Open Access Article
Open Access ArticleUpconversion of transparent glass ceramics containing β-NaYF4:Yb3+, Er3+ nanocrystals for optical thermometry
Xinyue Li*a,
Longyu Yanga,
Yiwen Zhua,
Jiasong Zhong a and
Daqin Chen
a and
Daqin Chen *b
*b
aCollege of Materials & Environmental Engineering, Hangzhou Dianzi University, Hangzhou, 310018, P. R. China. E-mail: lixy@hdu.edu.cn
bCollege of Physics and Energy, Fujian Normal University, Fuzhou, 350117, P. R. China. E-mail: dqchen@fjnu.edu.cn
First published on 11th March 2019
Abstract
β-NaYF4 nanocrystal embedded glass ceramics were fabricated by a melt-quenching method with subsequent heat-treatment. Structural characterizations and spectrographic techniques were performed to verify the successful precipitation of β-NaYF4 nanocrystals and partition of dopants. Upon excitation of 980 nm, bright green upconversion emission could be achieved in Yb3+, Er3+ codoped β-NaYF4 nanocrystal embedded glass ceramics. Furthermore, the temperature-dependent upconversion behaviour based on thermally coupled energy levels was also examined in the range of 300–773 K with the maximum relative sensitivity of 1.24% K−1 at 300 K. Accordingly, it has been proved to be a promising candidate for application in optical thermometry.
1. Introduction
Temperature, which is a fundamental parameter, is of unshakeable importance in various fields, covering daily life, science and technology, industrial manufacture and so on.1–8 In particular, the accuracy of temperature evaluation is urgently crucial. Recently, an ever-increasing focus has been paid to a new type of temperature-measurement technique, which is on the basis of temperature-dependent optical signals, involving luminescence intensity, peak position, full width at half maximum (FWHM) of emission bands, lifetime, fluorescence intensity ratio (FIR) and so on.9–13 In comparison with traditional temperature measurement methods, this optical thermometry technique possesses unique features such as non-invasiveness, high-resolution, and real time response.Especially, FIR technique is one of the most promising methods to be applied in practice, owing to its easy operation.14,15 When two distinguishable emission bands exhibit discrepant temperature behaviour, therefore, the values of their ratio also vary with respect to temperature. Benefit to the evaluation of ratio of two corresponding emission bands, FIR technique can get rid of measuring errors from measurement conditions, for instance fluctuations of excitation source, light scatting and reflection and the drifts of the optoelectronic system. The key factor for FIR technique is identification of two related emission bands. Generally, when the energy gap between two energy levels reaches in the range of 200–2000 cm−1, the signal of emission bands can be separated clearly, getting rid of overlapping of emission bands or losing thermal population from the upper level. As a result, the energy levels that meet this condition are also called the thermally coupled energy levels (TECLs). Coincidentally, lanthanide ions are endowed with enriched energy levels and extremely shaped spectral lines, owing to their 4f–4f transitions. In this way, it would come distinctly that lanthanide ions, for instance Er3+, Ho3+, Dy3+ et al., are suitable for FIR-based optical thermometry.16–20
But beyond all that, it is quite essential to select suitable host materials. Lanthanide ions doped glass ceramics (GCs) have been honoured as a new class of superior bulk materials, deriving from their favourable integration of low-cost and easy-synthesis of glass and crystal-like optical performance.21–23 Furthermore, their stable physical and chemical properties have greatly expanded the areas for practical applications. Up to now, oxyfluorides GCs have garnered wide attentions, due to their low phonon energies and high luminescent efficiency.24–26 Among investigated host materials, β-NaYF4 is a typical example of fluorides.27,28 It has been recognized as one of the most efficient host materials for upconversion (UC), with the incorporation of Yb3+, Er3+ for green UC emission and Yb3+, Tm3+ for blue UC emission. In β-NaYF4 crystal lattice, there exists two distinct sites for Y3+. As depicted in Fig. 1(a), half of Y3+ is occupied by a fixed site, while the other is randomly occupied with Na+. Every Y3+ is surrounded by nine F− anions. When lanthanide ions incorporated into the lattice, they prefer to substitute the sites of Y3+ consciously. These unique features make β-NaYF4 one of the most efficient host materials for UC.29,30
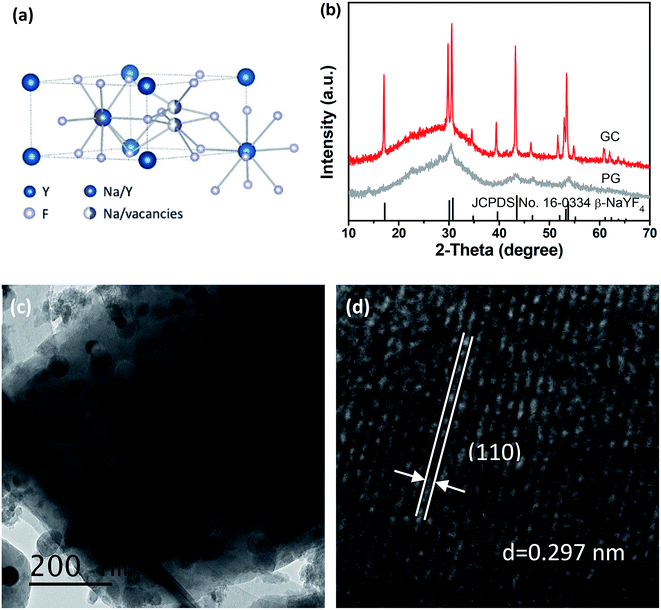 | ||
| Fig. 1 (a) Crystal structure of β-NaYF4. (b) XRD patterns of PG and GC samples with standard card of β-NaYF4 (JCPDS no. 16–0334). (c) TEM and (d) HRTEM of β-NaYF4 nanocrystal embedded glass ceramics. | ||
However, it is still not easy to grow β-NaYF4 nanocrystals owing to its cubic-to-hexagonal phase transition during cooling. To our best knowledge, the research on FIR-based optical thermometry in β-NaYF4 GC has not yet been reported. In our case, self-crystallized β-NaYF4:Yb3+, Er3+ nanocrystal embedded GCs were successfully synthesized by traditional melt-quenching method followed by heat-treatment. The precipitation of β-NaYF4 nanocrystals from glass matrix were verified by structural characterizations. Eu3+ was incorporated into the β-NaYF4 GC sample to serve as a super-sensitive probe, and the spectrographic feature suggests the partition of dopants. The GC containing β-NaYF4:Yb3+, Er3+ nanocrystals exhibited bright UC emission, when excited by 980 nm diode laser. The purpose of this work is to discuss the UC emission properties and mechanism of Yb3+, Er3+ codoped β-NaYF4 nanocrystal embedded glass ceramics, and to examine its temperature-dependent behaviour based on FIR technique serving as a candidate for optical thermometry.
2. Experimental section
Traditional melt-quenching method and subsequent heat-treatment was employed to prepare glass ceramics containing β-NaYF4 nanocrystals. Chemical compositions were carefully designed as 55SiO2–10Al2O3–17Na2O–17NaF–8YF3, according to the mechanism for phase-selective growth of NaYF4 reported in our previous studies.31 All of the raw materials were mixed together and melt at 1450–1550 °C for 30 min, and then poured onto a copper preheated at 300 °C to form precursor glass. In order to release inner stress, the precursor glasses, labelled as PGs, were maintained at 400 °C for 10 h. Glass ceramics, donated as GCs, could be gained after glass crystallization at 650 °C for 2 h.The crystalline phases of samples were conducted by X-ray diffractometer (Rigaku-TTR-III) with nickel-filtered Cu Kα radiation (λ = 0.15418 nm) in the 2θ range from 10° to 70°. A transmission electron microscopy (TEM, JEM-2010) was employed to characterize the microstructure of GCs. Additionally, scanning transmission electron microscopy (STEM) operated in the high-angle annular dark-field (HAADF) mode was also performed. Furthermore, when excited at 980 nm, the UC emission spectra were recorded by a Jobin-Yvon HRD-1 double monochromator equipped with a Hamamatsu R928 photomultiplier. An Opolette 355 LD laser (410–2200 nm, a spectral line-width of 4–7 cm−1) was chosen as the excitation source for the lifetime measurement. For the measurement of temperature-dependent UC emission spectra, the sample was loaded on a copper host equipped with a temperature controller (OMRON E5CC-800). The temperature, ranging from 300 K to 773 K, was controlled by a type-K thermocouple and a heating tube.
3. Results and discussion
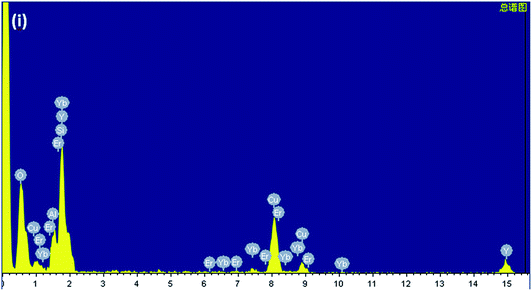
Fig. 1(b) presents the XRD patterns of PG and GC samples. For the PG, a small quantity of diffraction peaks, assigned to β-NaYF4 (JCPDS no. 16–0334), could be found superimposed on the amorphous hump of glass, suggesting the β-NaYF4 nanocrystals have been already formed in the precursor glass. After heat-treatment, both of the amount and size of β-NaYF4 nanocrystals are increased, so the diffraction peaks of GC are enhanced and sharpened. This crystallization behaviour of glass is call “self-crystallization”.4 Furthermore, morphological features of GC sample were conducted by TEM and high-resolution TEM (HRTEM) images. As exhibited in Fig. 1(c), the β-NaYF4 nanocrystals precipitated homogenously from glass matrix have sized mainly in the range of 30–50 nm. Notably, it can be seen that well-demarcated lattice fringes appear in HRTEM image in Fig. 1(d). The corresponding interplanar spacing d is given as 0.297 nm, which matches perfectly with the (110) lattice plane of β-NaYF4. This is a direct evidence for glass crystallization.Moreover, STEM-HAADF was also performed to examine the element mapping in GC sample. As presented in Fig. 2, elements of Na, Y, F could be found in nanocrystals with introduction of Yb and Er, while Si, Al maintain in the glass matrix. Impressively, Na and F exist in both the nanocrystals and glass. In Fig. 3, EDS spectrum of GC sample also confirms the existence of all above elements. All these results evidence the successful precipitation of β-NaYF4 nanocrystals from aluminosilicate glass and the partition of dopants into β-NaYF4 nanocrystals.
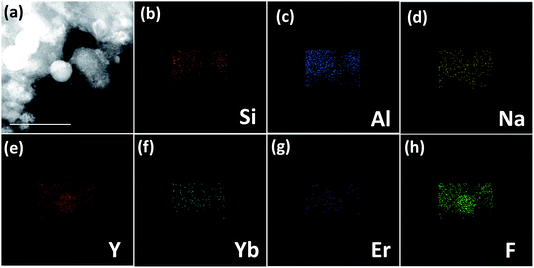 | ||
| Fig. 2 (a) STEM-HAADF images of GC sample with (b) Si, (c) Al, (d) O, (e) Y, (f) Yb, (g) Er and (h) F elemental mapping. | ||
As is well known, Eu3+ can serve as a super-sensitive probe to explore the local field it occupies, due to its magnetic dipole transition of 5D0 → 7F1 and electric dipole transition of 5D0 → 7F2.32 Therefore, Eu3+ is introduced into the GC sample to examine the glass crystallization and partition of dopants, as presented in Fig. 4. Notably, the emission intensities are strongly enhanced after glass crystallization. The ratios of the transitions of 5D0 → 7F1 to 5D0 → 7F2 are evaluated to be 2.3 for PG and 1.1 for GC, respectively. In addition, the lifetime of Eu3+ in GC sample is also lengthened compared to that of PG. Although the PG sample has self-crystallized, the proportion of ordered nanocrystals to disordered glass is still small, remaining residual Eu3+ in the glass. After glass crystallization, the percentage of β-NaYF4 nanocrystals has gone up, hence, a majority of Eu3+ ions are concentrated in the β-NaYF4 nanocrystals, resulting in an ordered local surrounding. These results provide a compelling evidence for incorporation of dopants into β-NaYF4 nanocrystals.
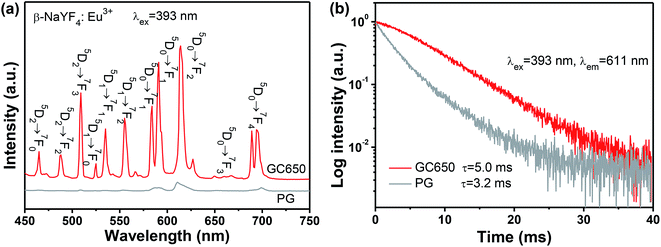 | ||
| Fig. 4 (a) The emission spectra and (b) the decay curves of GC and PG samples with introduction of Eu3+ as a structural probe, under excitation upon 393 nm. | ||
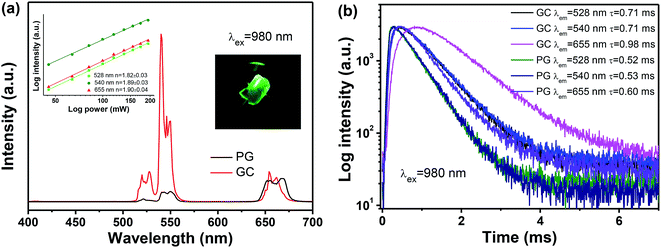
Under 980 nm laser irradiation, both PG and GC samples exhibit distinct green and red UC emissions of Er3+, which are centred at 520 nm (4S3/2 → 4I15/2), 545 nm (2H11/2 → 4I15/2) and 659 nm (4F9/2 → 4I15/2), respectively. In spite of self-crystallized PG in melt-quenching process, Er3+ in the PG sample still exhibit inhomogeneously broadened emissions, which is attributed to small amounts of β-NaYF4 crystals and large amounts of residual dopants in the glass matrix. After glass crystallization, the emission bands of GC are quite enhanced and narrowed, compared to that of PG. Notably, obvious Stark spitting can be also observed for GC sample. All these spectroscopic features indicate that Yb3+ and Er3+ ions are partitioned into β-NaYF4 nanocrystal embedded glass ceramics. In particular, the green UC emission dominates for GC sample, as evidenced by the inserted photograph in Fig. 5(a), suggesting a huge superiority for TECLs-based optical thermometry of Er3+ among various luminescent materials. Furthermore, Fig. 5(b) plots the decay curves of Er3+ monitoring at 545 nm and 659 nm. The fitting lifetime of Er3+ in GC are demonstrated to be longer than that in PG, which is also a favourable signal for partition of Er3+ into β-NaYF4 nanocrystals.
In order to investigate the UC mechanism, the UC emission intensities related to the pump power were measured. It is noteworthy that the relationship between UC emission intensity and the excitation power could be described as the following relation:
| I ∝ Pn | (1) |
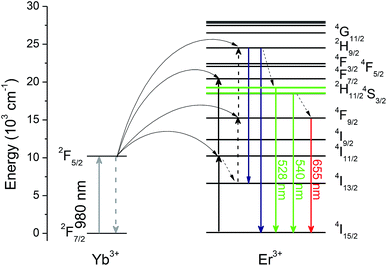
Fig. 6 illustrates the energy levels diagram of Yb3+ and Er3+, as well as possible energy transfer routes for UC. Upon excitation on 980 nm, Yb3+ ions act as sensitizers, whose role is to transfer their absorbed energy from excitation source to other activators. In Yb–Er system, Er3+ is a typical activator. Two-step energy transfer processes from Yb3+ to Er3+ occur: 2F5/2 + 4I15/2 → 2F7/2 + 4I11/2 and 2F5/2 + 4I11/2 → 2F7/2 + 4F7/2, and then relax to the 2H11/2 and 4S3/2 emitting states by non-radiative relaxation, producing green UC emission. On the contrary, Er3+ populated to 4I11/2 state can also relax to the 4I13/2 state firstly, and populated to the 4F9/2 emitting state through another energy transfer process 2F5/2 + 4I11/2 → 2F7/2 + 4F9/2, producing red UC emission. It is noteworthy that green and red UC emissions of Er3+ originate two-photon process, which is consistent with the power dependence in inset in Fig. 5(a).
As mentioned above, luminescent centre is considerably responsible for its performance as a temperature sensor. For Er3+ ions, the dominating UC emission bands centred at 520 nm and 540 nm are assigned to the transitions from the emitting 2H11/2 and 4S3/2 states, whose energy gap reaches 700–800 cm−1 approximately, to the ground 4I15/2 state. Undoubtedly, it is quite suitable for TECLs FIR-based optical thermometry. When it works, the relative population of the 2H11/2 and 4S3/2 energy levels should obey the Boltzmann distribution after reaching a fairly rapid thermal equilibrium, whose ratio corresponds to temperature uniquely. The temperature could be achieved by the ratio. The value of temperature-dependent FIR can be presented as follows:
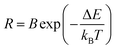 | (2) |
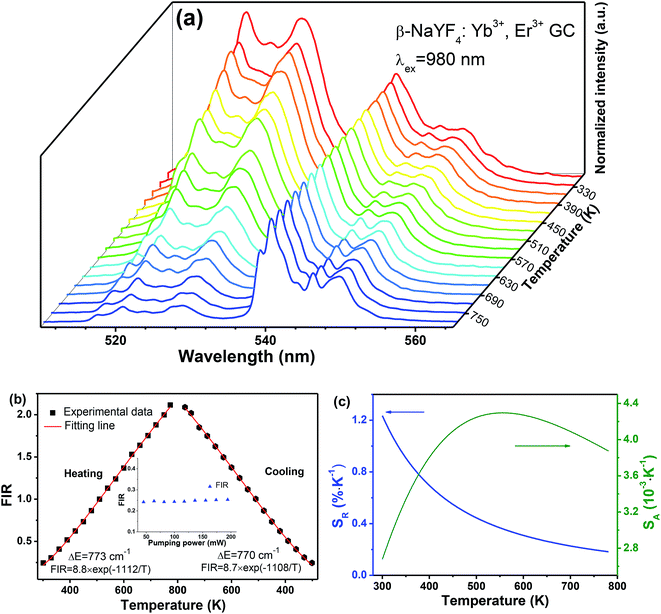
As a consequence, the temperature-dependence UC behaviour was investigated systematically in the range of 300–773 K to conduct its FIR-based temperature evaluation performance, as depicted in Fig. 7. Notably, it is of considerably significance to select suitable pumping power, in order to avoid the heating effect from excitation source. As presented in the inset of Fig. 7(b), the values of FIR were obtained without any obvious fluctuation under different pumping power ranging from 45 mW to 200 mW. In the case of ensuring the good signal-to-noise ratio of the temperature-dependent UC emission spectra, pumping power of 63.9 mW was finally chose as the excitation source for optical thermometry. For clarity, the intensities of the 4S3/2 → 4I15/2 transition In Fig. 7(a) are normalized. It can be obviously found that the values of FIR are gradually enhanced, as temperature increasing. Considering its Boltzmann's distributed thermal population, exponential function eqn (1) is employed to fit the experiment data exhibiting the relationship between FIR and temperature. As depicted in Fig. 7(b), the fitting energy gap between the 4S3/2 and 2H11/2 energy levels could be gained as 773 cm−1 during heating process and 770 cm−1 during cooling process. Impressively, they are almost the same, suggesting that the value of FIR just depends on temperature rather than measuring processes.
In general, sensitivity S would be employed to evaluate the temperature-dependent performance of a luminescent materials, including the absolute sensitivity SA and the relative sensitivity SR. In general, the absolute sensitivity SA represents the average change in corresponding temperature interval, while the relative sensitivity SR represents the slope of optical change with temperature. Compared to the absolute sensitivity SA, the relative sensitivity SR can be served as a more general form of evaluation on temperature behaviour. In FIR technique, the SA and SR could be given as eqn (3) and (4):
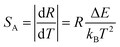 | (3) |
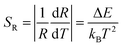 | (4) |
Fig. 7(c) depicts the temperature sensitivities curves, where the blue curve represents the relative sensitivity and the olive-green one represents the absolute sensitivity. Excitedly, the maximum of relative sensitivity is given as 1.24% K−1 at 300 K, which is a pretty good result among various FIR-based optical thermometry.33–36
As tabulated in Table 1, several typical systems for optical thermometry based TCELs of Er3+ were listed. It is not difficult to find that the GC is superior to the glass in terms of the relative sensitivity, owing to its crystal-like local field of Er3+. The narrowed and enhanced emission bands of GC make it more suitable for optical thermometry than glass. Interestingly, temperature evaluation could be achieved excellently in Yb3+–Er3+ codoped NaYF4 system, including α-NaYF4 GC,35 β-NaYF4 GC, β-NaYF4 phosphors36 or β-NaYF4 nanoparticles.37 Nevertheless, β-NaYF4 has proved to be more efficient than the α-NaYF4,31 on the other hand, GC exhibits more stable properties compared to the phosphors and nanoparticles due to its reliable glass network. As a consequence, Yb3+, Er3+ codoped β-NaYF4 GC is an outstanding alternative for optical thermometry. Notably, after several cyclic heating and cooling processes, Yb3+, Er3+ codoped β-NaYF4 nanocrystal embedded GC exhibit excellent repeatability, which is also a significant indicator for practical application in optical thermometry.
| Yb–Er system | Temperature range (K) | Energy gap (cm−1) | Relative sensitivity (% K−1) | Ref. |
|---|---|---|---|---|
| TeO2–WO3 glasses | 300–690 | 679 | 977/T2 | 33 |
| YF3–BaF2–Ba(PO3)2 glasses | 77–500 | 390 | 559/T2 | 34 |
| α-NaYF4 GC | 298–693 | 775 | 1117/T2 | 35 |
| β-NaYF4 phosphors | 160–320 | 752 | 1082/T2 | 36 |
| β-NaYF4 nanoparticles | 299–336 | 714 | 1028/T2 | 37 |
| β-NaYF4 GC | 300–773 | 773 | 1112/T2 | This work |
4. Conclusion
In summary, hexagonal NaYF4 nanocrystal embedded transparent glass ceramics, which is considered as one of the most optimal host materials for UC, were successfully fabricated via melt-quenching method with heat-treatment subsequently. Structural and spectrographic characterizations were performed to indicate the precipitation of β-NaYF4 nanocrystals and partition of dopants from glass matrix. The GC samples exhibit intense UC emission under the excitation of 980 nm, which is favourable for the performance of FIR technique based on the thermal couple levels. The ratio of UC emission originated from the transitions of 4S3/2 → 4I15/2 and 2H11/2 → 4I15/2 of Er3+ exhibited dramatic temperature dependence in the range of 300–773 K, while the maximum relative sensitivity was obtained as 1.24% K−1 at 300 K, suggesting that transparent glass ceramics containing β-NaYF4:Yb3+, Er3+ nanocrystals offer convincing facts in applications for optical thermometry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (51802064 and 51572065), Zhejiang Provincial Natural Science Foundation of China (LY18E020006 and LQ19E020008) and Scientific Research Foundation of Hangzhou Dianzi University (KYS205617012).References
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Adv. Opt. Mater., 2018, 6, 1701318 CrossRef.
- X. D. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- X. F. Wang, Q. Liu, Y. Y. Bu, C. S. Liu, T. Liu and X. H. Yan, RSC Adv., 2015, 5, 86219–86236 RSC.
- J. S. Zhong, D. Q. Chen, Y. Z. Peng, Y. D. Lu, X. Chen, X. Y. Li and Z. G. Ji, J. Alloys Compd., 2018, 763, 34–48 CrossRef CAS.
- L. H. Fischer, G. S. Harms and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 4546–4551 CrossRef CAS PubMed.
- E. J. McLaurin, V. A. Vlaskin and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 14978–14980 CrossRef CAS PubMed.
- Y. J. Cui, F. L. Zhu, B. L. Chen and G. D. Qian, Chem. Commun., 2015, 51, 7420–7431 RSC.
- S. S. Zhou, C. K. Duan, M. Yin, X. L. Liu, S. Han, S. B. Zhang and X. M. Li, Opt. Express, 2018, 26, 27339–27345 CrossRef PubMed.
- H. Kusama, J. S. Ojar and Y. Taisuke, Jpn. J. Appl. Phys., 1976, 15, 2345 CrossRef.
- X. Y. Li, G. C. Jiang, S. S. Zhou, X. T. Wei, Y. H. Chen, C. K. Duan and M. Yin, Sens. Actuators, B, 2014, 202, 1065–1069 CrossRef CAS.
- S. S. Zhou, X. Y. Li, X. T. Wei, C. K. Duan and M. Yin, Sens. Actuators, B, 2016, 231, 641–645 CrossRef CAS.
- B. M. Walsh and B. D. Bartolo, J. Lumin., 2015, 158, 265–267 CrossRef CAS.
- W. P. Chen, F. F. Hu, R. F. Wei, Q. G. Zeng and H. Guo, J. Lumin., 2017, 192, 303–309 CrossRef CAS.
- L. Li, X. H. Tang, Z. J. Wu, Y. F. Zheng, S. Jiang, X. Tang, G. T. Xiang and X. J. Zhou, J. Alloys Compd., 2019, 780, 266–275 CrossRef CAS.
- Y. X. Hao, S. C. Lv, Z. J. Ma and J. R. Qiu, RSC Adv., 2018, 8, 12165–12172 RSC.
- R. S. Yadav, D. Kumar, A. K. Singh, E. Raia and S. B. Rai, RSC Adv., 2018, 8, 34699–34711 RSC.
- J. K. Cao, X. M. Li, Z. X. Wang, Y. L. Wei, L. P. Chen and H. Guo, Sens. Actuators, B, 2016, 224, 507–513 CrossRef CAS.
- X. Y. Li, S. Yuan, F. F. Hu, S. Q. Lu, D. Q. Chen and M. Yin, Opt. Mater. Express, 2017, 7, 3023–3033 CrossRef CAS.
- Z. M. Cao, S. S. Zhou, G. C. Jiang, Y. H. Chen, C. K. Duan and M. Yin, Curr. Appl. Phys., 2014, 14, 1067–1071 CrossRef.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743–4756 CrossRef CAS.
- J. B. Zhao, X. L. Zheng, E. P. Schartner, P. Lonescu, R. Zhang, T. Nguyen, D. Y. Jin and H. Ebendorff-Heidepriem, Adv. Opt. Mater., 2016, 4, 1507–1517 CrossRef CAS.
- D. Q. Chen, Z. Y. Wan, Y. Zhou, X. Zhou, Y. Yu, J. S. Zhong, M. Y. Ding and Z. G. Ji, ACS Appl. Mater. Interfaces, 2015, 7, 19484–19493 CrossRef CAS PubMed.
- C. G. Lin, C. Bocker and C. Rüssel, Nano Lett., 2015, 15, 6764–6769 CrossRef CAS PubMed.
- A. Sarakovskis and G. Krieke, J. Eur. Ceram. Soc., 2015, 35, 3665–3671 CrossRef CAS.
- D. Q. Chen, Y. Zhou, Z. Y. Wan, H. Yu, H. W. Lu, Z. G. Ji and P. Huang, Phys. Chem. Chem. Phys., 2015, 17, 7100–7103 RSC.
- A. Herrmann, M. Tylkowski, C. Bocker and C. Rüssel, Chem. Mater., 2013, 25, 2878–2884 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- S. Fischer, N. D. Bronstein, J. K. Swabeck, E. M. Chan and A. P. Alivisatos, Nano Lett., 2016, 16, 7241 CrossRef CAS PubMed.
- Y. S. Liu, D. T. Tu, H. M. Zhu, R. F. Li, W. Q. Luo and X. Y. Chen, Adv. Mater., 2010, 22, 3266 CrossRef CAS PubMed.
- X. Y. Li, D. Q. Chen, F. Huang, G. C. Chang, J. J. Zhao, X. V. Qiao, X. H. Xu, J. C. Du and M. Yin, Laser Photonics Rev., 2018, 12, 1800030 CrossRef.
- X. Y. Li, X. Chen, S. Yuan, S. Liu, C. Wang and D. Q. Chen, J. Mater. Chem. C, 2017, 5, 10201–10210 RSC.
- A. Pandey, V. K. Rai, V. Kumar, V. Kumar and H. C. Swart, Sens. Actuators, B, 2015, 209, 352 CrossRef CAS.
- B. Y. Lai, L. Feng, J. Wang and Q. A. Su, Opt. Mater., 2010, 32, 1154–1160 CrossRef CAS.
- S. Jiang, P. Zeng, L. Q. Liao, S. F. Tian, H. Guo, Y. H. Chen, C. K. Duan and M. Yin, J. Alloys Compd., 2014, 617, 538–541 CrossRef CAS.
- S. S. Zhou, K. M. Deng, X. T. Wei, G. C. Jiang, C. K. Duan, Y. H. Chen and M. Yin, Opt. Commun., 2013, 291, 138–142 CrossRef CAS.
- F. Vetrone, R. Naccache, A. Zamarrón, A. J. de la Fuente, F. Sanz-Rodríguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Solé and J. A. Capobianco, ACS Nano, 2010, 4, 3254–3258 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |