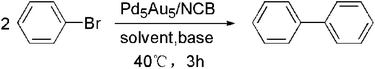Open Access Article
Open Access ArticlePdAu alloy nanoparticles supported on nitrogen-doped carbon black as highly active catalysts for Ullmann coupling and nitrophenol hydrogenation reactions†
Fengyan Han‡
 *ab,
Jiawei Xia‡a,
Xinglong Zhangb and
Yongsheng Fu
*ab,
Jiawei Xia‡a,
Xinglong Zhangb and
Yongsheng Fu a
a
aKey Laboratory for Soft Chemistry and Functional Materials of Ministry Education, Nanjing University of Science and Technology, Nanjing 210094, China
bCollege of Science, Institute of Materials Physics and Chemistry, Nanjing Forestry University, Nanjing 210037, China. E-mail: fengyanhan@njfu.edu.cn
First published on 5th June 2019
Abstract
Noble metal-based catalysts have been proven to be active for catalytic organic reactions. The selectivity and conversion can be improved by integration with proper carrier materials, and further modulated by tuning the composition as well as the electronic structure of the active noble metals. Compared with unsupported monometallic catalysts, the synergistic interactions between neighboring metals and the combined effects between the carrier materials and the active components often give rise to positive influences on the enhancement of the catalytic efficiency and selectivity. In this work, we report a facile process for the fabrication of nitrogen-doped carbon black (NCB) supported PdAu bimetallic nanoparticles (NPs) with a uniform dispersion and narrow size distribution. The PdAu/NCB catalyst with a Pd/Au mole ratio of 1/1 shows the highest activity towards both Ullmann coupling reactions of aryl halides and the hydrogenation reaction of nitrophenols. Moreover, this bimetallic catalyst also exhibits a superior recycling durability to that of monometallic Pd/NCB and Au/NCB catalysts. The enhanced catalytic performance of the bimetallic catalyst is mainly due to the large BET specific surface area (125.45 m2 g−1) and the synergy between the individual components of the catalyst.
Introduction
The synthesis of symmetrical di- or poly-aromatic compounds is one of the important processes in industry, and shows a wide application in the production of pharmaceuticals, natural products and organic conductors.1–3 The Pd-catalyzed Suzuki, Stille, Negishi and Ullmann coupling reactions are commonly used for the fabrication of carbon–carbon bonds to generate biaromatic compounds.4–7 Among the reactions above, the homogeneous Ullmann coupling of aryl halides is one of the most versatile strategies for the synthesis of symmetrical biaromatic compounds. However, the traditional homogeneous Ullmann coupling reaction is always carried out at a relatively high temperature in an organic medium with a high boiling point, leading to extensive energy-consumption and complicated post-processing. Hence, the development of highly efficient catalysts and the exploration of mild reaction conditions are in great demand. Even though a variety of homogeneous catalysts have been explored recently for homogeneous Ullmann coupling reactions,8–11 the exorbitant price of the complex catalysts, non-recyclable property and complicated post-processing are still the main blocks for such a homogeneous catalysis.To date, with the rapid development of nanotechnology, more and more attention has been paid to the design and synthesis of nanomaterials with unique structures as catalysts for organic reactions. Metallic NPs were widely studied in the areas of the hydrogenation reaction, coupling reaction, etc.12,13 Compared with monometallic NPs, multimetallic NPs often reveal enhanced optical, electronic and catalytic activity on account of the synergistic effect between different metal atoms.14–18 As a result, remarkable progress was achieved in the controllable synthesis and potential applications of bimetallic catalysts on the basis of a well-understood design strategy. The most studied bimetallic catalysts, such as Pd-based bimetallic catalysts, including Pd–Au,19,20 Pd–Ni,21 Pd–Ag22 and Pd–Cu,23 have been widely investigated for varied catalytic applications in oxidation, reduction and coupling reactions. Among these bimetallic catalysts, bimetallic PdAu materials have attracted extensive attention in the field of heterogeneous Ullmann coupling reactions due to their excellent catalytic activity and durability. For example, Sakurai et al. synthesized bimetallic Au/Pd nanoclusters for the Ullmann coupling of chloroarenes under an argon atmosphere at a low temperature with a high yield.24 Wang and co-workers prepared Au–Pd alloyed nanochain networks by using 4-aminopyridine as the structure-directing agent for the Ullmann intermolecular and intramolecular homocoupling and the catalyst exhibited a high activity and good stability.25
It is reported that the combination of active components with some certain carrier materials can largely improve the activity and stability of the catalysts. The carrier materials can help disperse the active components and prevent their aggregation, and meanwhile reduce the dosage of active components, especially for the precious metals, which is in favor of a controlled catalysis cost. Among various carrier materials, such as hydrotalcite,26 graphitic carbon nitride,27 metal–organic frameworks,28 graphene29,30 and carbon materials,31 XC-72 carbon black is one of the most promising in the near future because of many admirable properties, such as permeable pores, large surface area, excellent conductivity, good stability and chemical inertness.32–34 As reported, nitrogen doping is considered one of the most effective ways to further modify the properties of carbon materials, where the more negatively-charged nitrogen atoms can help to gain interactions between the active components and the substrates.35–39 The lone pair electrons in the nitrogen atoms can also act as Lewis base sites, which can partly modulate the electronic properties, basicity and hydrophilicity of carbon black.40,41 To the best of our knowledge, few works have been reported about the well-dispersed nitrogen-doped carbon black (NCB) supported bimetallic PdAu NPs in organic catalysis.
In this work, we report a facile, mild and surfactant-free process for the synthesis of a well-dispersed NCB supported bimetallic PdAu catalyst with an average particle size of 3.4 nm. The Pd/Au mole ratios can be easily tuned through controlling the amounts of the metal precursors. The PdAu/NCB catalyst with a Pd/Au ratio of 1/1 exhibits the most activity for both the Ullmann coupling reaction and the hydrogenation reaction of nitrophenols. Compared with the monometallic Pd/NCB or Au/NCB catalysts, the synergistic effect between two different metals and the combined effect between the nanoparticles and NCB lead to an enhanced catalytic performance, achieving a cost-effective Ullmann coupling reaction and nitrophenol hydrogenation reaction.
Experimental details
Materials
XC-72 carbon black (CB) was supplied by the Cabot Corporation. Bromobenzene (AR., 99%) and magnesium sulfate anhydrous (MgSO4, AR.) were purchased from the Aladdin Industrial Cooperation. Ethylene glycol (EG, 99.0%), nitric acid (HNO3, 65–68%), isopropanol (IPA) and potassium carbonate (K2CO3) were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. L-Ascorbic acid (AR.) was purchased from Xilong Scientific Co., Ltd. Potassium tetrachloropalladate (K2PdCl4, 99.99%) and chloroauric acid (HAuCl4·9H2O, 99.99%) were obtained from Alfa Aesar. All the chemicals in this work were used without further purification, and deionized water was used for the preparation of various solutions throughout the entire study.Synthesis of PdAu/NCB
Nitrogen-doped carbon black (NCB) was first synthesized through a hydrothermal process according to our previous work.42 PdxAuy/NCB nanocatalysts with a metal loading of 10 wt% were fabricated through a co-reduction process in the liquid system, where x and y are the relative molar ratios of Pd and Au, respectively, and x + y = 10. Typically, 20 mg of NCB was dispersed in 20 mL of ethylene glycol (EG) with sonication for 10 min to obtain a uniform suspension. Then 5 mL of L-ascorbic acid (1.0 mg mL−1) aqueous solution was dropped in with continuous stirring. After that, 5 mL of a fresh aqueous solution of metal precursors which contains the required amounts of K2PdCl4 and HAuCl4·9H2O was added into the above mixture with vigorous stirring at room temperature. After 2 h, the solid residue was filtered, washed with water and freeze-dried for further use, labelled as PdxAuy/NCB. The schematic fabrication process is shown in Scheme 1. | ||
| Scheme 1 Synthetic scheme of PdAu/NCB NPs for the Ullmann coupling of bromobenzene and reduction of 4-nitrophenol. | ||
According to the amounts of Pd and Au precursor in the solution, catalysts with different molar ratios of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au were obtained, and were set as 1
Au were obtained, and were set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 3
9, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 7
5, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 9
3 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Moreover, Pd/NCB and Au/NCB were also prepared in the same way but with only the Pd or Au precursor in the solution.
1, respectively. Moreover, Pd/NCB and Au/NCB were also prepared in the same way but with only the Pd or Au precursor in the solution.
Characterization
The morphology and microstructure of the catalyst were characterized by transmission electron microscopy (TEM, JEOL JEM-2100). To further identify the state of Pd and Au in the nanohybrid, PdAu/NCB was observed by high-angle annular dark-field scanning TEM (HAADF-STEM) and energy dispersive X-ray spectroscopy (EDX) mapping analysis (Tecnai G2 F30 S-Twin TEM). The structure of the catalyst was characterized by X-ray diffraction (XRD, Bruker D8 Advance diffractometer) analysis using Cu Kα radiation (λ = 0.15406 nm). The chemical state of each element was obtained by X-ray photoelectron spectroscopy (XPS) spectra on a PHI-5000C ESCA system (Thermo ESCALAB 250) with an Al Kα X-ray (E = 1486.6 eV) as the excitation light source. The nitrogen adsorption–desorption isotherm and BJH pore size distribution were measured by using a TriStar II3020 automated gas adsorption analyzer at 77 K. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) was performed on an ICP-OES analyzer (Agilent 730). The yield of biphenyl was measured by high-performance liquid chromatography (HPLC, Agilent 1260 Infinity, Eclipse XDB-C18 column) analysis according to an external standard method. The absorbance was measured by a UV-vis spectrophotometer (PERSEE TU-1900).Ullmann coupling reaction
The Ullmann coupling reaction was carried out at 40 °C in air for 3 hours. Typically, 1.0 mmol of aryl halide (1 equiv.), 3.0 mmol of base (3 equiv.) and 4 mg of the as-obtained catalyst were added into a mixed solution of 4 mL of IPA and 4 mL of water. Parts of mixture were taken out every 30 minutes for determination with HPLC. For the optimization of the reaction conditions, 200 μL of the above mixture was added into 4 mL of methanol followed by syringe filtration. The yield of biphenyl was measured by HPLC analysis according to an external standard method. While for the catalyst applicability study, the catalyst was removed by simple filtration, and then the remaining solution was extracted with ethyl acetate three times. The collected organic layers were combined, dehydrated with MgSO4 and finally rotary evaporated. The resulting crude product was separated by column separation to give an isolated yield.Nitrophenol hydrogenation reaction
Nitrophenol hydrogenation in the presence of an excess amount of NaBH4 was carried out in an aqueous solution. Typically, 100 mg of NaBH4 was mixed with the 20 mL of an aqueous solution of nitrophenol (100 mg L−1), and then 85 μL of the catalyst suspension (1 mg catalyst/1 mL H2O) was added to the above solution to start the reaction. In order to monitor the hydrogenation process, 3.0 mL of the solution was taken out of the reaction mixture at specific time intervals (2 minutes) followed by syringe filtration and finally measured by a UV-vis spectrophotometer to give time-dependent UV-vis spectra.Results and discussion
Morphology and structure
As shown in Fig. 1A, the Pd5Au5 alloy nanoparticles are uniformly dispersed on the surface of the NCB, with an average particle size of 3.4 nm and a narrow size distribution ranging from 2 to 5 nm (Fig. 1B). The inset of 1A exhibits the high-resolution TEM image of the Pd5Au5/NCB catalyst. The lattice plane distance of the Pd5Au5 particle is estimated as 0.230 nm, which is between the lattice plane distance of Pd (111) and Au (111), 0.223 nm and 0.234 nm, respectively, indicating the formation of an alloy structure.43,44 Furthermore, the elemental composition of the Pd5Au5/NCB was further investigated by the HAADF-STEM technique (Fig. 1C and D). The STEM-EDX mapping results confirm that C, N, O, Pd and Au elements are apparently observed in Pd5Au5/NCB. A homogeneous distribution of both Pd and Au is found (Fig. 1D), indicating the formation of a PdAu alloy on the surface of NCB. | ||
| Fig. 1 (A) TEM image, the inset is a HRTEM image; (B) particle size distribution; (C) HAADF-STEM images of Pd5Au5/NCB; (D) EDS mapping of C, N, O, and Pd and Au element. | ||
XRD analysis was used to further study the structure of the catalysts. Fig. 2A shows the XRD patterns of NCB, Pd/NCB, Au/NCB and PdAu/NCB catalysts with different Pd–Au molar ratios. For all the PdAu/NCB catalysts with different Pd/Au molar ratios, a broad peak centered at 2θ = 25° can be observed, which is ascribed to C (002) in the NCB material. As for Pd/NCB, the diffraction peaks centered at 2θ = 40.09°, 46.60°, 68.00° and 81.90° are assigned to the (111), (200), (220), and (311) lattice planes of face-centered cubic Pd, respectively. Similarly, for Au/NCB, the peaks located at 38.18°, 44.44°, 64.65°, 77.56° and 81.77 correspond to (111), (200), (220), (311) and (222). For the PdAu/NCB samples, an obvious shift (Fig. 2B) can be observed for the main peaks with an increase in the Pd content, suggesting the formation of a PdAu alloy rather than a core–shell structure.45–47 Meanwhile, the decreased intensity of the peaks implies a lower crystallization degree.
The typical IUPAC type IV pattern with a hysteresis loop in Fig. 2C shows that the Pd5Au5/NCB has a Brunauer–Emmett–Teller (BET) surface area (SBET) of 125.45 m2 g−1 and a pore volume of 0.24 cm3 g−1. The corresponding pore size distribution (Fig. 2D) shows that the NCB material itself has a pore size of around 3.60 nm, while the mesopore with a size of 34.96 nm simply reflects the average size of the voids between the aggregated particles. According to these results, the large specific surface area and abundant pores are expected to enhance the catalytic performance.
XPS characterization was employed to obtain the superficial element information of the as-prepared Pd5Au5/NCB catalyst. The XPS survey scans of Pd/NCB, Au/NCB and Pd5Au5/NCB are shown in Fig. 3A. The peaks of C, O, Pd and Au can be seen clearly on the Pd5Au5/NCB composite. Additionally, a weak peak of N 1s can also be observed, which indicates that nitrogen is successfully doped into carbon black via the facile hydrothermal treatment. The high-resolution XPS fitting spectrum of C 1s concludes that C exists in the form of a sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, a defect peak or sp2 C
C, a defect peak or sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–OH, C
N, C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C–N and O–C
O or C–N and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with the binding energy (BE) centered at 284.6, 285.2, 286.1, 287.1 and 289.1 eV, respectively (Fig. 3B).48–50 The convoluted result of the N 1s spectra shows three types of N species, including pyridinic N (398.8 eV, 39.9%), pyrrolic nitrogen (399.8 eV, 48.7%) and amino functional groups (401.3 eV, 11.4%), respectively (Fig. 3C).48,51 Also, the corresponding data of the atomic percentage of each nitrogen type indicated that the major nitrogen types in our support material are pyridinic N and pyrrolic N. As for the Au 4f spectrum of the Pd5Au5/NCB catalyst, it is well fitted with two pairs of peaks (Fig. 3D). One doublet located at 83.8 and 87.5 eV (ref. 52 and 53) is assigned to Au0, while the other at BE = 84.9 and 88.6 eV corresponds to Au+. Similarly, Pd0 (BE = 340.3 and 335.1 eV), Pd2+ (BE = 336.5 and 341.7 eV) and Pd4+ (BE = 337.8 and 343.0 eV) are three typical Pd species that appear in the Pd5Au5/NCB composite54,55 (Fig. 3E). It is worth noting that, compared with Au/NCB and Pd/NCB (Fig. 3D and E), both XPS spectra of Au 4f and Pd 3d in Pd5Au5/NCB have shifted to a lower binding energy, suggesting the electron transfer to Pd and Au metals. Due to the conductivity of NCB, we can deduce that these electrons came from NCB, confirming the interaction between PdAu NPs and NCB.56 Compared with the PdAu/CB sample (Fig. S1†), the Pd and Au peak positions of PdAu/NCB shift to higher binding energies, which is attributed to the metal-support interaction (MSI) between PdAu nanoparticles and NCB,57 suggesting an enhanced attraction force between the PdAu atoms and the NCB support.48
O with the binding energy (BE) centered at 284.6, 285.2, 286.1, 287.1 and 289.1 eV, respectively (Fig. 3B).48–50 The convoluted result of the N 1s spectra shows three types of N species, including pyridinic N (398.8 eV, 39.9%), pyrrolic nitrogen (399.8 eV, 48.7%) and amino functional groups (401.3 eV, 11.4%), respectively (Fig. 3C).48,51 Also, the corresponding data of the atomic percentage of each nitrogen type indicated that the major nitrogen types in our support material are pyridinic N and pyrrolic N. As for the Au 4f spectrum of the Pd5Au5/NCB catalyst, it is well fitted with two pairs of peaks (Fig. 3D). One doublet located at 83.8 and 87.5 eV (ref. 52 and 53) is assigned to Au0, while the other at BE = 84.9 and 88.6 eV corresponds to Au+. Similarly, Pd0 (BE = 340.3 and 335.1 eV), Pd2+ (BE = 336.5 and 341.7 eV) and Pd4+ (BE = 337.8 and 343.0 eV) are three typical Pd species that appear in the Pd5Au5/NCB composite54,55 (Fig. 3E). It is worth noting that, compared with Au/NCB and Pd/NCB (Fig. 3D and E), both XPS spectra of Au 4f and Pd 3d in Pd5Au5/NCB have shifted to a lower binding energy, suggesting the electron transfer to Pd and Au metals. Due to the conductivity of NCB, we can deduce that these electrons came from NCB, confirming the interaction between PdAu NPs and NCB.56 Compared with the PdAu/CB sample (Fig. S1†), the Pd and Au peak positions of PdAu/NCB shift to higher binding energies, which is attributed to the metal-support interaction (MSI) between PdAu nanoparticles and NCB,57 suggesting an enhanced attraction force between the PdAu atoms and the NCB support.48
 | ||
| Fig. 3 (A) Global XPS spectrum of Pd5Au5/NCB; XPS spectra of C 1s (B), N 1s (C), Au 4f (D) and Pd 3d (E). | ||
Ullmann coupling reaction
In our work, the Ullmann coupling of bromobenzene was employed as a model reaction to conduct the optimization of the reaction conditions with PdAu/CB and PdAu/NCB composites as the catalysts. The activities of PdAu/NCB with different Pd/Au molar ratios as well as their monometallic counterparts are summarized in Table 1. Monometallic Au/NCB was totally inactive for the Ullmann coupling of bromobenzene, and the monometallic Pd/NCB could only give a yield of 6%. Herein, it can be inferred that Pd is the only active site for the coupling reaction. By alloying Au with Pd, a great enhancement of the catalytic activity can be obtained. At an Au/Pd ratio of 5/5, the biphenyl yield reaches the highest value of 95% within 3 h. The yield of biphenyl over Pd5Au5/CB was only 16% under the same conditions. Hence, Pd5Au5/NCB was considered the best catalyst for further study.| a Reaction conditions: bromobenzene (1 mmol), K2CO3 (3 mmol), IPA (4 mL), H2O (4 mL), PdAu/NCB (4 mg, 10 wt% PdAu loading), 40 °C, 3 h. | |||||||
|---|---|---|---|---|---|---|---|
| Catalyst | Au | Pd1Au9 | Pd3Au7 | Pd5Au5 | Pd7Au3 | Pd9Au1 | Pd |
| Yield (%) | 0 | 49 | 74 | 95 | 51 | 18 | 6 |
As the bases and solvents play an important role in the Ullmann coupling reaction, a control variate method was employed to study the influence of each factor. As shown in Table 2, various bases, such as K2CO3, KOH, NaHCO3, NH4HCO3, NaOH, C5H5N, HCOONa and Et3N (entries 5–12), were employed in the Ullmann coupling reaction. Among these bases, K2CO3 was the most efficient for the Ullmann reaction in the IPA/H2O system with a 94.6% yield of biphenyl (entry 12). So, we used K2CO3 as the base in the subsequent experiments because of its high efficiency and low cost. Organic solvents such as IPA, EtOH, C4H4O and H2O gave moderate yields (entries 1–4). Interestingly, a mixed water-organic solvent can tremendously increase the yield of biphenyl (entries 12–14) in the presence of K2CO3 as a base, especially, the highest yield of 95% can be obtained by using the IPA/H2O system (entry 12). Because the base and organic reactants are more easily dissolved in the mixed solutions, organopalladium intermediates are easily formed, thus facilitating the reaction. Additionally, the desired product of biphenyl will separate out because of its limited solubility in the IPA/H2O medium, driving the reaction equilibrium in the forward direction. Hence, the IPA/H2O system is considered to be the best solvent because it is safe, easily available and environmentally friendly. So, the optimized reaction conditions are as follows: bromobenzene (1 mmol), K2CO3 (3 mmol), Pd5Au5/NCB (4 mg), IPA (4 mL), and water (4 mL) at 40 °C for 3 h.
| Entry | Base | Solvent (mL mL−1) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: bromobenzene (1 mmol), base (3 mmol), solvent (8 mL), PdAu/NCB(4 mg, 10 wt% Pd5Au5 loading, the calculated moles of Pd5Au5 is 0.0013 mmol), 40 °C, 3 h. Yields were determined by HPLC according to the standard curve based on biphenyl. | |||
| 1 | K2CO3 | IPA | 52 |
| 2 | K2CO3 | EtOH | 70 |
| 3 | K2CO3 | C4H4O | 60 |
| 4 | K2CO3 | H2O | 52 |
| 5 | KOH | IPA/H2O = 4/4 | 55 |
| 6 | NaHCO3 | IPA/H2O = 4/4 | 30 |
| 7 | NH4HCO3 | IPA/H2O = 4/4 | 10 |
| 8 | Et3N | IPA/H2O = 4/4 | 8 |
| 9 | NaOH | IPA/H2O = 4/4 | 60 |
| 10 | C5H5N | IPA/H2O = 4/4 | 20 |
| 11 | HCOONa | IPA/H2O = 4/4 | 17 |
| 12 | K2CO3 | IPA/H2O = 4/4 | 94 |
| 13 | K2CO3 | EtOH/H2O = 4/4 | 82 |
| 14 | K2CO3 | C4H4O/H2O = 4/4 | 75 |
The generality of the catalyst for the Ullmann coupling reaction was examined by extending different bromobenzene derivatives containing electron-donating and electron-withdrawing functional groups under the optimized conditions. As shown in Table 3, the activity of 4-OCH3-substituted aryl benzenes follows the sequence of I > Br > Cl because of the difference between the electronegativity of halogen atoms (Cl > Br > I), where higher electronegativity leads to a stronger carbon–halogen bond and thus a lower activity (entries 1–3).58 This result is consistent with the work reported by Zhang et al.,59 in which ion exchange resin supported Au alloyed with Pd single atoms was explored to serve as an effective and robust catalyst for the Ullmann reaction of aryl halides. For other OCH3-substituted bromobenzenes (entries 4–5), the 2-OCH3 substituted bromobenzene gives a lower yield of 85.1% due to steric hindrance.60 Moreover, for para-position substituted aryl bromides with electron-donating (–OH and –CH3) or electron-withdrawing (–CN, –CHO, –NO2) functional groups, desirable yields can be obtained under required conditions (entries 6–10). In addition, 4-NO2 iodobenzene can give the corresponding symmetrical products with a yield of 88.6% in 2 h (entry 11). However, Dhital et al.24,61 reported that Au–Pd alloy nanoclusters stabilized by PVP could efficiently catalyze the Ullmann coupling of chloroarenes but not for bromoarenes and iodide arenes, and aryl iodide was a stronger inhibitor by forming a stable complex with a gold component. Obviously, the as-prepared PdAu/NCB catalysts are more stable and adaptable to different substrates.
| Entry | Aryl halide | Product | Time | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: aryl halide(1 mmol), K2CO3 (3 mmol), IPA (4 mL), H2O (4 mL), Pd5Au5/NCB (4 mg, 10 wt% Pd5Au5 loading), 40 °C, 3 h, isolated yield. | ||||
| 1 | 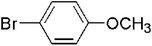 |
 |
3 h | 95 |
| 2 | 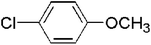 |
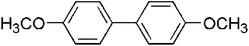 |
5 h | 85 |
| 3 |  |
 |
1 h | 97 |
| 4 | 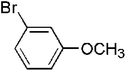 |
 |
3 h | 92 |
| 5 |  |
 |
3 h | 85 |
| 6 |  |
 |
3 h | 81 |
| 7 |  |
 |
3 h | 90 |
| 8 | 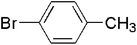 |
 |
3 h | 92 |
| 9 |  |
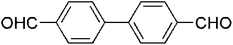 |
3 h | 88 |
| 10 |  |
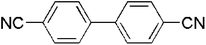 |
3 h | 87 |
| 11 |  |
 |
2 h | 88 |
A possible pathway for the Ullmann coupling of aryl halides catalyzed by the PdAu/NCB is proposed in Scheme 2. First, the aryl halides are adsorbed onto the surface of PdAu NPs followed by the activation (step A) and cleavage of the carbon–halogen (C–X) bond to generate metal-aryl and metal-halogen intermediates (step B). Later, a carbon–carbon bond is formed by reductive elimination (step C) and the bi-aryl product is desorbed from the active site and into the solution, accompanying the recovery of alloy nanoparticles by isopropanol (step D).62–64 As demonstrated by many reports, alcohols (such as ethanol or isopropanol) can be used as reducing agents for Ullmann homocoupling.65–67
Based on the above investigation, the excellent activity of PdAu/NCB in Ullmann coupling can probably be attributed to the unique nanostructure of the catalyst and the synergistic effect between individual components: (1) the abundant oxygen containing functional groups and large surface area on of NCB could anchor the PdAu NPs, which is in favor of the dispersion and stability of the catalyst. Moreover, nitrogen-doping can increase the interaction between the PdAu and NCB, thus enhancing the catalytic durability.68–70 (2) In the case of the Ullmann coupling reaction, Pd appears to be more catalytically active than Au. The synergistic effect creates highly active sites, which is beneficial to the oxidation addition of aryl halides.52 (3) For the Ullmann coupling reaction itself, the almost biphenyl with a noncoplanar conjugated structure, poor polarity and limited solubility will quickly escape from the surface of the catalyst, driving the reaction in the forward direction.71
Hydrogenation of nitrophenols
The PdAu/NCB alloy catalyst is not only active for the Ullmann coupling reaction, but also behaves well in the hydrogenation reaction of nitro compounds. Here, the catalytic reduction of p-nitrophenol (4-NP) to p-aminophenol (4-AP) was set as a model reaction to estimate the activity of the catalysts. The reaction was carried out in an aqueous solution using an excess amount of NaBH4 as the reductant. As shown in Fig. 4A, the maximum UV-vis adsorption wavelength of 4-NP is at 400 nm under alkaline conditions, while the peak maximum of 4-AP is around 303 nm.53 In the presence of NaBH4 and catalyst, the peak assigned to 4-NP decreases as the reaction proceeds, and meanwhile the peak of 4-AP increases concomitantly, which can be used to monitor the reaction process. Taking into account the excessive amount of NaBH4, the pseudo-first-order kinetics related to 4-NP can be used to evaluate the kinetic rate constant, and the corresponding kinetic equation can be written as eqn (1):
 | (1) |
Catalysts with different molar ratios of Pd and Au were synthesized in order to further investigate the catalytic activity for the hydrogenation of 4-NP to 4-AP. The ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c/c0 vs. reaction time over PdAu/NCB with various Pd/Au molar ratios is shown in Fig. 4B. Combining the results of Fig. 4B with Table 4, we can clearly conclude that all of the PdAu catalysts showed a higher catalytic activity compared with the corresponding Pd/NCB and Au/NCB, indicating the synergistic effect between Pd and Au nanoparticles in the PdAu alloy. Notably, the Pd5Au5/NCB catalyst exhibited the highest catalytic activity within 12 min to finish the reaction. However, only a 20% conversion of 4-NP could be obtained over Pd5Au5/CB within 12 min [Fig. S2†]. Obviously, N-doping can improve the catalytic performance due to an enhanced attraction force between PdAu atoms and the NCB support.48,57
c/c0 vs. reaction time over PdAu/NCB with various Pd/Au molar ratios is shown in Fig. 4B. Combining the results of Fig. 4B with Table 4, we can clearly conclude that all of the PdAu catalysts showed a higher catalytic activity compared with the corresponding Pd/NCB and Au/NCB, indicating the synergistic effect between Pd and Au nanoparticles in the PdAu alloy. Notably, the Pd5Au5/NCB catalyst exhibited the highest catalytic activity within 12 min to finish the reaction. However, only a 20% conversion of 4-NP could be obtained over Pd5Au5/CB within 12 min [Fig. S2†]. Obviously, N-doping can improve the catalytic performance due to an enhanced attraction force between PdAu atoms and the NCB support.48,57
| Entry | Catalyst | k (min−1) | R2 |
|---|---|---|---|
| a Reaction conditions: 4-NP (20 mL, 100 mg L−1), catalyst suspension (85 μL, 1 mg mL−1), NaBH4 (50 mg), 25 °C. | |||
| 1 | Pd5Au5/NCB | 0.2895 | 0.9961 |
| 2 | Pd9Au1/NCB | 0.1930 | 0.9724 |
| 3 | Pd7Au3/NCB | 0.1543 | 0.9813 |
| 4 | Pd3Au7/NCB | 0.1137 | 0.9902 |
| 5 | Pd1Au9/NCB | 0.0872 | 0.9893 |
| 6 | Pd/NCB | 0.0575 | 0.9785 |
| 7 | Au/NCB | 0.0372 | 0.9812 |
We also investigate the effect of temperature on the hydrogenation reaction. Four different temperatures ranging from 20 to 35 °C were chosen for the reduction of 2-NP, 3-NP and 4-NP. The activation energy (Ea) can be calculated according to the Arrhenius equation (eqn (2)):
 | (2) |
Thermodynamic parameters, including the activation of entropy (ΔS#), enthalpy (ΔH#) and Gibbs energy change (ΔG#), are calculated following eqn (3) and (4), where kB is the Boltzmann constant (1.38 × 10−23 J K−1) and h is the Planck constant (6.63 × 10−34 J).72 For Pd5Au5/NCB, the values of ΔS#, ΔH# and ΔG# are listed in Table 5. The positive values of activation of enthalpy show that the reduction of 2-NP, 3-NP and 4-NP is an endothermic reaction and the positive ΔG# values indicate that the hydrogenation needs the catalyst.73 The negative entropy (ΔS#) values indicate that randomness on the catalyst-solution interface decreased during the catalytic reduction process.74 In addition, the ΔG# values increase as the temperature rises, which indicates that the reduction process of 2-NP, 3-NP and 4-NP needs energy.75–77
 | (3) |
| ΔG# = ΔH# − TΔS# | (4) |
| Substrate | T (K) | K (min−1) | Ea (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|
| 2-NP | 293 | 0.1643 | 55.12 | 44.8 | −122.34 | 80.64 |
| 298 | 0.2071 | 81.25 | ||||
| 303 | 0.3233 | 81.86 | ||||
| 308 | 0.4829 | 82.48 | ||||
| 3-NP | 293 | 0.1467 | 65.13 | 57.1 | −80.6 | 80.31 |
| 298 | 0.1971 | 80.71 | ||||
| 303 | 0.3385 | 81.11 | ||||
| 308 | 0.5217 | 81.52 | ||||
| 4-NP | 293 | 0.1912 | 20.88 | 19.13 | −212.9 | 82.58 |
| 298 | 0.2114 | 84.70 | ||||
| 303 | 0.2540 | 86.83 | ||||
| 308 | 0.2896 | 88.96 |
Based on the experimental results and the literature,78,79 the high activity of Pd5Au5/NCB can be attributed to a synergistic effect and the unique nanostructure. The nitro group as an electron-withdrawing group enhances the π–π stacking interactions by reducing the electron density of π electrons, diminishing the repulsive electrostatic interactions between the aromatic rings.80 It provides a higher concentration of nitrophenol molecules near to the PdAu NPs on N-doping carbon black, leading to a highly efficient contact between the reactant molecules and the active sites, thus enhancing activity.81
Cycling performance
The recoverability and reusability of the catalyst are of great importance. The Pd5Au5/NCB was used in the Ullmann coupling of bromobenzene at 40 °C and the reduction of 4-NP at 25 °C over 10 cycles. As shown in Fig. 6, both the conversion efficiency of 4-NP and yield of the Ullmann coupling over Pd5Au5/NCB maintained at over 80%, suggesting a good reusability of Pd5Au5/NCB in the reaction. | ||
| Fig. 6 Cycling performance of Pd5Au5/NCB for the reduction of 4-NP and the Ullmann coupling of bromobenzene. | ||
The recovered catalyst was re-characterized using XPS, TEM HAADF and ICP. XPS analysis (Fig. S3†) reveals that PdAu nanoparticles maintain their state after the tenth use. TEM HAADF images of the tenth cycle of catalysis (Fig. S4†) show the PdAu particles had slightly grown to 4–6 nm, indicating the mild agglomeration of the particles, which may have caused the reduction in the catalytic activity after the tenth use. Metal leaching of the catalyst before and after the reaction was studied using ICP. The Au and Pd contents of the Pd5Au5/NCB are shown in Table S1† before reaction and after ten reaction cycles. These results demonstrate that the amount of metal loss can be 5%, which may be cause the decrease of the activity of Pd5Au5/NCB NPs.82
Furthermore, we performed a filtration test in the Ullmann coupling of PhBr at 40 °C. The Pd5Au5/NCB was filtrated out using a syringe filter after 1 h. As indicated in Fig. S5,† the catalytic reaction did not occur after the catalyst was filtered, which confirmed the heterogeneous nature of the catalysis.
Conclusion
In summary, a series of PdAu alloy catalysts was prepared with N-doped carbon black as the support via a co-reduction method. Due to the surfactant-free formation process, the PdAu/NCB catalysts are clean, and effective for the Ullmann coupling of aryl halides and for nitrophenol hydrogenation. According to the results, the PdAu/NCB nanoparticles are more active compared to the corresponding monometallic counterparts; moreover, Pd5Au5/NCB exhibits the highest activity. The price of the catalyst is low due to the low price of NCB. The enhanced catalytic performance can be ascribed to the unique nanostructure of the catalyst including the large BET surface, small size of PdAu NPs, excellent PdAu dispersion, strong adsorption of organic molecules on the surface of NCB, and the synergistic effect between two different metals and the combined effect between the nanoparticles and NCB.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 30916014103), NSF of China (No. 51572125), the Natural Science Foundation of Jiangsu Province (No. BK20171423) and PAPD of Jiangsu.References
- J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1470 CrossRef CAS PubMed.
- R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS PubMed.
- F. Yang, Y. Li, T. Liu, K. Xu, L. Zhang, C. Xu and J. Gao, Chem. Eng. J., 2013, 226, 52–58 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- Y. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef PubMed.
- S. Y. Xu, Y. B. Ruan, X. X. Luo, Y. F. Gao, J. S. Zhao, J. S. Shen and Y. B. Jiang, Chem. Commun., 2010, 46, 5864–5866 RSC.
- M. Opanasenko, P. Stepnicka and J. Cejka, RSC Adv., 2014, 4, 65137–65162 RSC.
- G. Cravotto, M. Beggiato, A. Penoni, G. Palmisano, S. Tollari, J. M. Lévêque and W. Bonrath, Tetrahedron Lett., 2005, 46, 2267–2271 CrossRef CAS.
- L. Shao, Y. Du, M. Zeng, X. Li, W. Shen, S. Zuo, Y. Lu, X. M. Zhang and C. Qi, Appl. Organomet. Chem., 2010, 24, 421–425 CAS.
- Z. Zhou and W. Xue, J. Organomet. Chem., 2009, 694, 599–603 CrossRef CAS.
- A. P. I. BhatI, F. Inam and B. R. Bhat, RSC Adv., 2013, 3, 22191–22198 RSC.
- J. Long, Y. Zhou and Y. Li, Chem. Commun., 2015, 51, 2331–2334 RSC.
- W. Zhao, L. Dong, C. Huang, Z. M. Win and N. Lin, Chem. Commun., 2016, 52, 13225–13228 RSC.
- F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 4, 8009–8020 RSC.
- X. W. Teng, Q. Wang, P. Liu, W. Q. Han, A. I. Frenkel, W. Wen, N. Marinkovic, J. C. Hanson and J. A. Rodriguez, J. Am. Chem. Soc., 2008, 130, 1093–1101 CrossRef CAS PubMed.
- Y. Sohn, D. Pradhan and K. T. Leung, ACS Nano, 2010, 4, 5111–5120 CrossRef CAS PubMed.
- S. Zhang, Y. Y. Shao, H. G. Liao, J. Liu, I. A. Aksay, G. P. Yin and Y. H. Lin, Chem. Mater., 2011, 23, 1079–1081 CrossRef CAS.
- M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kielye and G. J. Hutchings, Chem. Soc. Rev., 2012, 41, 8099–8139 RSC.
- S. Sarina, S. Bai, Y. Huang, C. Chen, J. Jia, E. Jaatinen, G. A. Ayoko, Z. Bao and H. Zhu, Green Chem., 2014, 16, 331–341 RSC.
- N. Agarwal, S. J. Freakley, R. U. McVicker, S. M. Althahban, N. Dimitratos, Q. He, D. J. Morgan, R. L. Jenkins, D. J. Willock, S. H. Taylor, C. J. Kiely and G. J. Hutchings, Science, 2017, 358, 223–227 CrossRef CAS PubMed.
- J. W. Xia, Y. S. Fu, G. Y. He, X. Q. Sun and X. Wang, Appl. Catal., B, 2017, 200, 39–46 CrossRef CAS.
- H. Jing and H. Wang, Chem. Mater., 2015, 27, 2172–2180 CrossRef CAS.
- J. J. Lv, Z. J. Wang, J. J. Feng, R. Qiu, A. J. Wang and X. Xu, Appl. Catal., A, 2016, 522, 188–193 CrossRef CAS.
- R. N. Dhital, C. Kamonsatikul, E. Somsook, K. Bobuatong, M. Ehara, S. Karanjit and H. Sakurai, J. Am. Chem. Soc., 2012, 134, 20250–20253 CrossRef CAS PubMed.
- Z. J. Wang, X. Wang, J. J. Lv, J. J. Feng, X. H. Xu, A. J. Wang and Z. W. Liang, New J. Chem., 2017, 41, 3894–3901 RSC.
- J. Wang, A. J. Xu, M. L. Jia, S. Bai, X. D. Cheng and B. Zhao, New J. Chem., 2017, 41, 1905–1909 RSC.
- W. Fang, Y. C. Deng, L. Tang, G. M. Zeng, Y. Y. Zhou, X. Xie, J. J. Wang and Y. Wang, J. Colloid Interface Sci., 2017, 490, 834–843 CrossRef CAS PubMed.
- H. L. Jiang, T. Akita, T. Ishida, M. Haruta and Q. Xu, J. Am. Chem. Soc., 2011, 133, 1304–1306 CrossRef CAS PubMed.
- X. M. Chen, Z. X. Cai, X. Chen and M. Oyama, J. Mater. Chem. A, 2014, 2, 5668–5674 RSC.
- R. Bajpai, S. Roy S, P. Kumar, P. Bajpai, N. Kulshrestha, J. Rafiee, N. Koratkar and D. S. Misra, ACS Appl. Mater. Interfaces, 2011, 3, 3884–3889 CrossRef CAS PubMed.
- L. Perini, C. Durante, M. Favaro, V. Perazzolo, S. Agnoli, O. Schneider, G. Granozzi and A. Gennaro, ACS Appl. Mater. Interfaces, 2015, 7, 1170–1179 CrossRef CAS PubMed.
- D. T. Liu, W. James and A. M. Rami, Nat. Geosci., 2017, 10, 184–188 CrossRef CAS.
- J. Yan, T. Wei and B. Shao, Carbon, 2010, 48, 1731–1737 CrossRef CAS.
- P. J. Li, J. H. Wu and J. M. Lin, Sol. Energy, 2009, 83, 845–849 CrossRef CAS.
- J. Liu, P. Song and W. Xu, Carbon, 2017, 115, 763–772 CrossRef CAS.
- V. Z. Radkevich, T. L. Senko, K. Wilson, L. M. Grishenko, A. N. Zaderko and V. Y. Diyuk, Appl. Catal., A, 2008, 335, 241–251 CrossRef CAS.
- M. Zhu and G. Diao, Nanoscale, 2011, 3, 2748–2767 RSC.
- Z. Li, J. Liu, Z. Huang, Y. Yang, C. Zia and F. Li, ACS Catal., 2013, 3, 839–845 CrossRef CAS.
- A. A. Deshmukh, R. U. Islam, M. J. Witcomb, W. A. Otterlo and N. J. Coville, ChemCatChem, 2010, 2, 51–54 CrossRef CAS.
- N. Sun, M. L. Wang and J. F. Chang, Front. Energy, 2017, 11, 310–317 CrossRef.
- Y. Z. Dong and J. H. Li, Chem. Commun., 2015, 51, 572–575 RSC.
- J. W. Xia, Y. S. Fu, G. Y. He, X. Q. Sun and X. Wang, Mater. Chem. Phys., 2018, 209, 86–94 CrossRef CAS.
- S. H. Wang, Z. L. Xin, X. H. Huang, W. Z. Yu and S. N. Niu, Phys. Chem. Chem. Phys., 2017, 19, 6164–6168 RSC.
- W. Hong, J. Wang and E. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 9481–9487 CrossRef CAS PubMed.
- X. Y. Liu, A. Q. Wang, X. Q. Mou and T. Zhang, Chem. Commun., 2008, 44, 3187–3189 RSC.
- W. J. Li, A. Q. Wang, X. Y. Liu and T. Zhang, Appl. Catal., A, 2012, 433, 146–151 CrossRef.
- L. L. Zhang, A. Q. Wang, J. T. Miller, X. Y. Liu, X. F. Yang, W. T. Wang, L. L. Li, Y. Q. Huang, C. Y. Mou and T. Zhang, ACS Catal., 2014, 4, 1546–1553 CrossRef CAS.
- H. Y. Qian, H. J. Huang and X. Wang, J. Power Sources, 2015, 275, 734–741 CrossRef CAS.
- Y. Wang, Y. Y. Shao, D. W. Matson, J. H. Li and Y. H. Lin, ACS Nano, 2010, 4, 1790–1798 CrossRef CAS PubMed.
- G. Z. Hu, F. Nitze, T. Sharifi, H. R. Barzegara and T. Wågberg, J. Mater. Chem., 2012, 22, 8541–8548 RSC.
- J. W. Xia, L. L. Zhang, Y. S. Fu, G. Y. He, X. Q. Sun and X. Wang, J. Mater. Sci., 2018, 53, 4467–4481 CrossRef CAS.
- C. H. Liu, R. H. Liu, Q. J. Sun, J. B. Chang, X. Gao, Y. Liu, S. T. Lee, Z. H. Kang and S. D. Wang, Nanoscale, 2015, 7, 6356–6362 RSC.
- A. Villa, N. Dimitratos, C. E. Chan-Thaw, C. Hammond, G. M. Veith, D. Wang, M. Manzoli, L. Prati and G. J. Hutchings, Chem. Soc. Rev., 2016, 45, 4953–4994 RSC.
- L. S. Kibis, A. I. Stadnichenko, S. V. Koscheev, V. I. Zaikovskii and A. I. Boronin, J. Phys. Chem. C, 2012, 116, 19342–19348 CrossRef CAS.
- X. N. Guo, P. Brault, G. J. Zhi, A. Caillard, G. Q. Jin, C. Coutanceau, S. Baranton and X. Y. Guo, J. Phys. Chem. C, 2011, 115, 11240–11246 CrossRef CAS.
- X. M. Chen, Z. X. Cai, X. Chen and M. Oyama, J. Mater. Chem. A, 2014, 2, 5668–5675 RSC.
- Z. Y. He, B. Q. Dong, W. Wang, G. X. Yang, Y. H. Cao, H. J. Wang, Y. H. Yang, Q. Wang, F. Peng and H. Yu, ACS Catal., 2019, 9, 2893–2901 CrossRef CAS.
- L. Zhang, C. Feng, S. Gao, Z. Wang and C. Wang, Catal. Commun., 2015, 61, 21–25 CrossRef CAS.
- L. L. Zhang, A. Q. Wang, J. Miller, X. F. Yang, W. T. Wang, L. Li, Y. Q. Huang, C. Y. Mou and T. Zhang, ACS Catal., 2014, 4, 1546–1553 CrossRef CAS.
- H. Song, Q. Zhu, X. Zheng and X. Chen, J. Mater. Chem. A, 2015, 3, 10368–10377 RSC.
- R. N. Dhital, C. Kamonsatikul, E. Somsook, Y. Satod and H. Sakurai, Chem. Commun., 2013, 49, 2542–2544 RSC.
- C. Kamonsatikul, T. Khamnaen and P. Phiriyawirut, Catal. Commun., 2012, 26, 1–5 CrossRef CAS.
- J. Wang, A. X. Xu, M. L. Jia, S. Bai, X. D. Cheng and G. T. Zhao, New J. Chem., 2017, 41, 1905–1910 RSC.
- M. F. Zeng, Y. J. Du, L. J. Shao, C. Z. Qi and X. M. Zhang, J. Org. Chem., 2010, 75, 2556–2563 CrossRef CAS PubMed.
- J. Hassan, V. Penalva, L. Lavenot, C. Gozzi and M. Lemaire, Tetrahedron, 1998, 54, 13793–13804 CrossRef CAS.
- L. Shao, Y. Du, M. Zeng, X. Li, W. Shen, S. Zuo, Y. Lu, X. Zhang and C. Qi, Appl. Organomet. Chem., 2010, 24, 421–425 CAS.
- B. Z. Yuan, Y. W. Li, B. L. Yin and H. F. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054–4058 CrossRef CAS PubMed.
- Y. K. Zhou, K. Neyerlin, T. S. Olson, S. Pylypenko, J. Bult, H. N. Dinh, T. Gennett, Z. P. Shao and R. O'Hayre, Energy Environ. Sci., 2010, 3, 1437–1446 RSC.
- K. Kakaei and K. Marzang, J. Colloid Interface Sci., 2016, 462, 148–153 CrossRef CAS PubMed.
- N. Liu, L. Z. Ding, M. J. Jia, W. X. Zhang, N. H. An and X. L. Yuan, J. Colloid Interface Sci., 2017, 490, 677–684 CrossRef CAS PubMed.
- S. I. Yamamoto, H. Kinoshita, H. Hashimoto and Y. Nishina, Nanoscale, 2014, 6, 6501–6505 RSC.
- V. K. Gupta, N. Atar, M. Yola, Z. Üstündag and L. Uzun, Water Res., 2014, 48, 210–217 CrossRef CAS PubMed.
- P. C. Guo, L. Tang, J. Tang, G. M. Zeng, B. B. Huang, H. R. Dong, Y. Zhang, Y. Y. Zhou, Y. C. Deng, L. L. Ma and S. R. Tan, J. Colloid Interface Sci., 2016, 469, 78–85 CrossRef CAS PubMed.
- V. K. Gupta, N. Atar, M. L. Yola, Z. Üstündağ and L. Uzun, Water Res., 2014, 48, 210–217 CrossRef CAS PubMed.
- M. Kohantorabi and M. R. Gholami, Ind. Eng. Chem. Res., 2017, 56, 1159–1167 CrossRef CAS.
- T. Zhou, T. T. Liu, Z. Q. Zhang, G. D. Zhang, F. W. Wang, X. F. Wang, S. Z. Liu, H. Z. Zhang, S. S. Wang and J. Ma, J. Solid State Chem., 2018, 263, 11–17 CrossRef CAS.
- M. Kohantorabi and M. R. Gholami, Appl. Phys. A, 2018, 124, 441–445 CrossRef.
- C. Wen, A. Yin and W. Dai, Appl. Catal., B, 2014, 160, 730–741 CrossRef.
- X. Li, K. Wu, Y. Ye and X. Wei, Nanoscale, 2013, 5, 3648–3653 RSC.
- Q. S. Liu, T. Zheng, P. Wang, J. P. Jiang and N. Li, Chem. Eng. J., 2010, 157, 348–356 CrossRef CAS.
- J. W. Xia, G. Y. He, L. L. Zhang, X. Q. Sun and X. Wang, Appl. Catal., B, 2016, 180, 408–415 CrossRef CAS.
- H. Park, D. A. Reddy, Y. Kim, S. Lee, R. Ma, M. Lim and T. K. Kim, Appl. Surf. Sci., 2017, 401, 314–322 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01685f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |