie Wang*b,
Jessica A. Smith d,
Yan Dange and
Dawn E. Holmesf
d,
Yan Dange and
Dawn E. Holmesf
aOcean College, Zhejiang University, Zhoushan 316021, P. R. China. E-mail: dsun@zju.edu.cn
bKey Laboratory of Environmental Biotechnology, Research Center for Eco-Environmental Sciences, China Academy of Sciences, Beijing 100084, P. R. China. E-mail: ajwang@rcees.ac.cn
cCollege of Life Sciences, Zhejiang University, Hangzhou 310058, P. R. China
dDepartment of Biomolecular Sciences, Central Connecticut State University, 1615 Stanley Street, New Britain, CT 06050, USA
eBeijing Key Laboratory for Source Control Technology of Water Pollution, Engineering Research Center for Water Pollution Source Control and Eco-remediation, College of Environmental Science & Engineering, Beijing Forestry University, 35 Tsinghua East Road, Beijing 100083, China
fDepartment of Physical and Biological Sciences, Western New England University, 1215 Wilbraham Rd, Springfield, MA 01190, USA
First published on 19th August 2019
Abstract
Geobacter anodireducens is unique in that it can generate high current densities in bioelectrochemical systems (BES) operating under high salt conditions. This ability is important for the development of BES treating high salt wastewater and microbial desalination cells. Therefore, the genome of G. anodireducens was characterized to identify proteins that might allow this strain to survive in high salt BES. Comparison to other Geobacter species revealed that 81 of its 87 c-type cytochromes had homologs in G. soli and G. sulfurreducens. Genes coding for many extracellular electron transfer proteins were also detected, including the outer membrane c-type cytochromes OmcS and OmcZ and the soluble c-type cytochrome PgcA. G. anodireducens also appears to have numerous membrane complexes involved in the translocation of protons and sodium ions and channels that provide protection against osmotic shock. In addition, it has more DNA repair genes than most Geobacter species, suggesting that it might be able to more rapidly repair DNA damage caused in high salt and low pH anode environments. Although this genomic analysis provides invaluable insight into mechanisms used by G. anodireducens to survive in high salt BES, genetic, transcriptomic, and proteomic studies will need to be done to validate their roles.
1 Introduction
Extracellular electron transfer (EET) is an important bioprocess that plays a role in global biogeochemical cycles, bioremediation applications, as well as anaerobic digestion.1 EET is primarily carried out by dissimilatory metal-reducing bacteria (DMRB), which have the ability to transfer electrons from the cytoplasm to the outer cell surface in order to reduce extracellular electron acceptors including Fe(III), neighboring microorganisms via direct interspecies electron transfer (DIET), or electrodes in microbial fuel cells (MFCs).2,3MFCs rely on microorganisms that colonize the anode to transfer electrons from the oxidation of organic matter. MFC technology has been developed for a variety of bioelectrochemical system (BES) applications including hydrogen production,4 desalination,5 organic product synthesis,6 biosensors,7–9 and wastewater treatment.10 The study of EET by exoelectrogens has resulted in the development of the novel sub-discipline, electromicrobiology.11–13 However, mechanisms used to transfer electrons from a microorganism's cytoplasm out to extracellular electron acceptors like anodes still remains an area of controversy. Therefore, identification and characterization of microbes that are superior in their EET capabilities is essential for understanding the EET mechanisms favored in the environment and for practical applications.
Mechanisms for EET have been most thoroughly studied in the genera Geobacter and Shewanella.14 The model exoelectrogenic Shewanella species, S. oneidensis, uses a single known pathway to transfer electrons from an inner membrane cytochrome (CymA) to the MtrCAB porin-multiheme c-type cytochrome complex.15 The Mtr proteins directly reduce flavins, which can act as soluble electron shuttles.16 Approximately 75% of EET by S. oneidensis results from electron shuttles, which allows it to produce current in MFCs even though it cannot form thick biofilms.17–19 Geobacter species, on the other hand, form very thick biofilms, and a recent study showed that G. sulfurreducens produced 1047% times more maximum current than S. oneidensis.20
Geobacter spp. are the most abundant microorganisms in anaerobic soils and sediments where microbial reduction of insoluble Fe(III) oxides is important.1 To date, a total of 21 Geobacter species have been characterized (NCBI Taxonomy browser). Nearly all of the research regarding mechanisms of EET by Geobacter has been conducted in G. sulfurreducens strain PCA because it was the first to have a genome sequence, it can be genetically manipulated, and its metabolic traits such as rapid growth with fumarate as the electron acceptor make it easy to cultivate in the lab.21,22 However, G. sulfurreducens is not the most environmentally representative strain as it does not reduce Fe(III) oxides or produce current as effectively as some other Geobacter species23–27 and it rarely shows up in environmental samples.3
Although it is apparent that Geobacter species do not use soluble flavin shuttles like Shewanella, there does not seem to be one single pathway for EET amongst the genus. All Geobacter genomes sequenced to date appear to have genes that code for abundant multiheme c-type cytochromes and electrically conductive type IV pili (e-pili).28–30 However, specific mechanisms used for EET seem to vary between species and even among strains of the same species.31–33 For example, the soluble extracellular c-type cytochrome PgcA is required for Fe(III) oxide reduction in some strains of G. sulfurreducens34 and in the absence of e-pili,32 but it is not significant in other strains.35 In addition, OmcS facilitates electron transfer to Fe(III) oxide33 and anodes with thin biofilms formed by G. sulfurreducens,36 while a different c-type cytochrome, OmcZ, is involved in EET in thick anode biofilms.37,38 G. metallireducens does not have OmcS, although several other c-type cytochromes as well as e-pili are essential for EET.32,39,40 Furthermore, some Geobacter strains that are capable of Fe(III) oxide reduction, are completely incapable of current generation. For example, G. bemidjiensis contains both e-pili and a c-type cytochrome in the OmcZ family, however it is not able to produce current.23,41
Geobacter anodireducens was the first Geobacter species isolated from an anode biofilm based on its ability to generate current.26 In cyclic voltammetry, electrochemical impedance spectroscopy, and biomass tests, G. anodireducens SD-1 performed better than G. sulfurreducens PCA, G. metallireducens GS-15, and a mixed culture (wastewater). Current production was even greater in BESs operating with high salt concentrations.42 Geobacter anodireducens SD-1 will be useful for providing additional insights into superior EET mechanisms and current generation in BESs. As more is learned, development of various new BES technologies can emerge. In this study, the genome of G. anodireducens SD-1 was analyzed and compared to genomes from closely related Geobacter species that are also able to generate current in MFCs in order to identify genes that might lead to the enhanced EET capabilities of SD-1.
2 Experimental
2.1 Physiological characterization
Geobacter anodireducens SD-1, Geobacter sulfurreducens PCA (ATCC 51573), and Geobacter metallireducens GS-15 (ATCC 53774) were acquired from Dr Bruce Logan's laboratory culture collection at Pennsylvania State University and grown anaerobically on bicarbonate-buffered medium (BCM-30)43 with acetate (1 g L−1) provided as the electron donor and Fe(III) citrate (20 mM) as the electron acceptor. All incubations were conducted under an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 N2
20 N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 atmosphere at 30 °C in the dark.26
CO2 atmosphere at 30 °C in the dark.26
Electrical activities of SD-1, PCA, and GS-15 were tested in mini-BESs (5 ml) with graphite plate anodes and stainless steel mesh cathodes as previously described.42,44 Microbial electrolysis cell (MEC) chambers were filled with either 50 mM phosphate buffer (PBM-50), 200 mM phosphate buffer (PBM-200), or saline water (SW, 50 mM PBS with 3.8% NaCl) and 1 g L−1 sodium acetate was provided as the electron donor. Reference electrodes (Ag/AgCl; +200 mV vs. standard hydrogen electrode (SHE); BASi) were used to record anode and cathode potentials. An applied potential of 0.7 V was supplied to each MEC. MECs were operated with a programmable power supply (model 3645A; Circuit Specialists Inc), and each circuit contained a 10 Ω resistor.
Voltage was recorded at 20 minute intervals with a multimeter (model 2700; Keithley Instruments Inc.), and current was calculated using Ohm's law (I = V/R). Volumetric current density (IV; A m−3) was determined by dividing current by liquid volume, and current density per area (IA; A m−2) was calculated by dividing current by anode surface area.
Fe(III) reduction was monitored by measuring Fe(II) that could be extracted in 0.5 M HCl after a 1 hour incubation with a ferrozine assay at an absorbance of 562 nm as previously described.45
2.2 G. anodireducens SD-1 genome sequencing, assembly and annotation
Total genomic DNA was extracted with a DNA extraction kit (Lifefeng), according to the manufacturer's protocol. The concentration and purity of DNA was measured with a NanoDrop spectrophotometer (ND 1000, Thermo Fisher Scientific, DE). The genome of strain G. anodireducens SD-1 was sequenced at the Life Science College of Zhejiang University using the PGM system (ABI, USA). The Ion Torrent data included a 350 bp paired-end library and a 3k bp mate-pair library, and a total of 0.8 G and 0.6 G of raw data were produced after filtering, respectively. These sequences were assembled into 2 contigs using the CLC Genomics Workbench 6.0 (CLCbio, Waltham, MA).Preliminary annotation was performed using Rapid Annotation Subsystem Technology (RAST)46 and NCBI. SignalIP v4.1 was used to identify genes with signal peptides, and THMMER 2.0 was used to define genes with transmembrane helices.47,48 Translated amino acids were assigned to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using KASS (KEGG Automatic Annotation Server).49
2.3 Comparative genomics
Genomes from Geobacter anodireducens SD-1 (NZ_CP014963 and NZ_CP014964), G. soli GSS01 (NZ_JXBL01000000), G. sulfurreducens PCA (NC_002939), and G. metallireducens GS-15 (NC_007517 and NC_007515) were downloaded from NCBI and screened by comparison to sequences in the NR database with the BLASTp algorithm50 using NCBI BLAST-2.2.31+ standalone software.51 C-type cytochrome proteins were identified by screening each protein for the heme binding motif (CXXCH). Tools on the IMG/M (Integrated Microbial Genomes & Microbiomes) website (https://img.jgi.doe.gov) were also used for comparison of the four genomes.3 Results and discussion
3.1 Similarities to Geobacter soli GSS01
Geobacter anodireducens SD-1 was isolated from a MFC biofilm inoculated with effluent collected from a primary clarifier at the Pennsylvania State University Wastewater Treatment Plant.52 Intriguingly, its genome is most similar to another Geobacter species, Geobacter soli GSS01, isolated from a completely different environment. Geobacter soli GSS01 was isolated from a soil sample taken from the humic layer of an underground ancient forest in China.27 Comparisons of the two genomes with the Pairwise ANI (ANI; a measure of nucleotide-level genomic similarity between the coding regions of two genomes) tool available on the IMG/MER website (www.img.jgi.org) revealed that 99.64% of the genes are similar.The physiologies of G. anodireducens and G. soli also appear to be quite similar (Table 1). Both strains can couple the oxidation of acetate with a variety of extracellular electron acceptors including Fe(III), S0, Mn(IV), and AQDS, and neither strain can respire fumarate. However, G. soli can grow with nitrate provided as an electron acceptor,27 while G. anodireducens cannot couple the oxidation of organic compounds with nitrate respiration.26
| Characteristic | SD-1 | PCA | GS-15 | GSS01 |
|---|---|---|---|---|
| a ND: not determined. | ||||
| Acetate (electron donor) | Yes | Yes | Yes | Yes |
| Hydrogen (electron donor) | Yes | Yes | No | ND |
| Ferric citrate (electron acceptor) | Yes | Yes | Yes | Yes |
| Fumarate (electron acceptor) | No | Yes | No | No |
| Nitrate (electron acceptor) | No | No | Yes | Yes |
| Sulfur (electron acceptor) | Yes | Yes | No | Yes |
| Mn(IV) (electron acceptor) | Yes | Yes | Yes | Yes |
| AQDS (electron acceptor) | Yes | Yes | Yes | Yes |
| MFC anode (electron acceptor) | Yes | Yes | Yes | Yes |
| NaCl tolerance | 3.0% | 1.7% | 0.5% | ND |
| Presence of plasmid | Yes | No | Yes | No |
G. anodireducens and G. soli are both much more similar to G. sulfurreducens than to G. metallireducens. Pairwise ANI values from comparisons of G. anodireducens to G. sulfurreducens and G. metallireducens were 93.10 and 78.65, respectively. Similar to most members of the genus Geobacter, all four strains can utilize both soluble and insoluble Fe(III) as the sole electron acceptor coupled with the oxidation of acetate,25–27,53 and are capable of producing current on the anode of MFCs (Table 1).11,26,54
3.2 Physiological comparison of Geobacter anodireducens to G. sulfurreducens and G. metallireducens
Due to the in depth characterization and completed genome sequences of G. sulfurreducens PCA and G. metallireducens GS-15, further physiological comparisons with these species were done in order to uncover potential mechanisms used for enhanced EET in high salinity environments. As previous research has shown, G. anodireducens SD-1 grew fastest of the three strains with soluble Fe(III) citrate provided as the electron acceptor. It had a doubling time of only 5.4 hours, while generation times of G. metallireducens GS-15 and G. sulfurreducens PCA were only 6.11 and 9.11 hours, respectively (ESI Fig. S1A†).26 In addition, strain SD-1 consistently produced the highest currents in BES with high-salt solutions (50 or 200 mM phosphate buffer solution, and high salinity water) (ESI Fig. S1B†).42Geobacter anodireducens shares some physiological traits with both G. sulfurreducens and G. metallireducens. All three species can grow with a number of insoluble extracellular electron acceptors including current-harvesting anodes, Fe(III), Mn(IV), and AQDS (Table 1).25,26,35,53 However, among the three species only G. anodireducens and G. sulfurreducens can grow with elemental sulfur provided as an electron acceptor.25,26 Fumarate is a soluble electron acceptor that can only be used by G. sulfurreducens,25 while nitrate is a soluble electron acceptor that can only be utilized by G. metallireducens.53 Another important difference between these strains is that G. anodireducens is much more salt tolerant compared to the other two strains.42 Similar to G. metallireducens, a plasmid was identified in the G. anodireducens genome.
In terms of environmental niche, G. anodireducens SD-1 survived at pH and temperature ranges similar to those of G. metallireducens and G. sulfurreducens.25,26,53 However, SD-1 was able to grow with up to 3% NaCl,26 which is significantly higher than G. sulfurreducens and G. metallireducens which could only tolerate 1.7% NaCl25 and 0.5% NaCl,53 respectively. Furthermore, SD-1 generated significant current in BES with 200 mM phosphate buffer and 650 mM NaCl, while neither G. sulfurreducens nor G. metallireducens could grow in the high salt solution and both were significantly impaired in 200 mM phosphate solutions (ESI Fig. S1B†).42 Salt concentrations have been shown to influence microbial metabolism in MFCs and a recent study demonstrated that NaCl concentrations should remain below 0.1 M for optimal electricity generation.55 SD-1's ability to withstand such high salt concentrations will make the strain useful in future application of microbial desalination cells and microbial reverse electrodialysis cells56 as well as BES used for bioremediation of high salt solutions.57
3.3 General genomic features
The complete genome of SD-1 consists of a circular chromosome of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555
555![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 bp with a GC content of 61.8% and a circular plasmid of 110
507 bp with a GC content of 61.8% and a circular plasmid of 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 bp with a GC content of 52.2%. In total, 3434 genes have been identified on the chromosome and 130 genes have been detected on the plasmid. These genome statistics are similar to those from the G. soli, G. sulfurreducens, and G. metallireducens genomes, except that G. metallireducens is the only other species with a plasmid (Table 2).
507 bp with a GC content of 52.2%. In total, 3434 genes have been identified on the chromosome and 130 genes have been detected on the plasmid. These genome statistics are similar to those from the G. soli, G. sulfurreducens, and G. metallireducens genomes, except that G. metallireducens is the only other species with a plasmid (Table 2).
| Total number of bases | Number of genes | % G + C | |
|---|---|---|---|
| SD-1 chromosome | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555 555![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 507 |
3434 | 61.8% |
| SD-1 plasmid | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 507 |
130 | 52.2% |
| GSS01 chromosome | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 657![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3388 | 61.8% |
| PCA chromosome | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 814![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 128 |
3711 | 60.9% |
| GS-15 chromosome | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
3617 | 59.5% |
| GS-15 plasmid | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 762 |
18 | 52.5% |
The Function Category Comparison tool on the IMG/MER website was used to classify genes from various COG pathways in the four different genomes (ESI Table S1†). The comparison revealed that both the G. anodireducens and G. soli genomes have genes coding for two ATP synthase complexes, the F- and V-type ATPases. The only other Geobacter species that have both ATPase complexes are G. uraniireducens and Geobacter sp. M18.58 The F-type ATPase complex is found in most bacteria, eukaryotic mitochondria, and chloroplasts58–60 while the V-type complex is found in archaea, a few bacteria, and eukaryotic vacuoles.58,61–63 Studies have found that both of these ATPases can translocate protons or sodium ions across the membrane to drive the synthesis or hydrolysis of ATP.58,59,63 Analysis of subunit c from the F- and V-type ATPase complexes revealed that neither appears to have Na+-binding sites,58 suggesting that both of these ATPase complexes are proton-dependent.
Another difference among these genomes is the presence of genes for a multisubunit NA+/H+ antiporter in G. anodireducens, G. soli, and G. sulfurreducens, but not G. metallireducens. There are 7 Mrp (multiple resistance and pH) subunits (MrpA-G) required for enzymatic activity64 and G. anodireducens has genes for all 7 of these subunits (Ga0133348_111263–Ga0133348_111270). The Mrp complex is a Na+/H+ antiporter that utilizes the proton motive force to efflux intracellular sodium ions, and its functions include sodium tolerance and pH homeostasis.65–68 This complex is likely to be important for the high salt tolerance of G. anodireducens.42
An additional notable difference is that the number of fatty acid metabolism genes is significantly higher (∼3 times) in the G. metallireducens genome than the other three genomes. This can be explained by the fact that G. metallireducens is able to utilize a number of aromatic hydrocarbons (i.e. benzoate, toluene, phenol, p-cresol, benzene) as electron donors53,69 and these pathways share many genes with the fatty acid degradation pathway.70–72
3.4 Metabolic pathways
All four Geobacter species have genes coding for all of the enzymes in the tricarboxylic acid (TCA) cycle (Fig. 1). The G. metallireducens genome, however, has significantly more TCA cycle genes (ESI Table S1†), as it has multiple copies of many of the enzymes. For example, G. metallireducens has two citrate synthase (gltA) genes and two sets of genes coding for the succinate dehydrogenase/fumarate reductase complex.24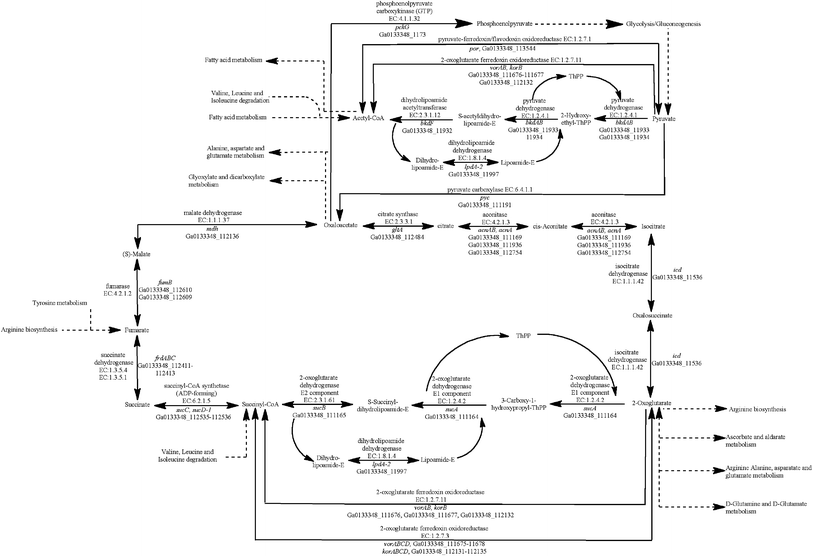 | ||
| Fig. 1 Identification of genes that code for proteins from the tricarboxylic acid (TCA) cycle in the G. anodireducens SD-1 genome. | ||
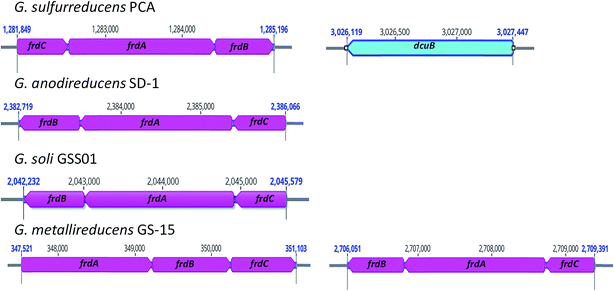
Similar to G. metallireducens, neither G. anodireducens nor G. soli are capable of growth with fumarate provided as the electron acceptor.26,27 Both of these organisms have genes that code for a succinate dehydrogenase complex which also functions as a respiratory fumarate reductase in G. sulfurreducens; Ga0133348_112411–112413 in G. anodireducens and Ga0077628_111869–111871 in G. soli (Fig. 2). However, their genomes lack the gene that codes for the anaerobic C4-dicarboxylate transporter (dcuB), which is required for the transport of aspartate, malate, fumarate, and succinate by many bacterial species.73–75 When the dcuB gene from G. sulfurreducens is expressed on a vector introduced into G. metallireducens, fumarate respiration is possible.76 It is likely that both of these strains would also be able to respire fumarate if the G. sulfurreducens dcuB gene was introduced into their chromosomes.
The respiratory nitrate reduction pathway has been characterized in G. metallireducens and genes for the nitrate reductase complex (narGYJI) and the nitrate/nitrite antiporter (narK) have been identified.24 Analysis of the other three genomes did not reveal the presence of any genes that code for proteins involved in nitrate respiration. This is consistent with the fact that neither G. sulfurreducens nor G. anodireducens can grow with nitrate provided as an electron acceptor. However, it is interesting that G. soli was reported to grow with nitrate provided as the sole electron acceptor.27 Further investigation into the mechanism for nitrate reduction used by G. soli is required.
3.5 Electron transport genes involved in extracellular electron transfer
Similar to other characterized Geobacter species, G. anodireducens appears to excel at its ability to exchange electrons with the extracellular environment.26 Numerous studies have identified c-type cytochromes that are involved in extracellular electron transfer to or from such substrates as Fe(III) oxide, Fe(III) citrate, Mn(IV) oxide, electrodes, Fe(0), Fe(III) containing sediments, and other microorganisms in such organisms as G. sulfurreducens,33,37,77–79 G. metallireducens,32,40 G. soli,54 and G. uraniireducens.35The G. anodireducens genome has 87 genes that could potentially code for c-type cytochrome proteins (ESI Table S2†). Four of these cytochromes are predicted to be localized to the inner membrane, 24 are extracellular or outer membrane associated, and 24 are periplasmic. More than half (53.5%) of these c-type cytochromes have 5 or more heme groups and 30.2% have 2 to 4 hemes. Many of these c-type cytochromes have homologs in both G. soli and G. sulfurreducens (ESI Table S2A†). In addition, many of the c-type cytochromes that genetic, transcriptomic, proteomic, and genomic studies have identified as being critical for extracellular electron exchange by Geobacter have homologs in G. anodireducens (Table 3, ESI Table S2†).
| Locus ID | SD-1 Locus ID | Abbreviation | Annotation | Impaired growth substrate(s) | References |
|---|---|---|---|---|---|
| GSU1761 | Ga0133348_111806 | pgcA | Soluble extracellular cytochrome c, class I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Fe(III) oxide | 31 and 34 |
| GSU2724–GSU2726 | No homologs | extEFG | Outer membrane electron conduit complex | Fe(III) citrate, | 88 |
| GSU2642–GSU2645 | Ga0133348_11946–11949 | extABCD | Porin–cytochrome (Pcc) complex | Anode | 88 |
| GSU2936–GSU2940 | Ga0133348_113069–113073 | extHIJKL | Porin–cytochrome (Pcc) complex | Fe(III) citrate, anode | 88 |
| GSU2731–GSU2739 | Ga0133348_11855–11861 | omcBC complex | Two tandem porin-cytochrome (Pcc) complexes | Fe(III) oxide, Fe(III) citrate, anode | 85 and 87–91 |
| GSU3259 | Ga0133348_113383 | imcH | Inner membrane c-type cytochrome protein | Fe(III) citrate, Mn(IV) oxide | 82 |
| GSU0274 | Ga0133348_11226 | cbcL | b/c-type cytochrome domain protein | Fe(III) oxide | 83 |
| GSU2504 | Ga0133348_111108 | omcS | Outer membrane c-type cytochrome | 33 | |
| GSU2076 | Ga0133348_111435 | omcZ | Outer membrane c-type cytochrome | Anode | 37 and 38 |
| GSU0618 | Ga0133348_11695 | omcE | Outer membrane c-type cytochrome | Fe(III) oxide, Mn(IV) oxide, anode, AQDS | 32 and 33 |
| GSU1496 | Ga0133348_112105 | pilA-N | Pilin domain protein | Fe(III) oxide, anode | 37, 39 and 101 |
| GSU0612 | Ga0133348_11688 | ppcA | Periplasmic cytochrome c, class III | Fe(III) citrate | 35 and 84 |
| GSU1394 | Ga0133348_112215 | ompB | Multicopper oxidase protein | Fe(III) oxide, Mn(IV) oxide | 100 |
| GSU2657 | No homolog | ompC | Multicopper oxidase protein | Fe(III) oxide | 99 |
| GSU1501 | Ga0133348_112101 | xapD | ATP dependent transporter involved in exopolysaccharide biosynthesis | Fe(III) oxide, anode | 105 and 106 |
| GSU1704 | Ga0133348_111888 | esnA | Mcp protein | Anode | 87 |
| GSU2220 | Ga0133348_111374 | esnB | cheW scaffolding protein | Anode | 87 |
| GSU2222 | Ga0133348_111372 | esnC | cheA histidine kinase | Anode | 87 |
| GSU3376 | Ga0133348_1182 | esnD | Diguanylate cyclase | Anode | 87 |
| Gmet_0557 | Ga0133348_113044 | omcP | Outer membrane c-type cytochrome | Fe(III) oxide | 32 and 35 |
| Gmet_0558 | No homolog | omcO | Outer membrane c-type cytochrome | Fe(III) oxide | 32 and 35 |
| Gmet_1867 | Ga0133348_111778 | c-type cytochrome | Fe(III) oxide | 32 | |
| Gmet_1868 | Ga0133348_111777 | — | c-type cytochrome | Fe(III) oxide | 32 |
| GSU2505 | Ga0133348_111106 | — | NHL repeat domain protein | Anode | 105 |
| GSU3361 | Ga0133348_1198 | — | Transglutaminase domain protein | Anode | 105 |
| Gmet_2029 | Ga0133348_111539 | — | Exopolysaccharide biosynthesis protein | Fe(III) oxide | 32 |
In order for Geobacter to use extracellular substrates as terminal electron acceptors, they need to be able to transfer electrons from the quinone/quinol pool in the inner membrane, across the periplasm and then across the outer membrane to the extracellular environment.3,80 Two inner membrane c-type cytochromes in G. sulfurreducens, the inner membrane cytochrome c (ImcH) and a cytochrome protein with b- and c-type domains (CbcL), appear to be involved in the early steps of electron transfer to extracellular substrates.81–83 ImcH is required for reduction of extracellular electron acceptors with reduction potentials greater than 0.1 V versus the standard hydrogen electrode (SHE) such as Fe(III) citrate and insoluble Mn(IV) oxides,82 while CbcL is important for electron transfer to low potential acceptors such as Fe(III) oxides.83 Homologs for both imcH and cbcL are present in the G. anodireducens, G. soli, and G. metallireducens genomes (Table 3, ESI Table S2B†). All four genomes also have homologs for PpcA, another periplasmic c-type cytochrome that is required for efficient reduction of Fe(III) citrate,84 but not insoluble Fe(III)-oxide or an electrode.35
Once periplasmic and inner membrane cytochromes shuttle electrons across the periplasm, they need to then be transferred across the outer membrane. Studies have suggested that porin cytochrome (Pcc) protein complexes, found in all Geobacter species that have been sequenced to date, act as electron conduits for transfer across the outer membrane.85,86 These conduits are composed of a periplasmic multiheme c-type cytochrome, a porin like outer membrane protein, and an outer membrane c-type cytochrome.85,87–91 The G. sulfurreducens genome has 5 gene clusters that code for putative Pcc complexes; ombB–omaB–omcB (GSU2737–GSU2739), ombC–omaC–omcC (GSU2731–GSU2733), extEFG (GSU2724–GSU2726), extABCD (GSU2642–GSU2645), and extHIJKL (GSU2936–GSU2940), each of which appears to shuttle electrons to different acceptors.87,88 The G. anodireducens, G. soli, and G. metallireducens genomes have homologs for all of the pcc complexes found in G. sulfurreducens except extEFG (Table 3, ESI Table S2B†).
OmcB, which is part of the ombB–omaB–omcB complex in G. sulfurreducens, is localized to the exterior surface of the outer membrane92 and is thought to transfer electrons to extracellular multiheme c-type cytochromes that act as terminal reductases.33,85 There is no homolog for omcB in G. metallireducens, although another c-type cytochrome (Gmet_0910) is found in a syntenous location.24 However, deletion of this gene did not impair Fe(III) oxide reduction.32 Both G. anodireducens (Ga0133348_11857) and G. soli (Ga0077628_11353) have omcB homologs with 84.09% and 83.82% amino acid sequence identity to that of G. sulfurreducens.
There also appears to be a considerable amount of variation in terminal c-type cytochromes between species and even within the same species. In G. sulfurreducens, the multiheme outer membrane c-type cytochrome, OmcS, is required for reduction of Fe(III)- and Mn(IV) oxides,33 the uptake of electrons during DIET79 and Fe(0),77 and anodes operated in fuel cell mode with thin biofilms.36 Immunogold labeling has shown that OmcS can localize along electrically conductive pili found on the G. sulfurreducens surface,93 and recent studies have suggested that it can form cytochrome based conductive filaments.94,95 OmcS also appears to be involved in electron transfer to insoluble Fe(III) oxide and anodes by G. soli.54 G. anodireducens also has a homolog for OmcS (Ga0133348_111108) with amino acid sequence identity of 95.16%. However, omcS homologs are not present in genomes from G. metallireducens or most other Geobacter species (Table 3, ESI Table S2†).3
Although most species within the genus Geobacter do not have OmcS, they all produce extracellular multiheme c-type cytochromes that can serve as terminal reductases. For example, the multiheme c-type cytochrome GscA (Gbem_3371) found in G. bemidjiensis was able to restore Fe(III) oxide reduction in the OmcS-deficient strain of G. sulfurreducens.96 In addition, while OmcS is required for efficient electron transfer from G. sulfurreducens to Fe(III) oxide,33 and in the reverse direction from an electron-donating partner during DIET,79 another extracellular multiheme c-type cytochrome (Gmet_2896) is needed for DIET and Fe(III) reduction by G. metallireducens.32,40 The outer membrane multiheme c-type cytochromes OmcP (GSU2913) and OmcO (GSU2912) are also not required for reduction of Fe(III) oxide by G. sulfurreducens,35 but their homologs (Gmet_0557 and Gmet_0558) are required for Fe(III) oxide respiration by G. metallireducens.32 G. anodireducens has a homolog for omcP (Ga0133348_113044) and two homologs for omcO (Ga0133348_113042 and Ga0133348_113043).
PgcA (GSU1761) is another extracellular c-type cytochrome that plays a role in Fe(III) reduction in some strains of Geobacter but not others.31,34 It is a soluble c-type cytochrome that is secreted into the extracellular environment to facilitate Fe(III) reduction.31,34 G. uraniireducens also releases a soluble electron shuttle that promotes Fe(III) oxide reduction,97 however, this shuttle was never characterized. The G. uraniireducens genome contains a gene that codes for a PgcA homolog (Gura_0706) which could have served as the shuttle and was significantly expressed in cells grown in Fe(III)-containing sediments.35,98 G. metallireducens does not have a PgcA homolog, however, homologs are found in both G. anodireducens (Ga0133348_111806) and G. soli (Ga0077628_111213) genomes (Table 3, ESI Table S2†).
Another multiheme outer surface c-type cytochrome, OmcZ, is required for electron transfer to anodes in flow-through systems with thick biofilms (>50 μm),37 but not for reduction of anodes with thin biofilms, Fe(III)- or Mn(IV) oxides by G. sulfurreducens.35,36 The homolog for omcZ in G. metallireducens (Gmet_0930) however, was essential for Fe(III) oxide reduction.32 Homologs for omcZ are found in all genomes analyzed in this study (Table 3, ESI Table S2†).
Other electron transport proteins that have been implicated in extracellular electron transfer from the G. sulfurreducens outer membrane include two outer membrane multicopper proteins (OmpB and OmpC),99,100 OmcE,33 and electrically conductive pili.101–103 All of the genomes have genes coding for OmcE and OmpB, but only the G. metallireducens genome has an ompC homolog (ESI Table S1†). A unique extracellular electron transfer component characteristic of Geobacter species is the presence of pilin monomers that have a structure that enables them to be electrically conductive.30,101–103 Conductive PilA subunits have >9% aromatic amino acid residues localized in specific regions along the protein and they do not have large gaps between the aromatic residues.29,30,104 Both the G. anodireducens and G. soli mature PilA monomers have 10.8% aromatic amino acids and their largest gap is 22 amino acids, which is the same as both G. metallireducens and G. sulfurreducens. Therefore, the G. anodireducens and G. soli pili are likely to be conductive.
3.6 Other proteins involved in extracellular electron transfer
In addition to the involvement of electron transport proteins in reduction of extracellular acceptors, proteins involved in biofilm formation also appear to be important. For example, XapD (GSU1501), which is an ATP-dependent transporter that is encoded by a gene located within an operon with extracellular polysaccharide (xapABCDEFGH) biosynthesis genes, is required for reduction of anodes and Fe(III) oxide.105,106 A number of genes involved in extracellular polysaccharide biosynthesis were also more significantly transcribed by G. metallireducens cells grown with Fe(III) oxide provided as the electron acceptor than cells grown with Fe(III) citrate.32 In fact, deletion of Gmet_2029 which codes for a lipopolysaccharide biosynthesis chain length determinant protein completely inhibited Fe(III) oxide reduction by G. metallireducens.32 All four Geobacter genomes in this study had genes coding for both the XapD protein and Gmet_2029 (Table 3, ESI Table S2B†).Another series of proteins involved in electron transfer to poised anodes are part of an electrode sensing network (Esn) and include an MCP (EsnA, GSU1704), a CheW-like scaffolding protein (EsnB, GSU2220), a CheA-like histidine kinase (EsnC, GSU2222), and a diguanylate cyclase (EsnD, GSU3376).87 It has been proposed that these Esn proteins regulate the accumulation of cyclic di-GMP, which is involved in biofilm formation.87,107,108 All of the genomes analyzed in this study have genes coding for all of the Esn proteins (Table 3, ESI Table S2B†).
Other proteins implicated in electrode and/or Fe(III) reduction by G. sulfurreducens that are not considered electron transport proteins include an NHL repeat domain protein (GSU2505) located just downstream from the omcS gene that might be required for proper assembly and/or expression of OmcS, and a transglutaminase domain protein (GSU3361) likely involved in posttranslational modification of proteins involved in extracellular electron transport.105 All four genomes from this study have both of these genes (Table 3, ESI Table S2B†).
3.7 Proteins involved in stress responses and DNA repair
One significant difference that was apparent when TIGR (The Institute for Genomic Research) categories were compared with the Function Category Comparison tool was that SD-1 has more genes coding for proteins involved in DNA replication, recombination and repair (143 genes compared to 86 in PCA, 94 in GS-15, and 98 in SS01). Many DNA recombination and repair genes (uvrA, uvrD, recJ, recN, polA, priA, mutS, and pmbA) are duplicated in the genome (ESI Table S3†), which is a genome trait that is not observed in the other 3 Geobacter species. Additionally, out of the 130 putative open reading frames detected in the plasmid genome, 17 genes coded for proteins involved in DNA replication, recombination or repair (ESI Table S4†). These proteins included the DNA repair proteins RadC and MutS, DNA primase, an ATP-dependent helicase and DNA polymerase III and IV subunits. This redundancy in DNA repair genes might give G. anodireducens an upper edge in stressful conditions like high salt environments or on anode surfaces where protons accumulate and lower the pH,109 as increased levels of these proteins might allow cells to rapidly repair DNA damage.The mechanosensitive ion channel (Msc) proteins are involved in protection from osmotic shock and are induced in response to high salt concentrations in some bacteria.110 Six different genes coding for mechanosensitive ion channel proteins were found in the G. anodireducens (Ga0133348_112933, Ga0133348_111255, Ga0133348_111279, Ga0133348_111853, Ga0133348_111966, Ga0133348_112030), G. soli (Ga0077628_112329, Ga0077628_11701, Ga0077628_11719, Ga0077628_111256, Ga0077628_111444, Ga0077628_111502) and G. sulfurreducens (GSU2794, GSU1557, GSU1633, GSU1723, GSU2316, GSU2357) genomes compared to only three in the G. metallireducens genome (Gmet_2522, Gmet_1942, Gmet_2581), suggesting that these proteins might also help Geobacter survive osmotically stressful environments like those with elevated salt concentrations.
Studies have also shown that many other stress response genes including oxidative stress, heat shock, and universal stress genes are more significantly expressed on anode surfaces and when Fe(III) serves as an electron acceptor.98,111,112 Analysis of other stress response genes (i.e. oxidative stress, heat shock proteins, and universal stress proteins) did not reveal any apparent differences among the four Geobacter species (ESI Table S3†).
4 Conclusions
G. anodireducens is a recently isolated Geobacter species that could be used for practical applications due to its generation of high current densities and its ability to withstand high salt concentrations in BESs. The genome of G. anodireducens was compared to G. sulfurreducens PCA, G. metallireducens GS-15, and G. soli GSS01 in order to uncover potential traits that give SD-1 its superior exoelectrogenic capabilities. The analysis identified many genes likely involved with EET including 87 genes encoding for c-type cytochromes, many of which are homologous to those previously found to be essential for EET in other Geobacter species. In addition, G. anodireducens has substantially more genes for DNA repair and osmotic shock protection than other Geobacter species, likely explaining its ability to survive in the stressful environment found in high salt BES. This study provides an instrumental foundation for future molecular and biochemical analyses of this strain.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant No. LY17D060004), the National Natural Science Foundation of China (Grant No. 51408541), and Joint Zhoushan City and Zhejiang University Cooperation Project (Grant No. 2017C82224).References
- D. R. Lovley, D. E. Holmes and K. P. Nevin, Adv. Microb. Physiol., 2004, 49, 219–286 CrossRef CAS PubMed.
- D. R. Lovley, Annu. Rev. Microbiol., 2017, 71, 643–664 CrossRef CAS PubMed.
- D. R. Lovley, T. Ueki, T. Zhang, N. S. Malvankar, P. M. Shrestha, K. A. Flanagan, M. Aklujkar, J. E. Butler, L. Giloteaux and A.-E. Rotaru, in Adv. Microb. Physiol., Elsevier, 2011, vol. 59, pp. 1–100 Search PubMed.
- H. Liu, S. Grot and B. E. Logan, Environ. Sci. Technol., 2005, 39, 4317–4320 CrossRef CAS PubMed.
- X. Cao, X. Huang, P. Liang, K. Xiao, Y. Zhou, X. Zhang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 7148–7152 CrossRef CAS PubMed.
- K. P. Nevin, T. L. Woodard, A. E. Franks, Z. M. Summers and D. R. Lovley, mBio, 2010, 1, e00103–e00110 CrossRef PubMed.
- B. H. Kim, I. S. Chang, G. C. Gil, H. S. Park and H. J. Kim, Biotechnol. Lett., 2003, 25, 541–545 CrossRef CAS PubMed.
- N. E. Stein, K. J. Keesman, H. V. Hamelers and G. van Straten, Biosens. Bioelectron., 2011, 26, 3115–3120 CrossRef CAS PubMed.
- D. P. Webster, M. A. TerAvest, D. F. Doud, A. Chakravorty, E. C. Holmes, C. M. Radens, S. Sureka, J. A. Gralnick and L. T. Angenent, Biosens. Bioelectron., 2014, 62, 320–324 CrossRef CAS PubMed.
- R. D. Cusick, B. Bryan, D. S. Parker, M. D. Merrill, M. Mehanna, P. D. Kiely, G. Liu and B. E. Logan, Appl. Microbiol. Biotechnol., 2011, 89, 2053–2063 CrossRef CAS PubMed.
- D. R. Lovley, Annu. Rev. Microbiol., 2012, 66, 391–409 CrossRef CAS PubMed.
- S. M. Strycharz-Glaven, R. M. Snider, A. Guiseppi-Elie and L. M. Tender, Energy Environ. Sci., 2011, 4, 4366–4379 RSC.
- N. S. Malvankar, M. T. Tuominen and D. R. Lovley, Energy Environ. Sci., 2012, 5, 8651–8659 RSC.
- L. Shi, H. Dong, G. Reguera, H. Beyenal, A. Lu, J. Liu, H.-Q. Yu and J. K. Fredrickson, Nat. Rev. Microbiol., 2016, 14, 651 CrossRef CAS PubMed.
- M. Breuer, K. M. Rosso, J. Blumberger and J. N. Butt, J. R. Soc., Interface, 2015, 12, 20141117 CrossRef PubMed.
- D. Coursolle, D. B. Baron, D. R. Bond and J. A. Gralnick, J. Bacteriol., 2010, 192, 467–474 CrossRef CAS PubMed.
- E. Marsili, J. B. Rollefson, D. B. Baron, R. M. Hozalski and D. R. Bond, Appl. Environ. Microbiol., 2008, 74, 7329–7337 CrossRef CAS PubMed.
- N. J. Kotloski and J. A. Gralnick, mBio, 2013, 4, e00553-12 CrossRef PubMed.
- K. Dolch, J. Danzer, T. Kabbeck, B. Bierer, J. Erben, A. H. Förster, J. Maisch, P. Nick, S. Kerzenmacher and J. Gescher, Bioresour. Technol., 2014, 157, 284–292 CrossRef CAS PubMed.
- C. E. A. Engel, F. Schattenberg, K. Dohnt, U. Schröder, S. Müller and R. Krull, Frontiers in Bioengineering and Biotechnology, 2019, 7, 60 CrossRef PubMed.
- B. Methe, K. E. Nelson, J. A. Eisen, I. T. Paulsen, W. Nelson, J. Heidelberg, D. Wu, M. Wu, N. Ward and M. Beanan, Scicence, 2003, 302, 1967–1969 CrossRef CAS PubMed.
- M. V. Coppi, C. Leang, S. J. Sandler and D. R. Lovley, Appl. Environ. Microbiol., 2001, 67, 3180–3187 CrossRef CAS PubMed.
- A.-E. Rotaru, T. L. Woodard, K. P. Nevin and D. R. Lovley, Frontiers in Microbiology, 2015, 6, 744 CrossRef PubMed.
- M. Aklujkar, J. Krushkal, G. DiBartolo, A. Lapidus, M. L. Land and D. R. Lovley, BMC Microbiol., 2009, 9, 109 CrossRef PubMed.
- F. Caccavo, D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz and M. J. McInerney, Appl. Environ. Microbiol., 1994, 60, 3752–3759 CAS.
- D. Sun, A. Wang, S. Cheng, M. Yates and B. E. Logan, Int. J. Syst. Evol. Microbiol., 2014, 64, 3485–3491 CrossRef PubMed.
- S. Zhou, G. Yang, Q. Lu and M. Wu, Int. J. Syst. Evol. Microbiol., 2014, 64, 3786–3791 CrossRef PubMed.
- J. E. Butler, N. D. Young and D. R. Lovley, BMC Genomics, 2010, 11, 40 CrossRef PubMed.
- D. E. Holmes, Y. Dang, D. J. Walker and D. R. Lovley, Microb. Genomics, 2016, 2, e000072 CrossRef PubMed.
- D. R. Lovley, Curr. Opin. Electrochem., 2017, 4, 190–198 CrossRef CAS.
- J. A. Smith, P.-L. Tremblay, P. M. Shrestha, O. L. Snoeyenbos-West, A. E. Franks, K. P. Nevin and D. R. Lovley, Appl. Environ. Microbiol., 2014, 80, 4331–4340 CrossRef PubMed.
- J. A. Smith, D. R. Lovley and P.-L. Tremblay, Appl. Environ. Microbiol., 2013, 79, 901–907 CrossRef CAS PubMed.
- T. Mehta, M. V. Coppi, S. E. Childers and D. R. Lovley, Appl. Environ. Microbiol., 2005, 71, 8634–8641 CrossRef CAS PubMed.
- L. A. Zacharoff, D. J. Morrone and D. R. Bond, Frontiers in Microbiology, 2017, 8, 2481 CrossRef PubMed.
- M. Aklujkar, M. Coppi, C. Leang, B. Kim, M. Chavan, L. Perpetua, L. Giloteaux, A. Liu and D. Holmes, Microbiology, 2013, 159, 515–535 CrossRef CAS PubMed.
- D. E. Holmes, S. K. Chaudhuri, K. P. Nevin, T. Mehta, B. A. Methé, A. Liu, J. E. Ward, T. L. Woodard, J. Webster and D. R. Lovley, Environ. Microbiol., 2006, 8, 1805–1815 CrossRef CAS PubMed.
- K. P. Nevin, B.-C. Kim, R. H. Glaven, J. P. Johnson, T. L. Woodard, B. A. Methé, R. J. DiDonato Jr, S. F. Covalla, A. E. Franks and A. Liu, PLoS One, 2009, 4, e5628 CrossRef PubMed.
- K. Inoue, X. Qian, L. Morgado, B.-C. Kim, T. Mester, M. Izallalen, C. A. Salgueiro and D. R. Lovley, Appl. Environ. Microbiol., 2010, 76, 3999–4007 CrossRef CAS PubMed.
- P. L. Tremblay, M. Aklujkar, C. Leang, K. P. Nevin and D. Lovley, Environ. Microbiol. Rep., 2012, 4, 82–88 CrossRef CAS PubMed.
- P. M. Shrestha, A.-E. Rotaru, Z. M. Summers, M. Shrestha, F. Liu and D. R. Lovley, Appl. Environ. Microbiol., 2013, 79, 2397–2404 CrossRef CAS PubMed.
- K. P. Nevin, D. E. Holmes, T. L. Woodard, E. S. Hinlein, D. W. Ostendorf and D. R. Lovley, Int. J. Syst. Evol. Microbiol., 2005, 55, 1667–1674 CrossRef CAS PubMed.
- D. Sun, D. Call, A. Wang, S. Cheng and B. E. Logan, Environ. Microbiol. Rep., 2014, 6, 723–729 CrossRef CAS PubMed.
- D. F. Call, R. C. Wagner and B. E. Logan, Appl. Environ. Microbiol., 2009, 75, 7579–7587 CrossRef CAS PubMed.
- D. F. Call and B. E. Logan, Biosens. Bioelectron., 2011, 26, 4526–4531 CrossRef CAS PubMed.
- E. J. Phillips and D. R. Lovley, Soil Sci. Soc. Am. J., 1987, 51, 938–941 CrossRef CAS.
- R. K. Aziz, D. Bartels, A. A. Best, M. DeJongh, T. Disz, R. A. Edwards, K. Formsma, S. Gerdes, E. M. Glass, M. Kubal, F. Meyer, G. J. Olsen, R. Olson, A. L. Osterman, R. A. Overbeek, L. K. McNeil, D. Paarmann, T. Paczian, B. Parrello, G. D. Pusch, C. Reich, R. Stevens, O. Vassieva, V. Vonstein, A. Wilke and O. Zagnitko, BMC Genomics, 2008, 9, 75 CrossRef PubMed.
- T. N. Petersen, S. Brunak, G. von Heijne and H. Nielsen, Nat. Methods, 2011, 8, 785 CrossRef CAS PubMed.
- A. Krogh, B. Larsson, G. von Heijne and E. L. L. Sonnhammer, J. Mol. Biol., 2001, 305, 567–580 CrossRef CAS PubMed.
- Y. Moriya, M. Itoh, S. Okuda, A. C. Yoshizawa and M. Kanehisa, Nucleic Acids Res., 2007, 35, 182–185 CrossRef PubMed.
- S. F. Altschul, T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller and D. J. Lipman, Nucleic Acids Res., 1997, 25, 3389–3402 CrossRef CAS PubMed.
- C. Camacho, G. Coulouris, V. Avagyan, N. Ma, J. Papadopoulos, K. Bealer and T. L. Madden, BMC Bioinf., 2009, 10, 421 CrossRef PubMed.
- D. Sun, D. F. Call, P. D. Kiely, A. Wang and B. E. Logan, Biotechnol. Bioeng., 2012, 109, 405–414 CrossRef CAS PubMed.
- D. R. Lovley, S. J. Giovannoni, D. C. White, J. E. Champine, E. Phillips, Y. A. Gorby and S. Goodwin, Arch. Microbiol., 1993, 159, 336–344 CrossRef CAS PubMed.
- X. Cai, L. Huang, G. Yang, Z. Yu, J. Wen and S. Zhou, Frontiers in Microbiology, 2018, 9, 1075 CrossRef PubMed.
- M. Miyahara, A. Kouzuma and K. Watanabe, AMB Express, 2015, 5, 34 CrossRef PubMed.
- B. E. Logan and K. Rabaey, Science, 2012, 337, 686–690 CrossRef CAS PubMed.
- D. R. Lovley, Energy Environ. Sci., 2011, 4, 4896–4906 RSC.
- A. Y. Mulkidjanian, M. Y. Galperin, K. S. Makarova, Y. I. Wolf and E. V. Koonin, Biol. Direct, 2008, 3, 13 CrossRef PubMed.
- R. A. Capaldi and R. Aggeler, Trends Biochem. Sci., 2002, 27, 154–160 CrossRef CAS PubMed.
- V. Kabaleeswaran, N. Puri, J. E. Walker, A. G. Leslie and D. M. Mueller, EMBO J., 2006, 25, 5433–5442 CrossRef CAS PubMed.
- V. Müller and G. Grüber, Cell. Mol. Life Sci., 2003, 60, 474–494 CrossRef.
- Ü. Coskun, Y. L. Chaban, A. Lingl, V. Müller, W. Keegstra, E. J. Boekema and G. Grüber, J. Biol. Chem., 2004, 279, 38644–38648 CrossRef PubMed.
- M. Nakanishi-Matsui and M. Futai, IUBMB Life, 2006, 58, 318–322 CrossRef CAS PubMed.
- M. Ito, M. Morino and T. A. Krulwich, Frontiers in Microbiology, 2017, 8, 2325 CrossRef PubMed.
- T. A. Krulwich, G. Sachs and E. Padan, Nat. Rev. Microbiol., 2011, 9, 330 CrossRef CAS PubMed.
- E. Padan, E. Bibi, M. Ito and T. A. Krulwich, Biochim. Biophys. Acta, Biomembr., 2005, 1717, 67–88 CrossRef CAS PubMed.
- D. G. Fuster and R. T. Alexander, Pflügers Archiv: European Journal of Physiology, 2014, 466, 61–76 CrossRef CAS PubMed.
- E. Padan and M. Landau, in The Alkali Metal Ions: Their Role for Life, Springer, 2016, pp. 391–458 Search PubMed.
- T. Zhang, T. S. Bain, K. P. Nevin, M. A. Barlett and D. R. Lovley, Appl. Environ. Microbiol., 2012, 78, 8304–8310 CrossRef CAS PubMed.
- J. E. Butler, Q. He, K. P. Nevin, Z. He, J. Zhou and D. R. Lovley, BMC Genomics, 2007, 8, 180 CrossRef PubMed.
- T. Zhang, P.-L. Tremblay, A. K. Chaurasia, J. A. Smith, T. S. Bain and D. R. Lovley, Appl. Environ. Microbiol., 2013, 79, 7800–7806 CrossRef CAS PubMed.
- M. Carmona, M. T. Zamarro, B. Blázquez, G. Durante-Rodríguez, J. F. Juárez, J. A. Valderrama, M. J. Barragán, J. L. García and E. Díaz, Microbiol. Mol. Biol. Rev., 2009, 73, 71–133 CrossRef CAS PubMed.
- S. Six, S. C. Andrews, R. E. Roberts, G. Unden and J. R. Guest, Biochem. Soc. Trans., 1993, 342S CrossRef CAS PubMed.
- N. Nógrády, A. Imre, I. Rychlik, P. A. Barrow and B. Nagy, Vet. Microbiol., 2003, 97, 191–199 CrossRef.
- R. Ullmann, R. Gross, J. Simon, G. Unden and A. Kröger, J. Bacteriol., 2000, 182, 5757–5764 CrossRef CAS PubMed.
- J. E. Butler, R. H. Glaven, A. Esteve-Núnez, C. Núnez, E. S. Shelobolina, D. R. Bond and D. R. Lovley, J. Bacteriol., 2006, 188, 450–455 CrossRef CAS PubMed.
- H.-Y. Tang, D. E. Holmes, T. Ueki, P. A. Palacios and D. R. Lovley, mBio, 2019, 10, e00303–e00319 CrossRef PubMed.
- J. A. Smith, K. P. Nevin and D. R. Lovley, Frontiers in Microbiology, 2015, 6, 121 Search PubMed.
- Z. M. Summers, H. E. Fogarty, C. Leang, A. E. Franks, N. S. Malvankar and D. R. Lovley, Scicence, 2010, 330, 1413–1415 CrossRef CAS PubMed.
- L. Shi, T. C. Squier, J. M. Zachara and J. K. Fredrickson, Mol. Microbiol., 2007, 65, 12–20 CrossRef CAS PubMed.
- C. E. Levar, C. L. Hoffman, A. J. Dunshee, B. M. Toner and D. R. Bond, ISME J., 2017, 11, 741 CrossRef CAS PubMed.
- C. E. Levar, C. H. Chan, M. G. Mehta-Kolte and D. R. Bond, mBio, 2014, 5, e02034 CrossRef CAS PubMed.
- L. Zacharoff, C. H. Chan and D. R. Bond, Bioelectrochemistry, 2016, 107, 7–13 CrossRef CAS PubMed.
- J. R. Lloyd, C. Leang, A. L. H. Myerson, M. V. Coppi, S. Cuifo, B. Methe, S. J. Sandler and D. R. Lovley, Biochem. J., 2003, 369, 153–161 CrossRef CAS PubMed.
- Y. Liu, Z. Wang, J. Liu, C. Levar, M. J. Edwards, J. T. Babauta, D. W. Kennedy, Z. Shi, H. Beyenal and D. R. Bond, Environ. Microbiol. Rep., 2014, 6, 776–785 CrossRef CAS PubMed.
- L. Shi, J. K. Fredrickson and J. M. Zachara, Frontiers in Microbiology, 2014, 5, 657 Search PubMed.
- C. H. Chan, C. E. Levar, F. Jiménez-Otero and D. R. Bond, J. Bacteriol., 2017, 199, e00340-17 CrossRef PubMed.
- F. J. Otero, C. H. Chan and D. R. Bond, J. Bacteriol., 2018, 200, e00347-18 CrossRef PubMed.
- Y. Liu, J. K. Fredrickson, J. M. Zachara and L. Shi, Frontiers in Microbiology, 2015, 6, 1075 Search PubMed.
- C. Leang, M. V. Coppi and D. Lovley, J. Bacteriol., 2003, 185, 2096–2103 CrossRef CAS PubMed.
- C. Leang and D. R. Lovley, Microbiology, 2005, 151, 1761–1767 CrossRef CAS PubMed.
- X. Qian, G. Reguera, T. Mester and D. R. Lovley, FEMS Microbiol. Lett., 2007, 277, 21–27 CrossRef CAS PubMed.
- C. Leang, X. Qian, T. Mester and D. R. Lovley, Appl. Environ. Microbiol., 2010, 76, 4080–4084 CrossRef CAS PubMed.
- D. J. Filman, S. F. Marino, J. E. Ward, L. Yang, Z. Mester, E. Bullitt, D. R. Lovley and M. Strauss, bioRxiv, 2018, 492645 Search PubMed.
- F. Wang, Y. Gu, J. P. O'Brien, M. Y. Sophia, S. E. Yalcin, V. Srikanth, C. Shen, D. Vu, N. L. Ing and A. I. Hochbaum, Cell, 2019, 177, 361–369 CrossRef CAS PubMed.
- J. Yun, N. S. Malvankar, T. Ueki and D. R. Lovley, ISME J., 2016, 10, 310–320 CrossRef CAS PubMed.
- Y. Tan, R. Y. Adhikari, N. S. Malvankar, J. E. Ward, K. P. Nevin, T. L. Woodard, J. A. Smith, O. L. Snoeyenbos-West, A. E. Franks and M. T. Tuominen, Frontiers in Microbiology, 2016, 7, 980 Search PubMed.
- D. E. Holmes, R. A. O'neil, M. A. Chavan, L. A. N'guessan, H. A. Vrionis, L. A. Perpetua, M. J. Larrahondo, R. DiDonato, A. Liu and D. R. Lovley, ISME J., 2009, 3, 216 CrossRef CAS PubMed.
- D. E. Holmes, T. Mester, R. A. O'Neil, L. A. Perpetua, M. J. Larrahondo, R. Glaven, M. L. Sharma, J. E. Ward, K. P. Nevin and D. R. Lovley, Microbiology, 2008, 154, 1422–1435 CrossRef CAS PubMed.
- T. Mehta, S. E. Childers, R. Glaven, D. R. Lovley and T. Mester, Microbiology, 2006, 152, 2257–2264 CrossRef CAS PubMed.
- G. Reguera, K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen and D. R. Lovley, Nature, 2005, 435, 1098–1101 CrossRef CAS PubMed.
- N. S. Malvankar, M. Vargas, K. P. Nevin, A. E. Franks, C. Leang, B.-C. Kim, K. Inoue, T. Mester, S. F. Covalla and J. P. Johnson, Nat. Nanotechnol., 2011, 6, 573–579 CrossRef PubMed.
- M. Vargas, N. S. Malvankar, P.-L. Tremblay, C. Leang, J. A. Smith, P. Patel, O. Synoeyenbos-West, K. P. Nevin and D. R. Lovley, mBio, 2013, 4, e00105–e00113 CrossRef PubMed.
- D. J. Walker, R. Y. Adhikari, D. E. Holmes, J. E. Ward, T. L. Woodard, K. P. Nevin and D. R. Lovley, ISME J., 2018, 12, 48 CrossRef CAS PubMed.
- J. B. Rollefson, C. E. Levar and D. R. Bond, J. Bacteriol., 2009, 191, 4207–4217 CrossRef CAS PubMed.
- J. B. Rollefson, C. S. Stephen, M. Tien and D. R. Bond, J. Bacteriol., 2011, 193, 1023–1033 CrossRef CAS PubMed.
- Z. F. Hallberg, X. C. Wang, T. A. Wright, B. Nan, O. Ad, J. Yeo and M. C. Hammond, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 1790–1795 CrossRef CAS PubMed.
- M. Valentini and A. Filloux, J. Biol. Chem., 2016, 291, 12547–12555 CrossRef CAS PubMed.
- A. E. Franks, K. P. Nevin, H. Jia, M. Izallalen, T. L. Woodard and D. R. Lovley, Energy Environ. Sci., 2009, 2, 113–119 RSC.
- E. S. Haswell, R. Phillips and D. C. Rees, Structure, 2011, 19, 1356–1369 CrossRef CAS PubMed.
- A. E. Franks, K. P. Nevin, R. H. Glaven and D. R. Lovley, ISME J., 2010, 4, 509–519 CrossRef PubMed.
- P. J. Mouser, D. E. Holmes, L. A. Perpetua, R. DiDonato, B. Postier, A. Liu and D. R. Lovley, ISME J., 2009, 3, 454–465 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary data related to this article can be found in the online version. See DOI: 10.1039/c9ra02343g |
| This journal is © The Royal Society of Chemistry 2019 |