Open Access Article
Open Access ArticleCoSx/C hierarchical hollow nanocages from a metal–organic framework as a positive electrode with enhancing performance for aqueous supercapacitors†
Weibin Zhou‡
ab,
Peng Wang‡ab,
Chunyang Liac,
Qinghong Huangc,
Jing Wangc,
Yusong Zhu*ac,
Lijun Fu *ac,
Yuhui Chenac and
Yuping Wu
*ac,
Yuhui Chenac and
Yuping Wu *ac
*ac
aState Key Laboratory of Materials-oriented Chemical Engineering, School of Energy Science and Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: zhuys@njtech.edu.cn; l.fu@njtech.edu.cn; wuyp@fudan.edu.cn
bInstitute of Advanced Materials (IAM), Nanjing Tech University, Nanjing 210009, China
cSchool of Energy Science and Engineering, Nanjing Tech University, Nanjing 211816, China
First published on 10th April 2019
Abstract
Benefiting from abundant redox chemistry and high electrochemical properties, metal sulfides have been broadly employed as electrode materials in supercapacitor systems. However, the predominant limitation in their performance, which arises from indifferent electron and ion dynamics for transportation and a rapid slash in capacitance, is of particular concern. Herein, we portray the cobalt sulfides/carbon (CoSx/C) hierarchical hollow nanocages using ZIF-67 nanocrystals coated with carbon from resorcinol–formaldehyde (ZIF-67@RF) as a self-sacrificial template. The RF acted as a hard framework to prevent the hollow structure from breaking and was transformed to a carbon layer to enhance the charge transfer process. When used as positive electrodes in supercapacitor systems with aqueous electrolytes, the optimized CoSx/C hierarchic hollow nanocages exhibited a considerable specific capacitance (618 F g−1 at 2 A g−1), superior rate performance (83.6% capacitance retention of the initial capacity when the current density was amplified from 2 A g−1 to 50 A g−1) and an extraordinary cycle stationarity along with an undiminished specific capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In this study, the meticulously designed hierarchical hollow structure that we conceived not only provides an outstanding electrochemical performance but also provides options for other related materials, such as various MOFs.
000 cycles. In this study, the meticulously designed hierarchical hollow structure that we conceived not only provides an outstanding electrochemical performance but also provides options for other related materials, such as various MOFs.
1. Introduction
Surpassing secondary batteries such as lithium ion batteries, sodium ion batteries and other metal ion batteries in power density, supercapacitors (SCs) are reckoned as irreplaceable and practical energy storage devices.1–5 Amidst them, electrical double layers capacitors (EDLCs) mainly store narrow energy,6–8 and are composed of carbon-based materials involving graphene, activated carbon and carbon nanotubes (CNTs). However, conductive polymers,9,10 metal oxides11–13 and sulfides,14–17 called pseudocapacitive materials, often demonstrate a relatively higher energy density, which is attributed to the fast absorption/desorption and redox reactions on the surface of active materials in supercapacitors. Therefore, advanced pseudocapacitive materials with an eminent electrochemical performance have become research hotspots in this field.Among miscellaneous pseudocapacitive materials ,8,18–21 the use of metal sulfides as bright electrode materials in supercapacitors (SCs) have dominated recent research discussions. Hence, the academic community has extensively explored metal sulfides in inner construction applications covering stoichiometric formulations, valence states and morphologies encompassing nanocrystalline morphologies and crystal frameworks, which enable them to deliver electro-chemical activities. Moreover, metal sulfides commonly offer more trivial electrical resistance as well as mechanically and thermally amended stability than their corresponding metal oxide counterparts.22 These unparalleled characters ensure a better electrochemical performance as electrodes compared to many other materials, consisting of carbonaceous materials and metal oxides. For instance, after the solvothermal operation, a flower-like β-NiS with a hierarchical architecture was collected, and then presented a specific capacity of 513 F g−1 at 5 A g−1.23 In addition to nickel sulfides, electrode materials from cobalt sulfides are similarly utilized for SCs.
Hybrid CoS/graphene with a 3D network highlighted a prosperous specific capacitance and a remarkable capacity retention of 82% when operated at 20 A g−1.24 Because metal ions and organic ligands can be combined to form a periodically poriferous structure, metal–organic frameworks (MOFs) were widely investigated for energy storage applications.25–32 For instance, the hierarchical CoS double-shelled hollow nanoboxes derived from a zeolitic imidazolate framework-67 (ZIF-67) demonstrated a high specific capacitance, and subsequently retained 60% of the initial capacitance at 20 A g−1.33 However, the limited rate performance and cycling stability required further improvement.
Due to the intrinsic poor electrical conductivity of metal oxides, carbon-based materials including multi-wall, double-wall and single-wall carbon nanotubes (CNTs) and reduced graphene oxide (rGO) are usually added into metal oxides to enhance the charge transfer process.34–37 Co3O4 in situ coating on CNTs were synthesized via a hydrothermal procedure and used as cathode materials in aqueous supercapacitors, which showed a specific capacity of 590 F g−1 at the ampere density of 15 A g−1 and a specific capacitance of 510 F g−1 at 100 A g−1.38 Metal sulfides, which have also been processed in this way, demonstrated a better electrochemical performance compared to their pristine counterparts.39–41 rGO-CNT-Co3S4 nanocomposites were optimized by adjusting the rGO concentration and the ratio of rGO/CNTs. These nanocomposites showed an optimally high specific capacitance due to an enhanced charge transfer procedure.42 Our previous study on the formation of the sandwich structures from rGO and cobalt sulfides also suggested that the addition of rGO had positive effects on the capacitance performance.43,44 Nevertheless, many studies focused on the charge transfer among large-size samples, such as micron-sized metal oxides particles, thus ignoring the high charge transfer resistance inside these particles.
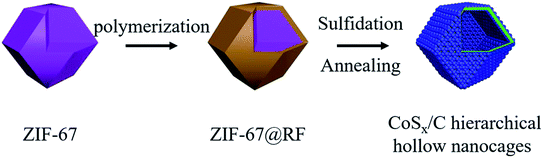
Herein, we designed a rational and versatile hierarchical hollow structure with cobalt sulfide nanoparticles having a diameter ca. 10 nm attached on the surface of the conductive hollow carbon layer. ZIF-67 coated with a RF layer was applied as a self-sacrificial template and subsequently transformed to the hierarchical hollow CoSx/C nanocages. The RF coating layer not only supported and stabilized the hollow structure during the sulfurization process, but was transformed to a conductive carbon layer during heat treatment to enhance the electrochemical performance as a positive electrode in aqueous supercapacitors. Due to the combination of the hollow structure and the conductive carbon shell, the resulting materials showed a superb rate performance and dramatically wonderful cycling stability. It is believed that this synthesis method and the unique hierarchical hollow structure could be extended to other related materials, such as various MOFs.
2. Experimental section
2.1 Preparation of ZIF-67@RF
All chemicals were obtained commercially and were used without additional purification. ZIF-67 was devised as the following: 2-methylimidazole (2-MI, 410 mg) and cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 294 mg) were added to a 10 mL and 30 mL solvent mixture of methanol and ethanol with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the former solution was poured into the later solution under vigorous stirring for 15 min and then, the mixture was kept motionless for 24 h. The product was obtained by suction filtration and washed with methanol three times, followed by drying at 60 °C overnight.
1. Then, the former solution was poured into the later solution under vigorous stirring for 15 min and then, the mixture was kept motionless for 24 h. The product was obtained by suction filtration and washed with methanol three times, followed by drying at 60 °C overnight.
ZIF-67@RF was prepared in line with the previous study:45 0.2 g of ZIF-8, 14 mL of deionized water and 6 mL of ethanol were mixed by ultrasonic treatment and stirred at room temperature. After 30 min, 0.23 g of cetyltrimethylammonium bromide (CTAB), 0.035 g of resorcinol and 0.1 mL of ammonium hydroxide were added in series. After another 30 min, 0.06 mL of a formaldehyde solution was added again. After 8 h, the resulting ZIF-67@resorcinol–formaldehyde (ZIF-67@RF) was achieved by washing with deionized water for 5 times. To change the RF content in ZIF-67@RF, the amount of both the resorcinol and formaldehyde solution was decreased to 1/2 and 1/4 without any other changes.
Hierarchical hollow CoSx/C nanocages were compounded via the solvothermal method. The obtained ZIF-67@RF (100 mg) was dispersed in 30 mL of ethanol solution by ultrasonic treatment, followed by the addition of thioacetamide (TAA, 150 mg) and stirring the mixture for 30 min. Then, the mixture was transferred to a Teflon-lined stainless-steel autoclave, which was subsequently heated at 120 °C for 4 h. After naturally cooling to room temperature, the prepared black precipitate was separated by filtration, washed with ethanol 3 times and dried in an oven. Finally, the as-prepared sample was annealed at 575 °C under nitrogen for 2 h at a ramping rate of 2 °C min−1. The final samples were denoted as CoSx/C-1, CoSx/C-2 and CoSx/C-3, while the carbon or RF content increased gradually. In term of the pristine CoSx, the synthesis procedure was the same except for that ZIF-67@RF was replaced with ZIF-67.
2.2 Material characterizations
Data on the morphology and structure of the samples were recorded using a field-emission scanning electron microscope (FESEM and JSM-7800F) and a high-resolution transmission electron microscope (HR-TEM, Tecnai 20UTwin) affiliated X-ray energy dispersive spectrometry (EDS). To characterize the structure and measure the chemical elements in the specimen, many instruments were incorporated, including an X-ray diffraction (XRD) (Rigaku D/Max-KA diffractometer with Cu Kα radiation, λ = 1.5418 Å), a Raman spectroscope (WITEC Alpha300M+), a thermogravimetric (TG)-differential scanning calorimeter (DSC, Netzsch STA 449 F5) and a Fourier-transform infrared spectroscope (FTIR) (Bruker ALPHA).2.3 Electrochemical measurements
Electrochemical metrics were implemented in a three-electrode system, where a nickel mesh impersonated as the counter electrode, a saturated calomel electrode (SCE) acted as the reference electrode and an aqueous solution dissolved with 1 M KOH represented part of the electrolyte. After the commixture of the obtained samples with poly-(tetrafluoroethylene) (PTFE) and acetylene black in a proportion of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight and the result was rolled into a thin film, the working electrode was achieved. After drying, a slice weighing ca. 2 mg was crushed into a nickel mesh, which represented the working electrode. Particularly, the mass loading for CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3 was about 2 mg, which was precisely weighed prior to testing. In accordance with our former reports,46 an electrochemical working station (CHI 660C) regulated the aggregation on the metrics of cyclic voltammograms (CV), electrochemical impedance spectroscopy (EIS) and charge–discharge surveying, while a Land CT2001A battery program-controlled test system (Land, Wuhan, China) recorded all the data during the cycling progress.
1 by weight and the result was rolled into a thin film, the working electrode was achieved. After drying, a slice weighing ca. 2 mg was crushed into a nickel mesh, which represented the working electrode. Particularly, the mass loading for CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3 was about 2 mg, which was precisely weighed prior to testing. In accordance with our former reports,46 an electrochemical working station (CHI 660C) regulated the aggregation on the metrics of cyclic voltammograms (CV), electrochemical impedance spectroscopy (EIS) and charge–discharge surveying, while a Land CT2001A battery program-controlled test system (Land, Wuhan, China) recorded all the data during the cycling progress.
3. Result and discussion
The strategy for synthesizing the hierarchical hollow CoSx/C nanocages is schematically depicted in Fig. 1 and the specifics are outlined in the experimental section. The obtained ZIF-67 with an average diameter of 200–400 nm has a smooth exterior surface, showing a typical rhombic dodecahedron shape, as shown in Fig. 2a. ZIF-67 nanocrystals could be coated by the RF due to the H-bonding from the ZIF-67 nanocrystals and the hydroxyl groups from RF. It can be clearly seen that the surface of ZIF-67@RF was rougher than that of the ZIF-67 nanocrystals and some RF flakes could be identified as shown in Fig. 2b. In order to confirm that the RF was covered on ZIF-67 during the polymerization process, ZIF-67@RF was acidified with 1 M HCl for 30 min. The resulting dull yellow product showed a hollow shell maintaining the shape of ZIF-67 (Fig. S1a†). To compare the difference in functional groups on the surface between ZIF-67, ZIF-67@RF and the RF hollow shell, the FTIR spectra were recorded, as shown in Fig. 2c. The band at 1578 cm−1 was attributed to the stretching of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, while the band at 3136 cm−1 was attributed to the stretching vibration of C–H from the aliphatic chain. Particularly, the band located at 424 cm−1 came from the stretching of the Co
N bond, while the band at 3136 cm−1 was attributed to the stretching vibration of C–H from the aliphatic chain. Particularly, the band located at 424 cm−1 came from the stretching of the Co![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. The bands at 2929 and 2848 cm−1 were assigned to the CH2 stretching and bending vibrations, respectively, whereas the band at 1605 cm−1 was assigned to the aromatic ring stretches. The results confirmed that ZIF-67 and RF coexisted and were blended effectively in ZIF-67@RF. Powder X-ray diffraction (XRD) patterns for both ZIF-67 and ZIF-67@RF are displayed in Fig. 2d, showing that the typical ZIF topologies are in agreement with previous reports.47
N bond. The bands at 2929 and 2848 cm−1 were assigned to the CH2 stretching and bending vibrations, respectively, whereas the band at 1605 cm−1 was assigned to the aromatic ring stretches. The results confirmed that ZIF-67 and RF coexisted and were blended effectively in ZIF-67@RF. Powder X-ray diffraction (XRD) patterns for both ZIF-67 and ZIF-67@RF are displayed in Fig. 2d, showing that the typical ZIF topologies are in agreement with previous reports.47
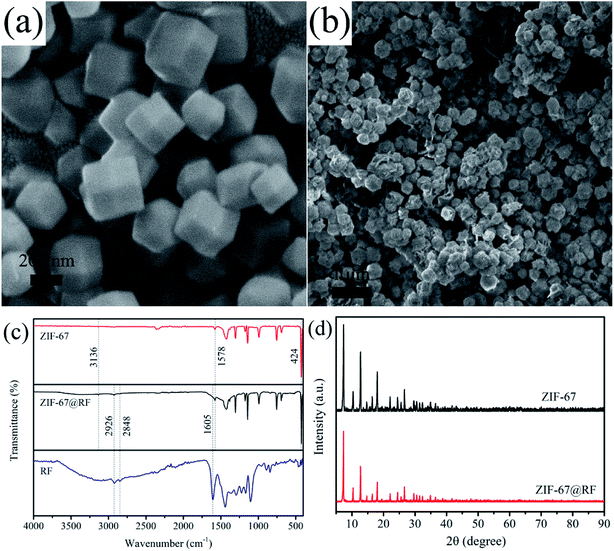 | ||
| Fig. 2 SEM images of (a) ZIF-67 and (b) ZIF-67@RF. (c) FTIR spectra of ZIF-67, ZIF-67@RF and RF. (d) XRD patterns of ZIF-67 and ZIF-67@RF. | ||
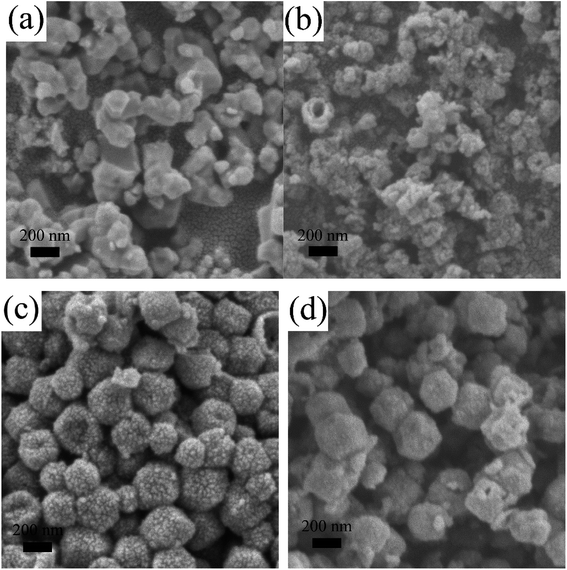
During the subsequent sulfurization process, the Co ion that dissociated from the ZIF-67 nanocrystals reacted with S2− hydrolyzed from TAA, which resulted in cobalt sulfides.48 In particular, the RF coating layer was supposed to act as the hard framework to form the hollow nanocages during the sulfurization process when the obtained cobalt sulfides nanoparticles could attach to the layer. The RF coating layer contained in the hollow nanocages was in situ transformed to a high-conductivity carbon layer after the heat treatment. To identify the positive influence that the RF coating layers exerted on the morphology of the ultimate products, various ZIF-67@RF samples with dissimilar proportion of RF were yielded after the same operation. These various samples were named CoSx/C-1, CoSx/C-2 and CoSx/C-3, with a gradual increase in the RF content. As shown in Fig. 3, CoSx without the RF coating layer was irregular and aggregated (Fig. 3a). A few broken hollow nanocages were recognized in CoSx/C-1 (Fig. 3b), while the hollow structure remained unbroken and uniform for CoSx/C-2 (Fig. 3c and Fig. S1b and c†) and CoSx/C-3 (Fig. 3d). This distinct trend indicates that the RF coating layer effectively promoted the formation of the hierarchical hollow nanocages.
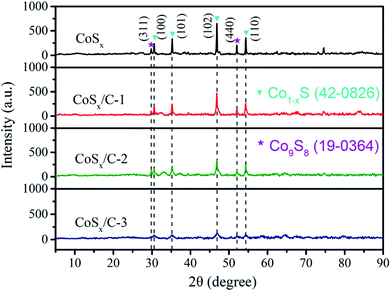
A closer inspection of Fig. 4 evidences the XRD patterns of CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3. At the limit of the resolution ratio for our X-ray diffractometer, the XRD pattern of CoSx/C-1 was more correlated with that of CoSx/C-2 and CoSx/C-3 compared with that of pristine CoSx, illustrating negligible influence on the framework of the cube from the incorporation of carbon layer. All examples showed that the featured peaks of the (311) and (440) planes originated from Co9S8 (JCPDS card no. 19-0364) at 2θ values of 39.5° and 52.0°, labeled with purple pentalpha, and the characteristic peaks of the (100), (101), (102) and (110) planes for the Co1−xS (JCPDS card no. 19-0364) at 2θ values of 30.5°, 35.2°, 46.8° and 54.3° are labelled with a blue mark in Fig. 4. On account of the inability to detect the other peaks belonging to crystalline carbon, the carbon was deemed as amorphous. Moreover, with the augment of the carbon content, there was a visible decrease in the intensity of the diffraction peaks from CoSx/C, which unveiled an obstructive effect on the grain growth of cobalt sulfides by amorphous carbon.49
To unearth the precise statistics for the carbon content, TG-DSC was used to interpret CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3 with temperature initiating from 25 °C and ending at 800 °C under the air atmosphere, as revealed in Fig. S2.† Along with the evaporation of water (adsorption from the air), an apparent weight loss was observed from 25 °C to 200 °C. Soon after, the weight loss trend transformed for temperatures higher than 300 °C. As pointed out in a previous research, a series of weight changes can be attributed to the complicated reaction between CoSx and oxygen, and the further pyrolytic degradation of some intermediate products results in the formation of Co3O4.49 To calculate the carbon content, the temperature of 575 °C was selected as a standard because all the samples temporarily steady in weight and the carbon in the CoSx/C could react with oxygen to form CO2. The calculation details are shown in Fig. S2e† and the calculated carbon contents were 2.5%, 5.3% and 11.0% in CoSx/C-1, CoSx/C-2 and CoSx/C-3, respectively.
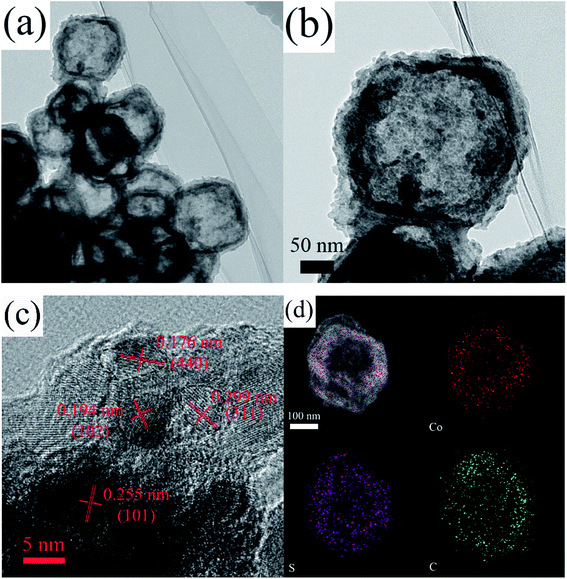
The microstructures of the CoSx/C-2 hierarchical hollow nanocages are depicted in detail in Fig. 5a and b. The well-defined carbon layer can be clearly seen, which supports the two uniform CoSx layers consisting of monodispersed nanoparticles with a diameter of ca. 10 nm. These outer nanoparticles were consistent with the rough surface of CoSx/C-2, as shown in Fig. 3c and S1c.† In addition, the composition of CoSx was assured by the HRTEM lattice image in Fig. 5c. The notable d-spacings of 0.176 nm and 0.299 nm were well-substantiated to those of the (440) and (311) planes of Co9S8, and the d-spacings of 0.194 nm and 0.255 nm corroborated to with those of the (102) and (101) planes of Co1−xS, which agreed well with the XRD pattern (Fig. 4).44 The elemental mapping of a single CoSx/C-2 hierarchical hollow nanocage confirmed the uniform presence of Co, S and C throughout the surface of the sample, as given in Fig. 5d. In addition, the atomic ratio of S and Co was 1.08 in this single hollow nanocages.
The electrochemical performance of the as-prepared CoSx/C electrode was evaluated using cyclic voltammetry (CV) and galvanostatic charge/discharge cycling with the assistance of a three-electrode system in an aqueous electrolyte containing 1 M KOH. Fig. 6a exhibits the CV curves of the CoSx/C-2 hollow nanocages at various scan rates from 5 mV s−1 to 100 mV s−1 within the potential window from 0 V to 0.5 V (vs. saturated calomel electrode, SCE). Apparently, the ampere density was gradually augmented along with the scan rate as the shape of the CV curve was well-preserved without any marked deformation. When the scan rate reached 5 mV s−1, the oxidation and reduction peaks were around 0.36 V and 0.29 V, respectively. The anodic peaks shifted in the anodic direction, while the cathodic peaks tended to shift in the opposite direction. Similar to the CV curves for CoSx (Fig. S3a†), all of the curves for CoSx/C-1 (Fig. S3b†) and CoSx/C-2 (Fig. S3c†) presented a clear pseudo capacitance featured with an unchanged shape. According to previous reports, the faradaic reactions with the incorporation of the cobalt sulfide-based materials in the alkaline solution system are shown in eqn (1) and (2).50–52 From the data in Fig. 6a, it is apparent that a pair of redox peaks (A2 and C2) associated with eqn (2) were highly reversible, while in the other pair of redox peaks (A1 and C1) the reduction peak C1 was almost invisible. This finding indicates that the reaction given in eqn (1) tends to mostly oxidize, which aligns well with the previous report.50 In particular, the curves of the CoSx/C-2 hollow nanocages still maintained a regular shape with much slighter peaks shifts than those of CoSx. This result occurred even though the scan rate is increased to 100 mV s−1, thus attesting the promotion to a fast redox reaction from the appropriate incorporation of the carbon layer. The comparison of the CV curves for CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3 at 10 mV s−1 is shown in Fig. S3d.† The relatively higher area of the closed CV curve for the CoSx/C-2 hollow nanocages surmised higher reactivities for the redox reactions. Within the potential window of 0 V to 0.45 V at various current densities, the galvanostatic charge–discharge investigation was disseminated to measure the specific capacitances of all specimens, as shown in Fig. 6b and S4.† The CoSx/C-2 hollow nanocages exhibited admirable specific capacitances of 618.4 F g−1, 608.4 F g−1, 594.4 F g−1, 584.3 F g−1 and 574.1 F g−1 at discharge current densities of 2 A g−1, 4 A g−1, 6 A g−1, 8 A g−1, and 10 A g−1, respectively, which exceeded those of the pristine CoSx (Fig. S4a†) (518.6 F g−1, 498.7 F g−1, 485.6 F g−1, 474.9 F g−1 and 461.3 F g−1 at the corresponding current densities). A comparison of the rate performance for all the samples is given in Fig. 6c, the specific capacitance of the CoSx/C-2 hollow nanocages was retained as high as 83.6% with the current density ranging from 2 A g−1 to 50 A g−1.
| CoSx + OH− ↔ CoSxOH + e− | (1) |
| CoSxOH + OH− ↔ CoSxO + H2O + e− | (2) |
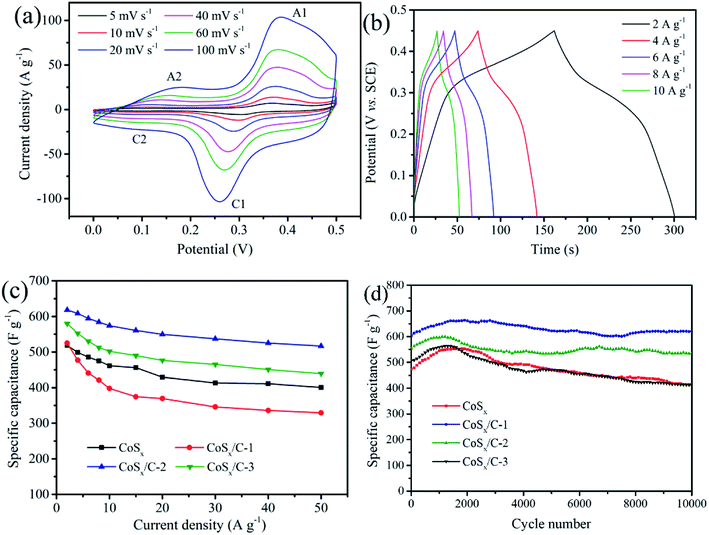 | ||
| Fig. 6 (a) CV curves and (b) galvanostatic charge–discharge curves of CoSx/C-2 hollow nanocages. (c) Rate performance and (d) cycling performance of CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3. | ||
This superior rate performance should be ascribed to the optimized charge transfer procedure, which is expounded by the EIS spectra and the corresponding equivalent circuit in Fig. 7. The resistance of the system (Rs), consisting of the ohmic resistance of the aqueous electrolyte, the electrolyte/electrode interface and active materials, was 0.33 Ω for the CoSx/C-2 hollow nanocages and 1.30 Ω for pristine CoSx. The Rs values for CoSx/C-1 and CoSx/C-3 was also smaller than that for pristine CoSx as shown in the magnified EIS spectra (inset, Fig. 7a), confirming that the interior high-conductivity carbon layer can effectively diminish the Rs. The charge transference resistance (Rct) of the CoSx/C-2 hollow nanocages with the incorporation of the carbon layer was dramatically decreased to 0.68 Ω compared to that of pristine CoSx (2.36 Ω). The decline in the Rct value also appeared for CoSx/C-1 (2.01 Ω) and CoSx/C-3 (0.86 Ω), revealing that the interior high-conductivity of the carbon layer can effectively diminish the charge transfer resistance. The schematic in Fig. 7c discloses the decreased charge transfer and improved the ion diffusion path for the CoSx/C-2 hollow nanocages. Moreover, the carbon layer of high-conductivity could effectively get the charges from the cobalt sulfide nanoparticles attached on the both side of the layer, which donated the unique charge transfer path compared to the aggregated regular CoSx. The hollow structure and cobalt sulfide nanoparticles enlarged the contact area between the electrolyte and electrode materials, providing more active redox sites in comparison to the aggregated pristine CoSx.53,54 These two factors were mainly responsible for the increased specific capacitance and enhanced rate performance. It is believed that the rational hierarchical hollow nanocages containing the high-conductive carbon layer support can be useful in boosting the electrochemical performance of more electrode materials.
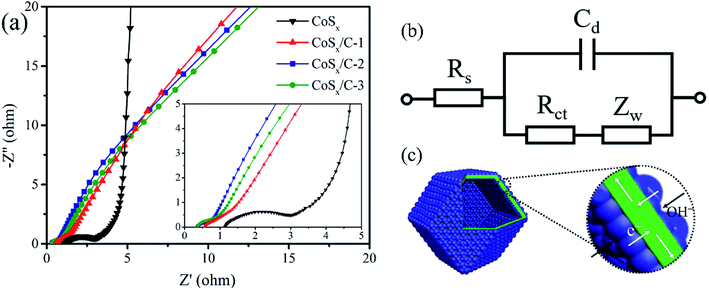 | ||
| Fig. 7 (a) EIS spectra and (b) equivalent circuits of CoSx, CoSx/C-1, CoSx/C-2 and CoSx/C-3. (c) Schematic models of the charge transfer and ion diffusion path of CoSx/C hollow nanocages. | ||
The cycling performance of the CoSx/C-2 hollow nanocage electrode was also analyzed via galvanostatic charge–discharge tests for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles when the current density was 4.0 A g−1, as exhibited in Fig. 6d. The specific capacitances of all the samples were elevated at the very beginning due to the activation of CoSx,43,50 then decreased to some content and later stabilized. After 10
000 cycles when the current density was 4.0 A g−1, as exhibited in Fig. 6d. The specific capacitances of all the samples were elevated at the very beginning due to the activation of CoSx,43,50 then decreased to some content and later stabilized. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the high specific capacitances of the CoSx/C-2 hollow nanocages were still retained without any evident capacity fading. The high specific capacitances were much better than that of the pristine CoSx (ca. 81.6%), CoSx/C-1 (ca. 87.6%) and CoSx/C-3 (ca. 94.7%), proving the long-term electrochemical stability of the hierarchical hollow nanocages. It should be noted that the hierarchically hollow structure was composed of a carbon layer framework and nano-sized cobalt sulfide particles, which maintained the morphology stability, prevented aggregation and eliminated deactivation during the iterative redox reactions. Recent reports on the use of cobalt sulfides as electrode materials for supercapacitors are enumerated in Table 1. Our optimized CoSx/C hierarchical hollow nanocages demonstrated an excellent electrochemical performance compared to previous works.39–42,50,51,55–58
000 cycles, the high specific capacitances of the CoSx/C-2 hollow nanocages were still retained without any evident capacity fading. The high specific capacitances were much better than that of the pristine CoSx (ca. 81.6%), CoSx/C-1 (ca. 87.6%) and CoSx/C-3 (ca. 94.7%), proving the long-term electrochemical stability of the hierarchical hollow nanocages. It should be noted that the hierarchically hollow structure was composed of a carbon layer framework and nano-sized cobalt sulfide particles, which maintained the morphology stability, prevented aggregation and eliminated deactivation during the iterative redox reactions. Recent reports on the use of cobalt sulfides as electrode materials for supercapacitors are enumerated in Table 1. Our optimized CoSx/C hierarchical hollow nanocages demonstrated an excellent electrochemical performance compared to previous works.39–42,50,51,55–58
| Material | Specific capacitance (F g−1) | Cycling performance | Reference |
|---|---|---|---|
| CoxS@PC/rGO | 455.0 (2 A g−1) | 99.7% (4000 cycles, 1 A g−1) | 43 |
| CoSNC | 360 (1.5 A g−1) | 90% (2000 cycles, 12 A g−1) | 55 |
| Co9S8/GPs | 536 (1 A g−1) | 91.8% (2500 cycles, 10 A g−1) | 56 |
| Co9S8@C | 514 (1 A g−1) | 88% (1000 cycles, 8 A g−1) | 57 |
| Co9S8 nanotubes | 285.3 (2 A g−1) | 90.4% (1000 cycles, 2 A g−1) | 50 |
| Co9S8 nanospheres | 306.1 (0.1 A g−1) | — | 58 |
| 3D flower-like Co9S8 | 522 (0.5 A g−1) | 97.7% (1000 cycles, 1 A g−1) | 51 |
| CoSx/C-2 | 618.4 F g−1 (2 A g−1) | ca. 100% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 4 A g−1) 000 cycles, 4 A g−1) |
This study |
4. Conclusion
In summary, cobalt sulfide hierarchical hollow nanocages coated with a carbon layer were synthesized using ZIF-67 nanocrystals coated with RF as a self-sacrificial template. The morphology and electrochemical performance was investigated for the CoSx/C hierarchical hollow nanocages with different carbon contents of the RF coating layer after an in situ transformation to a conductive carbon layer. The results demonstrated that an appropriate RF coating layer could promote the formation of hollow nanocages. The modified CoSx/C hierarchical hollow nanocages demonstrated a superior rate performance (83.6% capacitance retention with a current density varying from 2 A g−1 to 50 A g−1) and an extraordinary cycling durability without the capacity fading after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The prominent electrochemical performance could be ascribed to the elaborately designed hierarchical hollow structure, which provided support and protection for the hollow shells and the conductive carbon layer. This technique could be generally extended to other related materials, such as various MOFs.
000 cycles. The prominent electrochemical performance could be ascribed to the elaborately designed hierarchical hollow structure, which provided support and protection for the hollow shells and the conductive carbon layer. This technique could be generally extended to other related materials, such as various MOFs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Key Project of MOST (2016YFB0700600), the National Natural Science Foundation Committee of China (Distinguished Youth Scientists Project of 51425301, U1601214, 51573013, 51773092 and 51772147), the 1000 Youth Talents Plan of the National Natural Science Foundation of China (51773092), the Research Foundation of State Key Lab (ZK201805 and ZK201717) and the Jiangsu Distinguished Professorship Program (2016).References
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Latest advances in supercapacitors: from new electrode materials to novel device designs, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- M. Winter and R. J. Brodd, What are batteries, fuel cells, and supercapacitors?, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, A review of electrode materials for electrochemical supercapacitors, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- P. Simon, Y. Gogotsi and B. Dunn, Where do batteries end and supercapacitors begin?, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- B. E. Conway, V. Birss and J. Wojtowicz, The role and utilization of pseudocapacitance for energy storage by supercapacitors, J. Power Sources, 1997, 66, 1–14 CrossRef CAS.
- L. L. Zhang and X. S. Zhao, Carbon-based materials as supercapacitor electrodes, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz and M. Thommes, Carbon-based supercapacitors produced by activation of graphene, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- E. Frackowiak, Carbon materials for supercapacitor application, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- G. A. Snook, P. Kao and A. S. Best, Conducting-polymer-based supercapacitor devices and electrodes, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Highly flexible and all-solid-state paperlike polymer supercapacitors, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- X. Lang, A. Hirata, T. Fujita and M. Chen, Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS PubMed.
- P. Zhang, X. Zhao, Z. Liu, F. Wang, Y. Huang, H. Li, Y. Li, J. Wang, Z. Su, G. Wei, Y. Zhu, L. Fu, Y. Wu and W. Huang, Exposed high-energy facets in ultradispersed sub-10 nm SnO2 nanocrystals anchored on graphene for pseudocapacitive sodium storage and high-performance quasi-solid-state sodium-ion capacitors, NPG Asia Mater., 2018, 10, 429–440 CrossRef CAS.
- C. Wei, R. Zhang, X. Zheng, Q. Ru, Q. Chen, C. Cui, G. Li and D. Zhang, Hierarchical porous NiCo2O4/CeO2 hybrid materials for high performance supercapacitors, Inorg. Chem. Front., 2018, 5, 3126–3134 RSC.
- X.-Y. Yu, L. Yu and X. W. D. Lou, Metal sulfide hollow nanostructures for electrochemical energy storage, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- L. Shen, L. Yu, H. B. Wu, X.-Y. Yu, X. Zhang and X. W. D. Lou, Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- Y. M. Chen, Z. Li and X. W. D. Lou, General formation of MxCo3−xS4(M= Ni, Mn, Zn) hollow tubular structures for hybrid supercapacitors, Angew. Chem., 2015, 127, 10667–10670 CrossRef.
- C. Z. Wei, Q. L. Ru, X. T. Kang, H. Y. Hou, C. Cheng and D. J. Zhang, Self-template synthesis of double shelled ZnS-NiS1.97 hollow spheres for electrochemical energy storage, Appl. Surf. Sci., 2018, 435, 993–1001 CrossRef CAS.
- T. Cottineau, M. Toupin, T. Delahaye, T. Brousse and D. Bélanger, Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors, Appl. Phys. A, 2006, 82, 599–606 CrossRef CAS.
- Z. Fan, D. Qi, Y. Xiao, J. Yan and T. Wei, One-step synthesis of biomass-derived porous carbon foam for high performance supercapacitors, Mater. Lett., 2013, 101, 29–32 CrossRef CAS.
- K. H. An, W. S. Kim, Y. S. Park, Y. C. Choi, S. M. Lee, D. C. Chung, D. J. Bae, S. C. Lim and Y. H. Lee, Supercapacitors using single-walled carbon nanotube electrodes, Adv. Mater., 2001, 13, 497–500 CrossRef CAS.
- C. Wei, N. Zhan, J. Tao, S. Pang, L. Zhang, C. Cheng and D. Zhang, Synthesis of hierarchically porous NiCo2S4 core-shell hollow spheres via self-template route for high performance supercapacitors, Appl. Surf. Sci., 2018, 453, 288–296 CrossRef CAS.
- C. H. Lai, M. Y. Lu and L. J. Chen, Metal sulfide nanostructures: synthesis, properties and applications in energy conversion and storage, J. Mater. Chem., 2012, 22, 19–30 RSC.
- J. Yang, X. Duan, Q. Qin and W. Zheng, Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors, J. Mater. Chem. A, 2013, 1, 7880–7884 RSC.
- J. Shi, X. Li, G. He, L. Zhang and M. Li, Electrodeposition of high-capacitance 3D CoS/graphene nanosheets on nickel foam for high-performance aqueous asymmetric supercapacitors, J. Mater. Chem. A, 2015, 3, 20619–20626 RSC.
- W. Xia, A. Mahmood, R. Zou and Q. Xu, Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion, Energy Environ. Sci., 2015, 8, 1837–1866 RSC.
- S. Bai, X. Liu, K. Zhu, S. Wu and H. Zhou, Metal–organic framework-based separator for lithium–sulfur batteries, Nat. Energy, 2016, 1, 16094 CrossRef CAS.
- F. Zheng, Y. Yang and Q. Chen, High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework, Nat. Commun., 2014, 5, 5261 CrossRef CAS PubMed.
- S. L. James, Metal-organic frameworks, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature, 1999, 402, 276 CrossRef CAS.
- B. Liu, H. Shioyama, H. Jiang, X. Zhang and Q. Xu, Metal–organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor, Carbon, 2010, 48, 456–463 CrossRef CAS.
- R. Díaz, M. G. Orcajo, J. A. Botas, G. Calleja and J. Palma, Co8-MOF-5 as electrode for supercapacitors, Mater. Lett., 2012, 68, 126–128 CrossRef.
- F. Yu, Z. Chang, X. Yuan, F. Wang, Y. Zhu, L. Fu, Y. Chen, H. Wang, Y. Wu and W. Li, Ultrathin NiCo2S4@graphene with a core-shell structure as a high performance positive electrode for hybrid supercapacitors, J. Mater. Chem. A, 2018, 6, 5856–5861 RSC.
- H. Hu, B. Y. Guan and X. W. Lou, Construction of complex CoS hollow structures with enhanced electrochemical properties for hybrid supercapacitors, Chem, 2016, 1, 102–113 CAS.
- C. Yuan, L. Yang, L. Hou, J. Li, Y. Sun, X. Zhang, L. Shen, X. Lu, S. Xiong and X. W. D. Lou, Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors, Adv. Funct. Mater., 2012, 22, 2560–2566 CrossRef CAS.
- Z. Chen, V. Augustyn, J. Wen, Y. Zhang, M. Shen, B. Dunn and Y. Lu, High-performance supercapacitors based on intertwined CNT/V2O5 nanowire nanocomposites, Adv. Mater., 2011, 23, 791–795 CrossRef CAS PubMed.
- J. Y. Lee, K. Liang, K. H. An and Y. H. Lee, Nickel oxide/carbon nanotubes nanocomposite for electrochemical capacitance, Synth. Met., 2005, 150, 153–157 CrossRef CAS.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review, Nanoscale, 2013, 5, 72–88 RSC.
- X. Wang, M. Li, Z. Chang, Y. Yang, Y. Wu and X. Liu, Co3O4@MWCNT nanocable as cathode with superior electrochemical performance for supercapacitors, ACS Appl. Mater. Interfaces, 2015, 7, 2280–2285 CrossRef CAS PubMed.
- T. Zhu, B. Xia, L. Zhou and X. W. D. Lou, Arrays of ultrafine CuS nanoneedles supported on a CNT backbone for application in supercapacitors, J. Mater. Chem., 2012, 22, 7851–7855 RSC.
- T. Zhu, H. B. Wu, Y. Wang, R. Xu and X. W. D. Lou, Formation of 1D hierarchical structures composed of Ni3S2 nanosheets on CNTs backbone for supercapacitors and photocatalytic H2 production, Adv. Energy Mater., 2012, 2, 1497–1502 CrossRef CAS.
- H. Zhang, X. Yu, D. Guo, B. Qu, M. Zhang, Q. Li and T. Wang, Synthesis of bacteria promoted reduced graphene oxide-nickel sulfide networks for advanced supercapacitors, ACS Appl. Mater. Interfaces, 2013, 5, 7335–7340 CrossRef CAS PubMed.
- A. Mohammadi, N. Arsalani, A. G. Tabrizi, S. E. Moosavifard, Z. Naqshbandi and L. S. Ghadimi, Engineering rGO-CNT wrapped Co3S4 nanocomposites for high-performance asymmetric supercapacitors, Chem. Eng. J., 2018, 334, 66–80 CrossRef CAS.
- Y. Wang, B. Chen, Z. Chang, X. Wang, F. Wang, L. Zhang, Y. Zhu, L. Fu and Y. Wu, Enhancing performance of sandwich-like cobalt sulfide and carbon for quasi-solid-state hybrid electrochemical capacitors, J. Mater. Chem. A, 2017, 5, 8981–8988 RSC.
- P. Wang, C. Li, W. Wang, J. Wang, Y. Zhu and Y. Wu, Hollow Co9S8 from metal organic framework supported on rGO as electrode material for highly stable supercapacitors, Chin. Chem. Lett., 2018, 29, 612–615 CrossRef CAS.
- S. Dong, C. Li, X. Ge, Z. Li, X. Miao and L. Yin, ZnS-Sb2S3@C core-double shell polyhedron structure derived from metal-organic framework as anodes for high performance sodium ion batteries, ACS Nano, 2017, 11, 6474–6482 CrossRef CAS PubMed.
- C. Li, W. Wu, P. Wang, W. Zhou, J. Wang, Y. Chen, L. Fu, Y. Zhu, Y. Wu and W. Huang, Fabricating an aqueous symmetric supercapacitor with a stable high working voltage of 2 V by using an alkaline–acidic electrolyte, Adv. Sci., 2018, 1801665 Search PubMed.
- J. Qian, F. Sun and L. Qin, Hydrothermal synthesis of zeolitic imidazolate framework-67(ZIF-67) nanocrystals, Mater. Lett., 2012, 82, 220–223 CrossRef CAS.
- Z. Jiang, W. Lu, Z. Li, K. H. Ho, X. Li, X. Jiao and D. Chen, Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors, J. Mater. Chem. A, 2014, 2, 8603–8606 RSC.
- X. Liu, H. Liu, Y. Zhao, Y. Dong, Q. Fan and Q. Kuang, Synthesis of the carbon-coated nanoparticle Co9S8 and its electrochemical performance as an anode material for sodium-ion batteries, Langmuir, 2016, 32, 12593–12602 CrossRef CAS PubMed.
- J. Yu, H. Wan, J. Jiang, Y. Ruan, L. Miao, L. Zhang, D. Xia and K. Xu, Activation mechanism study of dandelion-like Co9S8 nanotubes in supercapacitors, J. Electrochem. Soc., 2014, 161, A996–A1000 CrossRef CAS.
- L. Yin, L. Wang, X. Liu, Y. Gai, L. Su, B. Qu and L. Gong, Ultra-fast microwave synthesis of 3D flower-Like Co9S8 hierarchical architectures for high-performance supercapacitor applications, Eur. J. Inorg. Chem., 2015, 2457–2462 CrossRef CAS.
- T.-W. Lin, C.-S. Dai, T.-T. Tasi, S.-W. Chou, J.-Y. Lin and H.-H. Shen, High-performance asymmetric supercapacitor based on Co9S8/3D graphene composite and graphene hydrogel, Chem. Eng. J., 2015, 279, 241–249 CrossRef CAS.
- S. Peng, L. Li, H. B. Wu, S. Madhavi and X. W. D. Lou, Controlled Growth of NiMoO4 Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors, Adv. Energy Mater., 2015, 5, 1401172 CrossRef.
- G. Wang, L. Zhang and J. Zhang, A review of electrode materials for electrochemical supercapacitors, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- F. Cao, M. Zhao, Y. Yu, B. Chen, Y. Huang, J. Yang, X. Cao, Q. Lu, X. Zhang and Z. Zhang, Synthesis of two-dimensional CoS1.097/nitrogen-doped carbon nanocomposites using metal–organic framework nanosheets as precursors for supercapacitor application, J. Am. Chem. Soc., 2016, 138, 6924–6927 CrossRef CAS PubMed.
- D. Xiong, X. Li, Z. Bai, J. Li, Y. Han and D. Li, Vertically aligned Co9S8 nanotube arrays onto graphene papers as high-performance flexible electrodes for supercapacitors, Chem.–Eur. J., 2018, 24, 2339–2343 CrossRef CAS PubMed.
- T. W. Lin, H. C. Tsai, T. Y. Chen and L. D. Shao, Facile and controllable one-pot synthesis of hierarchical Co9S8 hollow microspheres as high-performance electroactive materials for energy storage and conversion, ChemElectroChem, 2018, 5, 137–143 CrossRef CAS.
- L. Zhang, Y. Wang, W. Zhou, G. Song and S. Cheng, Facile synthesis of hollow Co9S8 nanospheres for high performance pseudocapacitor, Int. J. Electrochem. Sci., 2016, 11, 1541–1548 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01167f |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2019 |