Open Access Article
Open Access ArticleProbing the glass transition in reversible cross-linked polymer composites†
Yong-jin Peng *abe,
Chen-ting Caib,
Chang-jun Wanga,
Zhong-fu Zuo*cde and
Xue-zheng Liu*ce
*abe,
Chen-ting Caib,
Chang-jun Wanga,
Zhong-fu Zuo*cde and
Xue-zheng Liu*ce
aCollege of Comprehensive Studies, Jinzhou Medical University, Jinzhou 121001, P. R. China
bCollege of Chemistry, Nankai University, Tianjin 300071, P. R. China
cDepartment of Anatomy, Histology and Embryology, Jinzhou Medical University, Jinzhou 121001, P. R. China. E-mail: jzmuzzf@163.com; jzmulxz@163.com
dDepartment of Anatomy, Histology and Embryology, Postdoctoral Research Station, Guangxi Medical University, Nanning 530021, P. R. China
eLiaoning Key Laboratory of Diabetic Cognitive and Perceptive Dysfunction, Jinzhou Medical University, Jinzhou 121001, P. R. China
First published on 16th May 2019
Abstract
Understanding the nature of glass transition is still a great challenge. Glass transition is widely observed in many glassy materials; however, it has never been unambiguously observed in reversible cross-linked polymer, which is an ideal model of the percolation process. Herein, we report the synthesis of a reversible cross-linked polymer incorporated with four-armed Diels–Alder (DA) dynamic covalent bonds, and the robust experimental observation of percolation-induced glass transition in this reversible four-armed cross-linked polymer (DAMF1). Temperature-modulated differential scanning calorimetry (TMDSC) experiment results clearly revealed the presence of a glass transition along with an endothermic or exothermic peak associated with DA/retro-DA (RDA) reaction related to the reconstitution/disassociation of the DAMF1's four-armed cross-linked network. In situ 13C variable-temperature solid-state NMR experiments further confirmed the DA/RDA reaction during glass transition at a molecular level. The above experimental results provide a direct experimental evidence for the recently developed percolation model of glass transition, which provides new insights into the nature of glass transition.
1 Introduction
The nature of glass transition is one of the most important unresolved problems. Although many theories have been proposed, the understanding of glass transition has remained unclear and is still a great challenge.1–4 Glass transition is widely observed in many glassy materials, such as homo-polymers, polymer blends and cross-linked polymers. However, it has hardly been unambiguously observed in a reversible cross-linked polymer, which is an ideal model of the percolation process.5–7 Reversible cross-linked polymers based on dynamic covalent bonds have been widely reported in the past decade; however, few of them have fast DA kinetics except the cross-linked polyketones.8–18 Herein, by the incorporation of Diels–Alder dynamic covalent bonds into a four-armed cross-linked polymer network, we have reported the experimental observation of percolation-induced glass transition in a reversible highly cross-linked polymer. The experimental results provide direct evidence for the recently developed percolation model of glass transition.19–24The glass transition of a glass-forming system can usually be realized through two kinds of methods: (i) a physical method that usually lowers the temperature or increases the pressure of the system, and (ii) a chemical method that, for example, can reduce the system's dynamism and make the system turn into the glassy state through the polymerization reaction.25–27
Recently, a dynamic percolated network structure indicated the beginning of the glass transition process, which was confirmed by several experimental results and theoretical simulation studies.28–31 Subsequently, several glass transition theories were built based on this significant experimental result, including the twinkling fractal theory by Wool et al.32,33 and the configuron percolation theory by Ojovan et al.22
Actually, the introduction of the concept of percolation into the explanation of the vitrification process has a long history. The earliest idea of using percolation to explain the glass transition may come from Cohen and Grest. Based on the percolation concept, they extended the free volume theory to describe the thermodynamic behavior of the glass forming system. Their theory involved the entropy of the system and was also an important application of the percolation theory. D. Long and F. Lequeux explained the dynamic heterogeneity of the system near the glass transition, and the difference in the glass transition behavior between 2D films and 3D bodies by introducing the percolation model of glass transition.34 Nevertheless, the details of the heterogeneous dynamic behavior within the dynamical percolated network structure have not been clearly elucidated.
The percolation model of glass transition was proposed by Wool et al., which stated that glass transition happening with the rigid percolation process has recently attracted significant attention. However, few experimental evidences have been reported for this theory. The simplest and well-known percolation model is shown in Scheme 1a. Percolation happens at a certain ratio of unclipped bonds to the whole bonds in the network. In this communication, a new strategy is proposed to simulate the cross-linked network shown in Scheme 1b. Our key idea is to construct a reversible cross-linked network using dynamic covalent bonds; thus, a percolation process could be directly observed in a real glassy material under the DA/RDA reaction. As the glass transition is reversible, there are two challenges for the design of such a DA polymer: (1) cross-linked network without any other disturbed transition and (2) fast DA/RDA kinetics related to the thermal behavior.
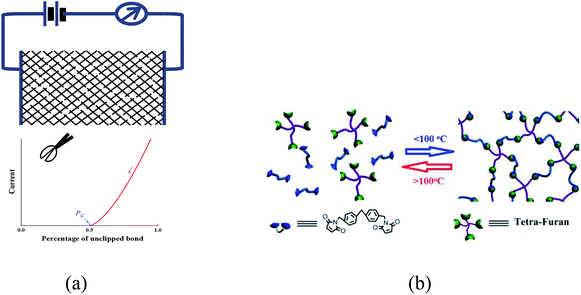 | ||
| Scheme 1 (a) A simple model of percolation. (b) Simplified 2D schematic depiction of reversible four-armed cross-linked polymer with dynamic DA covalent. | ||
2 Experiment
Materials and sample preparation
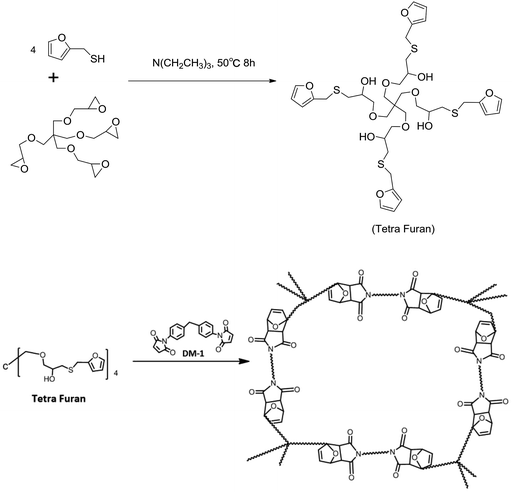
Pentaerythritol glycidyl ether, furfuryl mercaptan and triethylamine were purchased from J&K Scientific. Diphenylmethane bismaleimide (DM-1) was purchased from Sigma-Aldrich. All the reagents were used without any further purification.The synthesis pathway of the polymer is shown in Scheme 2, where pentaerythritol glycidyl ether (11.4 g, 0.1 mol) was reacted with furfuryl mercaptan (9.0 g, 0.025 mol) and triethylamine (0.4 g) under 50 °C for 8 h. The preliminary product was further purified by silica gel column chromatography, and tetra furan (0.6 g), which had a four-armed molecular with DA reactive end-groups, was obtained. The chemical structures were confirmed by liquid 1H and 13C NMR experiments (see ESI†). Then, a mixture of the cross-linker (DM-1) and tetra furan in dimethylacetamide (DMAc) with same DA equivalent was cast on a PTFE plate. Vacuum evaporation of the solvent at 65 °C, followed by the heating process, produced solid DA polymers (DAMF1). The resulting bulk polymers were all uniform and fully transparent and mechanically very hard due to high cross-link density.
DSC measurement
Mettler-Toledo DSC1 STARe type instrument with a high sensitivity sensor of the FRS5 was used for the conventional and modulated DSC experiments. The temperature and heat flow were calibrated before the measurement. In the conventional DSC experiments, all samples were heated and cooled several times at the rate of 10 °C min−1 in nitrogen atmosphere. The temperature of the midpoint, corresponding to the change in the specific heat, was taken as the glass transition temperature (Tg). In the modulated DSC experiments, all samples were heated and cooled for several times at the rate of 2 °C min−1 in a nitrogen atmosphere. The reversible and non-reversible heat flow information can be simultaneously obtained in one modulated DSC experiment.Dynamic mechanical analysis (DMA) studies
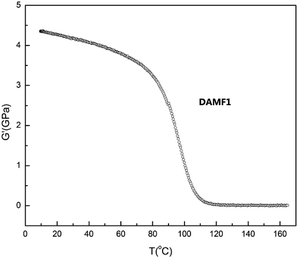
NETZSCH DMA242 Analyzer was used for DMA measurements in the stretching mode. The 10 × 2 × 0.5 mm3 samples were heated from 0 °C to 170 °C at 2 °C min−1 under nitrogen gas protection until the sample became soft. The storage modulus (G′) of the sample as a function of temperature is shown in Fig. 2.NMR analysis
Bruker-AVANCE III NMR spectrometer was used for the liquid NMR experiments. The resonant frequencies of 1H and 13C were 400.01 and 100.02 MHz, respectively, and the solvent was CDCl3. Varian Infinity plus-400 NMR spectrometer was used for the solid NMR experiment with the resonance frequencies of 399.68 and 100.12 MHz for 1H and 13C, respectively.3 Results and discussion
Fig. 1(a) shows the thermal behavior of DAMF1, which was measured by repeated DSC cycles to verify the reversible temperature dependence of the DA and RDA reaction. An overlapping of the obvious endothermic and exothermic transition at about 80–120 °C can be observed during successive thermal cycles. The observed repeated endothermic and exothermic transitions of DAMF1 could be predominantly attributed to the RDA and DA reactions, respectively. The slight deviations of the repeated DSC traces can be attributed to the incomplete reconstitution of the DA network within the relatively short time scale of the DSC experiments. It is noteworthy to mention that with respect to the thermal behavior of the previously reported DA polymers, few of them had such a fast DA kinetics, except the cross-linked polyketones. This polymer provides a clear example to examine the hidden glass transition in it.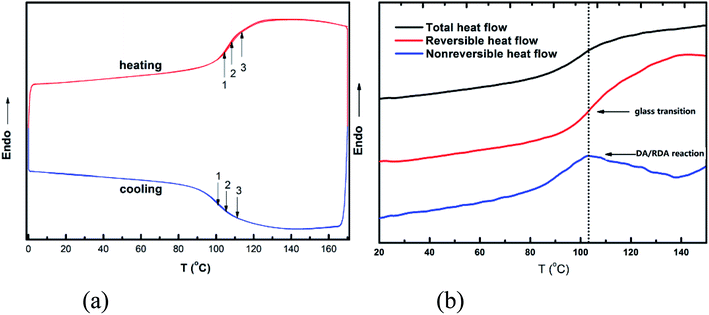 | ||
| Fig. 1 (a) DSC traces of DAMF1 in repeated cooling and heating cycles at rate of 10 °C min−1. (b) Modulated DSC (TOPEM) traces of DAMF1 at a cooling rate of 2 °C min−1. | ||
It can be seen in Fig. 1(b) that there was a heat flow step-up near 103 °C within the total heat flow curve. However, there were actually two processes combined in that step-up: glass transition and DA/RDA reaction, both of which cannot be distinguished in the total heat flow curve. The modulated DSC experiment can provide the reversible and non-reversible heat flow by measuring the samples with multiple frequencies. Thus, the step-up in the reversible heat flow showed there was a glass transition process near 103 °C and the peak in the non-reversible heat flow at this temperature range indicating that the DA/RDA reaction occurred at the same time. Thus, it is reasonable to deduce that with lowering (increasing) the temperature, the formation (fracture) of the critical percolation network owing to the DA (RDA) reaction in the system led to the glass transition process. Therefore, the experimental results indicated that the glass transition of this kind of polymer was a network percolation process and provided an evidence for the percolation model of glass transition.
Dynamic mechanical analysis (DMA) was further conducted in shear sandwich mode to gain an understanding of the thermo-mechanical properties of DAMF1. Fig. 2 shows the storage modulus (G′) of DAMF1 as a function of temperature. A clear transition can be observed ranging from 80 °C to 120 °C, which could be attributed to the RDA reactions in the system.
Variable temperature (VT), solid-state 13C direct polarization (DP), magic angle spinning (MAS), and cross polarization (CP) MAS NMR spectroscopy were further utilized to monitor the thermally reversible DA/RDA reactions in the solid DAMF1 at the molecular level, as used in our previous study. The results are shown in Fig. 3. Please refer to the ESI† or ref. 6 for the assignment of peaks with similar DA adducts. In Fig. 3, when heating the sample from 30 °C to 140 °C, it was clear that the peaks at 45, 76, 87 and 170 ppm in the 13C DPMAS spectrum disappeared at a higher temperature; moreover, the intensities of the peaks at 99, 103 and 144 ppm grew significantly. These results indicated the presence of un-reacted furan moieties resulting from the disconnection of cross-linked network through the RDA reaction. When cooling the sample from 140 °C to 30 °C, the 13C CPMAS spectrum was almost identical to the one before the heating process. The peaks of DA adducts appeared again and those of furan moieties disappeared, indicating almost complete reconstruction of all the disconnected linkages through the DA reaction. Therefore, the VT-NMR experiments strongly confirmed the reversible cross-linking of DAMF1 at a molecular level.
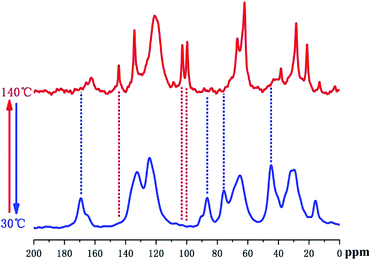 | ||
| Fig. 3 In situ solid-state 13C VT-NMR spectra of DAMF1 at thermal cycling of 30 °C (CPMAS), 140 °C (DPMAS), respectively. | ||
A similar phenomenon of percolation-induced glass transition was also detected in our recently designed DA polymer by TMDSC and SSNMR techniques (summarized in another study), which implied that the percolation-induced glass should be a robust phenomenon for reversible cross-linked polymers.
In summary, we reported the robust experimental observation of percolation-induced glass transition in a reversible four-armed cross-linked polymer, which was incorporated with Diels–Alder dynamic covalent. TMDSC clearly revealed the presence of a glass transition along with an endothermic and exothermic peak associated with DA/retro-DA reaction related to the reconstitution/disassociation of the four-armed cross-linked network. In situ 13C variable-temperature SSNMR experiments further confirmed the DA/retro-DA reaction during glass transition at a molecular level. The above experimental results provided a direct experimental evidence for the recently developed percolation model of glass transition, which provided new insights into the nature of glass transition.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the Natural Science Foundation of Liaoning Province (20180550512), National Natural Science Foundation of China (81571383) and China Postdoctoral Science Foundation (2017M612870).References
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- A. Ninarello, L. Berthier and D. Coslovich, Phys. Rev. X, 2017, 7, 021039 Search PubMed.
- S. Napolitano, E. Glynos and N. B. Tito, Rep. Prog. Phys., 2017, 80, 036602 CrossRef PubMed.
- L. Atia, D. Bi, Y. Sharma, J. A. Mitchel, B. Gweon, S. A. Koehler, S. J. DeCamp, B. Lan, J. H. Kim, R. Hirsch, A. F. Pegoraro, K. H. Lee, J. R. Starr, D. A. Weitz, A. C. Martin, J.-A. Park, J. P. Butler and J. J. Fredberg, Nat. Phys., 2018, 14, 613–620 Search PubMed.
- S. Yu, R. Zhang, Q. Wu, T. Chen and P. Sun, Adv. Mater., 2013, 25, 4912–4917 CrossRef CAS PubMed.
- S. Chen, F. Wang, Y. Peng, T. Chen, Q. Wu and P. Sun, Macromol. Rapid Commun., 2015, 36, 1687–1692 CrossRef CAS PubMed.
- Y.-J. Peng, X. He, Q. Wu, P.-C. Sun, C.-J. Wang and X.-Z. Liu, Polymer, 2018, 149, 154–163 CrossRef CAS.
- M. A. Tasdelen, Polym. Chem., 2011, 2, 2133–2145 RSC.
- Z. Stirn, A. Rucigaj and M. Krajnc, eXPRESS Polym. Lett., 2016, 10, 537–547 CrossRef CAS.
- J. Steinkoenig, M. M. Zieger, H. Mutlu and C. Barner-Kowollik, Macromolecules, 2017, 50, 5385–5391 CrossRef CAS.
- X. Shen, X. Liu, J. Wang, J. Dai and J. Zhu, Ind. Eng. Chem. Res., 2017, 56, 8508–8516 CrossRef CAS.
- L. M. Polgar, M. van Duin, A. A. Broekhuis and F. Picchioni, Macromolecules, 2015, 48, 7096–7105 CrossRef CAS.
- N. Okhay, C. Jegat, N. Mignard and M. Taha, React. Funct. Polym., 2013, 73, 745–755 CrossRef CAS.
- J. Kida, K. Imato, R. Goseki, M. Morimoto and H. Otsuka, Chem. Lett., 2017, 46, 992–994 CrossRef CAS.
- H. Hou, J. Yin and X. Jiang, Adv. Mater., 2016, 28, 9126–9132 CrossRef CAS PubMed.
- T. Engel and G. Kickelbick, Chem. Mater., 2013, 25, 149–157 CrossRef CAS.
- T. S. Coope, D. H. Turkenburg, H. R. Fischer, R. Luterbacher, H. Van Bracht and I. P. Bond, Smart Mater. Struct., 2016, 25, 084010 CrossRef.
- R. Araya-Hermosilla, G. Fortunato, A. Pucci, P. Raffa, L. Polgar, A. A. Broekhuis, P. Pourhossein, G. M. R. Lima, M. Beljaars and F. Picchioni, Eur. Polym. J., 2016, 74, 229–240 CrossRef CAS.
- R. P. Wool, in 6th International Conference on Times of Polymers, ed. A. Damore, L. Grassia and D. Acierno, 2012, pp. 5–7 Search PubMed.
- R. P. Wool, Abstracts of Papers of the American Chemical Society, 2004, vol. 228, p. U515 Search PubMed.
- D. S. Patil, M. Konale, S. Cozic, L. Calvez, V. Zima, T. Wagner, J. S. McCloy and D. Le Coq, J. Alloys Compd., 2019, 782, 375–383 CrossRef CAS.
- M. I. Ojovan, J. Non-Cryst. Solids, 2013, 382, 79–86 CrossRef CAS.
- Y.-j. Peng, Y.-l. Liu, Q. Wu and P.-c. Sun, Anal. Sci., 2017, 33, 1071–1076 CrossRef CAS PubMed.
- Y.-j. Peng, C.-t. Cai, R.-c. Zhang, T.-h. Chen, P.-c. Sun, B.-h. Li, X.-l. Wang, G. Xue and A.-C. Shi, Chin. J. Polym. Sci., 2016, 34, 446–456 CrossRef CAS.
- S. Caponi and S. Corezzi, in 6th International Conference on Times of Polymers, ed. A. Damore, L. Grassia and D. Acierno, 2012, pp. 33–35 Search PubMed.
- S. Corezzi, L. Comez, G. Monaco, R. Verbeni and D. Fioretto, Phys. Rev. Lett., 2006, 96, 255702 CrossRef CAS PubMed.
- S. Corezzi, D. Fioretto and P. Rolla, Nature, 2002, 420, 653–656 CrossRef CAS PubMed.
- Y. Yilmaz, A. Erzan and O. Pekcan, Eur. Phys. J. E: Soft Matter Biol. Phys., 2002, 9, 135–141 CrossRef CAS PubMed.
- A. R. C. Baljon, J. Billen and R. Khare, Phys. Rev. Lett., 2004, 93, 255701 CrossRef PubMed.
- J. C. Conrad, P. P. Dhillon, E. R. Weeks, D. R. Reichman and D. A. Weitz, Phys. Rev. Lett., 2006, 97, 265701 CrossRef PubMed.
- Y.-j. Peng, Y.-l. Liu, J.-h. Hao, R.-c. Zhang and P.-c. Sun, RSC Adv., 2017, 7, 56311–56316 RSC.
- R. P. Wool and A. Campanella, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 2578–2590 CrossRef CAS.
- R. P. Wool, in 5th International Conference on Times of Polymers Top and Composites, ed. A. Damore, D. Acierno and L. Grassia, 2010, pp. 215–217 Search PubMed.
- P. Sotta and D. Long, Eur. Phys. J. E: Soft Matter Biol. Phys., 2003, 11, 375–387 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01942a |
| This journal is © The Royal Society of Chemistry 2019 |