Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multifunctional BiF3:Ln3+ (Ln = Ho, Er, Tm)/Yb3+ nanoparticles: an investigation on the emission color tuning, thermosensitivity, and bioimaging†
Xinxin Yana,
Tiesheng Li *a,
Linna Guo
*a,
Linna Guo a,
Honglei Lia,
Penglei Chen
a,
Honglei Lia,
Penglei Chen b and
Minghua Liu
b and
Minghua Liu b
b
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, The Key Lab of Chemical Biology and Organic Chemistry of Henan Province, The Key Lab of Nano-information Materials of Zhengzhou, Zhengzhou, 450001, P. R. China. E-mail: lts34@zzu.edu.cn
bBeijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China
First published on 8th April 2019
Abstract
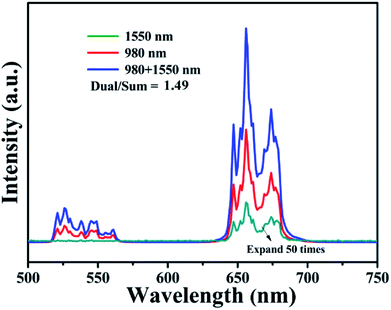
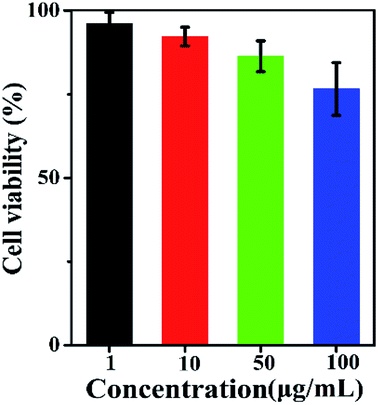
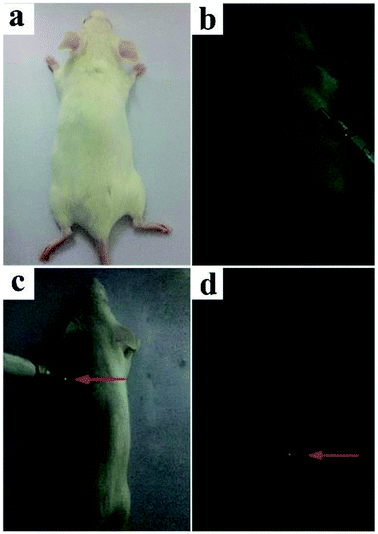
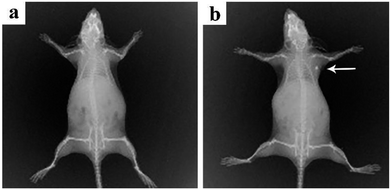
Pure cubic phase and uniform BiF3:Ln3+ (Ln = Ho, Er, Tm)/Yb3+ nanoparticles (NPs) were prepared by coprecipitation. The growth mechanism of BiF3:2%Er3+/20%Yb3+ NPs was proposed based on evolution analysis of the time-dependent morphology, in which BiF3:2%Er3+/20%Yb3+ was formed through the growth process of “nucleation to crystallization and Ostwald ripening”. The upconversion luminescence (UCL) properties and mechanism of BiF3:Ln3+ (Ln = Ho, Er, Tm)/Yb3+ under dual-wavelength excitation were also systematically investigated. The emission intensity of BiF3:2%Er3+/20%Yb3+ by dual-wavelength excitation (λ = 980 nm + 1550 nm) was 1.49 times more than that excited by 1550 nm or 980 nm individually. Furthermore, the properties of the bright white and multicolor UCL showed that yellow, purple, green, or pinkish light could be observed by controlling the doping concentration of Ln3+ (Ln = Yb, Er, Tm, and Ho), indicating that they had potential applications in backlight sources of color displays and security labeling. The temperature sensitivity of BiF3:2%Er3+/20%Yb3+ exhibited a downward tendency and its max value was about 0.0036 K−1 at 273 K. Cell toxicity tests showed that the UCNPs in phospholipid aqueous solution presented low cytotoxicity. Also, in vivo imaging and X-ray imaging revealed that the BiF3:2%Er3+/20%Yb3+ NPs had deep penetration and high contrast, which meant it could be used as a potential probe and contrast agent in in vivo optical bioimaging.
1. Introduction
Recently, UCNPs doped with lanthanides (Ln) have made considerable progress.1–7 Compared to organic dyes and conventional quantum dot materials, Ln3+-doped UCNPs are better in terms of chemical stability, toxicity, optical stability, luminous life, and emission peak, resulting in UCNPs having the advantages of weak auto-fluorescence, low radiation damage, and deep tissue penetration.8–16 Therefore, they have great potential in different fields.17 It is well known that UC emission can be accurately controlled through adjusting the concentrations of different activator ions (Er3+, Tm3+, Ho3+). Also, white and multicolor UCLNPs have attracted much attention due to their potential applications in backlight sources in color displays and security labeling.18,19It is well known that matrix selecting is an important factor to get desired UCL, in which various host materials, such as sulfide, fluorides, oxygenated compounds, can be used for UC emissions.20–22 Among these, fluorides are considered as excellent hosts because of their relatively lower cut-off phonon frequency and lower non-radiative relaxation.23–27 Zhang et al. reported the excellent matrix NaBiF4 without a rare earth ion fabricated by a super facile synthetic method, in which the reaction only took 1 min at room temperature.28 Yu et al. used the same method to synthesize NaBiF4:Yb3+/Er3+ NPs and BiF3:Yb3+/Er3+ NPs and investigated their UCL properties and temperature sensitivity.29,30 However, investigations on multicolor UCL, UCL excited by dual-wavelength, and the bio-imaging of BiF3:Yb3+/Er3+ have not been performed yet, but are very important for their application.5,18,31 Herein, this motivated us to further study these excellent matrix BiF3:Yb3+/Ln3+ (Ln = Ho, Er, Tm) NPs in a deeper investigation.
In this work, the cubic phase of BiF3:Ln3+ (Ln = Ho, Er, Tm)/Yb3+ UCNPs was designed and synthesized by the same facile strategy. Their UCL properties were investigated by excitation at 1550 nm and 980 nm, simultaneously. The growth mechanism and the effect of time on their morphology was also explored. The function of different doped concentrations of Er3+, Ho3+, and Tm3+ ions for the multicolor and white emission were also investigated. In vivo and X-ray imaging of the BiF3:2%Er3+/20%Yb3+ NPs were studied.
2. Experimental
2.1 Preparation
All reagents were purchased from Aladdin Chemical Reagent Factory (China) in this research and used without further treatment. The synthesis of the cubic BiF3:Ln3+/Yb3+ UCNPs proceeded as reported in ref. 30 (see ESI†).2.2 Characterization
Details on the instruments used are presented in the ESI.†2.3 In vitro cytotoxicity evaluation, and in vivo and X-ray imaging
The relevant evaluation32 of cytotoxicity in vitro and in vivo and X-ray imaging were presented in ESI.†3. Results and discussion
3.1 Characterization of the phase and morphology
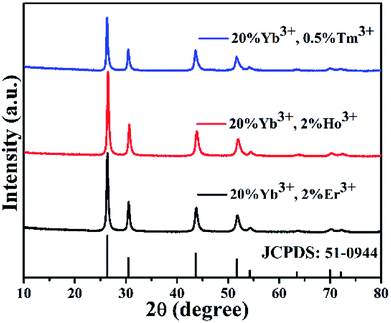
The crystal phases of BiF3:Ln3+/Yb3+ NPs were determined by XRD as shown in Fig. 1, in which they presented similar crystal cubic phases (JCPDS: 51-0944). The SEM image of BiF3:2%Er3+/20%Yb3+ NPs, as an example, exhibited regular NPs (Fig. 2a), which was confirmed by the TEM image (Fig. 2b). Fig. 2c shows that the d-spacing was 0.13 nm, which matched with the crystal plane (331) of the cubic phase BiF3 NPs. According to the results of element mapping (Fig. 2d–g), it was clear that the elements of Bi, Yb, Er, and F were uniform on the NPs. These elements were also confirmed by the EDX spectrum (Fig. 2h). BiF3:20%Yb3+,0.5%Tm3+/2% Ho3+ are presented in Fig. S1† and their SEM images are shown in Fig. S2,† which further illustrates the formation of BiF3:Ln3+(Ln = Ho, Er, Tm)/Yb3+ UCNPs.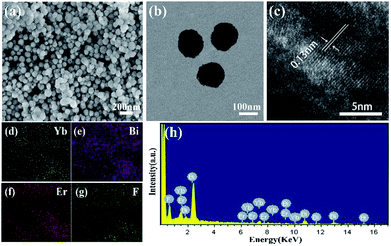 | ||
| Fig. 2 Characterization of BiF3:2%Er3+/20%Yb3+ NPs: (a) SEM image, (b) TEM image, (c) HRTEM image, (d–g) elemental mappings of Yb, Bi, Er, and F, (h) energy-dispersive X-ray (EDX) spectrum. | ||
The XPS of BiF3:2%Er3+/20%Yb3+ NPs was measured (Fig. S3a†). Two peaks centered at 159.9 eV and 165.1 eV were assigned to Bi 4f7/2 and Bi 4f5/2 (Fig. S3b†). The peaks located at 684.4, 165.3, and 186.1 eV were attributed to the BiF3:2%Er3+/20%Yb3+ NPs of Yb 4d, F 1s, and Er 4d, respectively (Fig. S3c–e†).
The stability of the BiF3:2%Er3+/20%Yb3+ NPs was investigated. The TG-DSC curve (see Fig. S4†) showed that the NPs had a mild loss of weight below 600 °C. The loss of water or crystalline water could be calculated at about 100 °C. When heated at 200 °C, the glycol on the surface of the sample gradually disappeared, and the surface defects of the sample gradually decreased. When the temperature reached up to 600 °C, the sample itself began to decompose and then the mass declined linearly.
To verify whether the reaction time affected the phase and morphology, XRD patterns and SEM/TEM images of BiF3:2%Er3+/20%Yb3+ NPs prepared at different reaction times were investigated (Fig. 3 and 4). The results showed that the diffraction peaks matched with BiF3 (JCPDS: 51-0944), indicating that the prepared compounds were single phase and had better crystallinity (Fig. 3). It can be seen from Fig. 3b that the diffraction peaks at 26.34° of Yb3+, Er3+-doped BiF3 had a slight shift compared to BiF3 (JCPDS: 51-0944). The reason should be that the ionic radii of both Er3+ (1.004 Å) and Yb3+ (0.985 Å) are smaller than that of Bi3+ (1.17 Å). In the BiF3 host lattice, the ions (Yb3+, Er3+) could enter the BiF3 crystal site through substituting for the Bi3+ ions or in the interstitial sites or could coexist in these two ways. Different doping methods could make the XRD peaks of the samples prepared at different time shift by different degrees. This indicated that Yb3+ and Er3+ had been doped into the BiF3 matrix.33 The XRD of BiF3:20%Yb3+,0.5%Tm3+/2%Ho3+ showed similar results, as shown in Fig. S5.†
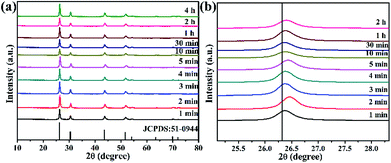 | ||
| Fig. 3 (a) XRD patterns of BiF3:2%Er3+/20%Yb3+ NPs obtained at different reaction times, (b) shifts of the main diffraction peaks of BiF3:2%Er3+/20%Yb3+ NPs. | ||
 | ||
| Fig. 4 SEM and TEM images of BiF3:2%Er3+/20%Yb3+ NPs obtained at different reaction times: (a) 2 min, (b) 3 min, (c) 4 min, (d) 5 min, (e) 10 min, (f) 30 min, (g) 1 h, (h) 2 h, and (i) 4 h. | ||
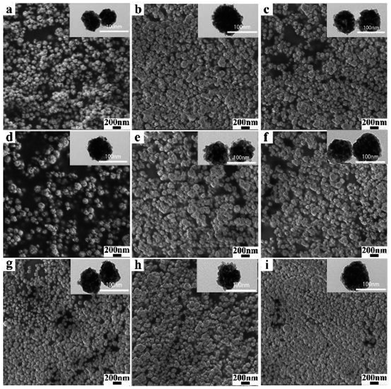
The SEM and TEM images of BiF3:2%Er3+/20%Yb3+ obtained at different reaction times are presented in Fig. 4. It can be clearly observed that there are a large number of well-dispersed NPs with similar morphologies, indicating that the morphologies were almost unchanged even as the time was prolonged, which was confirmed by the TEM images (insets of Fig. 4). In addition, BiF3:20%Yb3+,2%Ho3+/0.5%Tm3+ in Fig. S6 and S7† showed similar results.
In order to better understand the formation process of BiF3:2%Er3+/20%Yb3+ within 1 min, the growth mechanism was carefully studied by XRD and TEM at different reaction times (5, 30, 50, and 60 s), as shown in Fig. S8† and 5. Some impurity peaks emerged compared with the standard peaks in 5 s (Fig. S8†) and some small particles appeared in the TEM (Fig. 5a), indicating that the crystal nucleus was completed quickly. As the reaction time was further increased, the impurity peaks gradually disappeared (Fig. S8†) and the basic BiF3 spheroids gradually further grew or aggregated (see Fig. 5b and c). These could be deemed as indicating the ripening process of BiF3 NPs, meaning that larger spherical NPs were gradually formed due to the thermodynamic minimization of the surface energies of smaller particles, which is commonly known as Ostwald ripening.34 BiF3:2%Er3+/20%Yb3+ with regular morphologies were formed and the whole system achieved equilibrium when the time was 1 min (Fig. 5d). Meanwhile, XRD patterns were well matched with the pure cubic phase of BiF3 (Fig. S8†). Based on the morphology evolution, the possible growth mechanism is proposed as follows (Scheme 1). First, the precursor was converted to BiF3:2%Er3+/20%Yb3+ nuclei in the nucleation stage. Subsequently, the nuclei grew to rudimental NPs and then underwent the Ostwald ripening process until well-crystallized BiF3 NPs were formed, therefore the process could be described as “Nucleation → Crystallization → Ostwald ripening” (Scheme 1).
 | ||
| Fig. 5 TEM images of BiF3:2%Er3+/20%Yb3+ UCNPs obtained at different reaction times: (a) 5 s, (b) 30 s, (c) 50 s, and (d) 1 min. | ||
The influence of the temperature on the phase and morphology of BiF3:2%Er3+/20%Yb3+ were also investigated by XRD, SEM, and TEM (Fig. S9† and 6). It could be seen that the XRD spectra of NPs obtained at different temperatures (−25, 0, 30, 50, and 70 °C) matched well with the standard cubic phase (Fig. S9†). No impurities peaks were found in these patterns, indicating these samples had higher purity. However, a few new diffraction peaks appeared at 100 °C, indicating that the pure cubic phase of BiF3:2%Er3+/20%Yb3+ NPs tended to be formed at a lower temperature. Meanwhile, SEM images of BiF3:2%Er3+/20%Yb3+ NPs synthesized at different temperatures, sizes, and morphologies also showed no obvious changes, except that they became irregular at 100 °C (Fig. S9† and 6f). The corresponding TEM images are shown in Fig. 6g–l, in which the results were in good agreement with the XRD results. From the above analysis, it could be concluded that the regular BiF3:2%Er3+/20%Yb3+ NPs could be formed at lower temperature under co-precipitation conditions.
 | ||
| Fig. 6 SEM and TEM images of BiF3:20%Yb3+/2%Er3+ NPs obtained at different temperatures: (a and g) −25 °C, (b and h) 0 °C, (c and i) 30 °C, (d and j) 50 °C, (e and k) 70 °C, and (f and l) 100 °C. | ||
3.2 UCL of BiF3:Ln3+(Ln = Ho, Er, Tm)/Yb3+
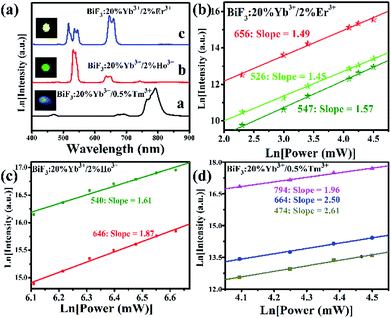 | ||
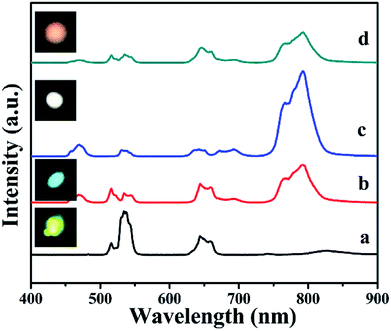
| Fig. 8 (a) UC emission spectra and corresponding luminescence photographs, (b–d) pump power dependence of blue, green, red, and NIR UCL emission intensities in BiF3:Ln3+ NPs excited at 980 nm. | ||
The possible UCL mechanism of Yb3+, Er3+, Ho3+, and Tm3+ ions are shown in Fig. S13.† For BiF3:2%Er3+/20%Yb3+ NPs, the Yb3+ ion absorbs a photon and is excited to 2F5/2, then drops back to the ground state and transfers the energy to a nearby Er3+ ion to populate the 4I11/2. The Yb3+ can then transfer the second photon to populate the 4F7/2 and relax to 2H11/2 or 4S3/2 levels non-radiatively. Through non-radiative relaxation, the 4I11/2 level can also relax to the 4I13/2 level and after that a transferred photon from Yb3+ ions can populate the 4F9/2. Red emission is transmitted through 4F9/2 → 4I15/2 of Er3+, as shown in Fig. S13a.† The red and blue emissions of BiF3:20%Yb3+/0.5%Tm3+ NPs could be confirmed as a three-photon process, as shown in Fig. S13b.† The 1G4 level could be populated by a three-step energy transfer of Yb3+ through 3H4 and 3F4. From the transitions of 1G4 → 3F4, 3H4 → 3H6 and 1G4 → 3H6, red, NIR, and blue emissions of Tm3+ could be obtained, respectively. As for BiF3:20%Yb3+/2%Ho3+ NPs, red emission through the bridge of the 5I7 level was pumped to the 5F5 level and the green emission was then a two-photon process (Fig. S13b†).18
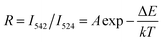 | (1) |
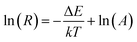 | (2) |
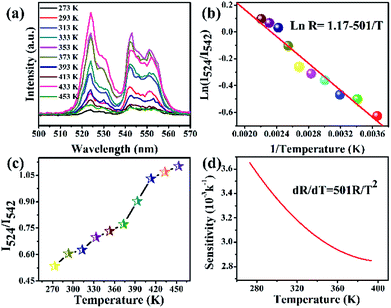
Fig. 11a presents the UC emission spectra of BiF3:2%Er3+/20%Yb3+ excited at 980 nm with various temperatures. For green (524 nm and 542 nm) emissions, the UC emission intensities changed with increasing temperature. Fig. 11b presents a plot of 1/T versus ln(R), in which the slope (−ΔE/k) is equal to −501 and the intercept is 1.17. It could be also seen from Fig. 11c that R increased with the increasing temperature and presented a certain rule. In addition, the sensor sensitivity is another very important factor. The sensor sensitivity (S) can be calculated by using the following formula:45–48
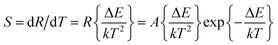 | (3) |
It was noteworthy that the sensitivity exhibited a downward tendency with the increasing temperature from 273 K to 453 K and its max value was about 0.0036 K−1 at 273 K (Fig. 11d), suggesting that the BiF3:2%Er3+/20%Yb3+ NPs had a certain thermometric property, which might be employed as a temperature sensor.
4. Conclusions
In summary, multi-functional BiF3 UCNPs were successfully synthesized by a facile co-precipitation method, both below and above room temperature (−25 °C, 100 °C). The growth mechanism indicated that the obtained BiF3 NPs were formed through the growth process of “Nucleation → Crystallization → Ostwald ripening”. Fluorescence intensity ratio technology was used to investigate the temperature sensitivity of BiF3:2%Er3+/20%Yb3+ and the max sensitivity was found to be 0.0036 K−1 at 273 K. The integrated emission of BiF3:2%Er3+/20%Yb3+ NPs excited by dual-wavelength simultaneously was 1.49 times more than that excited by two single wavelengths (1550 nm and 980 nm), respectively. Multicolor and bright white light could be obtained upon excitation at 980 nm by modulating the content of Ho3+, Er3+, and Tm3+, by which they might be used as security labeling and backlight sources for color displays. The low cytotoxicity of BiF3:2%Er3+/20%Yb3+ was confirmed by cell toxicity tests with phospholipid aqueous solution. BiF3:2%Er3+/20%Yb3+ NPs presented better properties of penetration and contrast in the in vivo imaging and X-ray imaging tests, indicating that they could be used as potential probe and contrast agents in in vivo optical bioimaging.Live subject statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhengzhou University and experiments were approved by the Animal Ethics Committee of Henan Laboratory Animal Center.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Chinese Academy of Sciences (Grants XDA09030203), the NSFH (142300410223) for their financial support.References
- Z. W. Wei, L. N. Sun, J. L. Liu, J. Z. Zhang, H. R. Yang, Y. Yang and L. Y. Shi, Biomaterials, 2014, 35, 387–392 CrossRef CAS PubMed.
- L. Y. Zeng, Y. W. Pan, R. F. Zou, J. C. Zhang, Y. Tian, Z. G. Teng, S. J. Wang, W. Z. Ren, X. S. Xiao, J. C. Zhang, L. L. Zhang, A. G. Li, G. M. Lu and A. G. Wu, Biomaterials, 2016, 103, 116–127 CrossRef CAS PubMed.
- Z. M. Deng, X. L. Li, Z. L. Xue, M. Y. Jiang, Y. B. Li, S. J. Zeng and H. R. Liu, Nanoscale, 2018, 10, 9393–9400 RSC.
- Z. L. Xue, Z. G. Yi, X. L. Li, Y. B. Li, M. Y. Jiang, H. R. Liu and S. J. Zeng, Biomaterials, 2017, 115, 90–103 CrossRef CAS PubMed.
- S. J. Zeng, Z. G. Yi, W. Lu, C. Qian, H. B. Wang, L. Rao, T. M. Zeng, H. R. Liu, H. J. Liu, B. Fei and J. H. Hao, Adv. Funct. Mater., 2014, 24, 4051–4059 CrossRef CAS.
- X. L. Li, Z. G. Yi, Z. L. Xue, S. J. Zeng and H. R. Liu, Mater. Sci. Eng., C, 2017, 75, 510–516 CrossRef CAS PubMed.
- J. Zhou, Z. G. Lu, G. G. Shan, S. H. Wang and Y. Liao, Biomaterials, 2014, 35, 368–377 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- P. Huang, W. Zheng, S. Y. Zhou, D. T. Tu, Z. Chen, H. M. Zhu, R. F. Li, E. Ma, M. D. Huang and X. Y. Chen, Angew. Chem., Int. Ed., 2014, 53, 1252–1257 CrossRef CAS PubMed.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834–3838 CrossRef CAS PubMed.
- H. H. Gorris and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 3584–3600 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Y. Li, Chem. Rev., 2014, 115, 395–465 CrossRef PubMed.
- G. F. Wang, Q. Peng and Y. D. Li, J. Am. Chem. Soc., 2009, 131, 14200–14201 CrossRef CAS PubMed.
- P. P. Lei, R. An, X. S. Zhai, S. Yao, L. L. Dong, X. Xu, K. M. Du, M. L. Zhang, J. Feng and H. J. Zhang, J. Mater. Chem. C, 2012, 18, 1580 Search PubMed.
- G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- Z. X. Zhang, R. F. Cao, L. N. Guo, P. Li, C. X. Liang and T. S. Li, New J. Chem., 2018, 42, 15215–15220 RSC.
- E. M. Chan, Chem. Soc. Rev., 2015, 44, 1653–1679 RSC.
- Z. Wang, J. Feng, M. Pang, S. h. Pan and H. J. Zhang, Dalton Trans., 2013, 42, 12101–12108 RSC.
- N. S. Singh, N. K. Sahu and D. Bahadur, J. Mater. Chem. C, 2014, 2, 548–555 RSC.
- X. Lu, M. Yang, L. Y. Yang, Q. L. Ma, X. T. Dong and J. Tian, J. Mater. Sci.: Mater. Med., 2015, 26, 4078–4084 CAS.
- Z. Yin, H. Li, W. Xu, S. B. Cui, D. L. Zhou, X. Chen, Y. S. Zhu, G. S. Qin and H. W. Song, Adv. Mater., 2016, 28, 2518–2525 CrossRef CAS PubMed.
- P. P. Lei, P. Zhang, Q. H. Yuan, Z. Wang, L. L. Dong, S. Y. Song, X. Xu, X. L. Liu, J. Feng and H. J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26346–26354 CrossRef CAS PubMed.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426–6436 CrossRef CAS PubMed.
- X. Teng, Y. H. Zhu, W. Wei, S. C. Wang, J. F. Huang, R. Naccache, W. B. Hu, A. L. Y. Tok, Y. Han, Q. C. Zhang, Q. L. Fan, W. Huang, J. A. Capobianco and L. Huang, J. Am. Chem. Soc., 2012, 134, 8340–8343 CrossRef CAS PubMed.
- Q. Liu, Y. Sun, T. S. Yang, W. Feng, C. G. Li and F. Y. Li, J. Am. Chem. Soc., 2011, 133, 17122–17125 CrossRef CAS PubMed.
- S. Sarkar, A. Dash and V. Mahalingam, Chem.–Asian J., 2014, 9, 447–451 CrossRef CAS PubMed.
- X. L. Wu, L. G. Xu, W. Ma, L. Q. Liu, H. Kuang, N. A. Kotov and C. L. Xu, Adv. Mater., 2016, 28, 5907–5915 CrossRef CAS PubMed.
- P. P. Lei, R. An, S. Yao, Q. S. Wang, L. L. Dong, X. Xu, K. M. Du, J. Feng and H. J. Zhang, Adv. Mater., 2017, 29, 1700505 CrossRef PubMed.
- P. Du, L. H. Luo, X. Y. Huang and J. S. Yu, J. Colloid Interface Sci., 2018, 514, 172–181 CrossRef CAS PubMed.
- P. Du and J. S. Yu, Microchim. Acta, 2018, 185, 237 CrossRef PubMed.
- P. Li, L. N. Guo, Z. X. Zhang, T. S. Li and P. L. Chen, Dyes Pigm., 2018, 154, 242–251 CrossRef CAS.
- B. H. Wang, Y. X. Zhai, J. J. Shi, L. Y. Zhuang, W. Liu, H. J. Zhang, H. L. Zhang and Z. Z. Zhang, J. Controlled Release, 2017, 268, 225–236 CrossRef CAS PubMed.
- P. Li, L. N. Guo, C. X. Liang, T. S. Li and P. L. Chen, Mater. Sci. Eng., B, 2018, 229, 20–26 CrossRef CAS.
- A. M. Li, D. K. Xu, H. Lin, S. H. Yang, Y. Z. Shao and Y. L. Zhang, Sci. Rep., 2016, 6, 31366 CrossRef CAS PubMed.
- L. N. Guo, Y. H. Wang, Y. Z. Wang, J. Zhang, P. Y. Dong and W. Zeng, Nanoscale, 2013, 5, 2491–2504 RSC.
- C. X. Liang, L. N. Guo, P. Li, T. S. Li, P. L. Chen, M. H. Liu and Y. J. Wu, J. Alloys Compd., 2017, 715, 37–42 CrossRef CAS.
- P. Chen, S. L. Yu, B. B. Xu, J. C. Wang, X. W. Sang, X. F. Liu and J. R. Qiu, Mater. Lett., 2014, 128, 299–302 CrossRef CAS.
- J. J. Zhou, J. Y. Deng, H. M. Zhu, X. Y. Chen, Y. Teng, H. Jia, S. Q. Xu and J. R. Qiu, J. Mater. Chem. C, 2013, 1, 8023–8027 RSC.
- Z. Chen, G. B. Wu, H. Jia, K. Sharafudeen, W. B. Dai, X. W. Zhang, S. F. Zeng, J. M. Liu, R. F. Wei, S. C. Lv, G. P. Dong and J. R. Qiu, J. Phys. Chem. C, 2015, 119, 24056–24061 CrossRef CAS.
- Z. Chen, X. W. Zhang, S. F. Zeng, Z. J. Liu, Z. J. Ma, G. P. Dong, S. F. Zhou, X. F. Liu and J. R. Qiu, Appl. Phys. Express, 2015, 8, 032301 CrossRef CAS.
- Y. H. Yao, C. Xu, Y. Zheng, C. S. Yang, P. Liu, J. X. Ding, T. Q. Jia, J. R. Qiu, S. A. Zhang and Z. R. Sun, RSC Adv., 2016, 6, 3440–3445 RSC.
- D. He, C. F. Guo, S. Jiang, N. M. Zhang, C. K. Duan, M. Yin and T. Li, RSC Adv., 2015, 5, 1385–1390 RSC.
- C. D. S. Brites, X. J. Xie, M. L. Debasu, X. Qin, R. F. Chen, W. Huang, J. Rocha, X. G. Liu and L. D. Carlos, Nat. Nanotechnol., 2016, 11, 851 CrossRef CAS PubMed.
- G. Y. Zhang, Q. P. Qiang, S. S. Du and Y. H. Wang, RSC Adv., 2018, 8, 9512–9518 RSC.
- W. Yu, W. Xu, H. W. Song and S. Zhang, Dalton Trans., 2014, 43, 6139–6147 RSC.
- J. Zhang, B. W. Ji, G. B. Chen and Z. H. Hua, Inorg. Chem., 2018, 57, 5038–5047 CrossRef CAS PubMed.
- J. Zhang, X. M. Jiang and Z. H. Hua, Ind. Eng. Chem. Res., 2018, 57, 7507–7515 CrossRef CAS.
- P. Li, L. N. Guo, C. X. Liang, T. S. Li, P. L. Chen, M. H. Liu and Y. J. Wu, RSC Adv., 2017, 7, 51233–51244 RSC.
- D. M. Yang, Y. L. Dai, J. H. Liu, Y. Zhou, Y. Y. Chen, C. X. Li, P. A. Ma and J. Lin, Biomaterials, 2014, 35, 2011–2023 CrossRef CAS PubMed.
- P. Ramasamy, P. Chandra, S. W. Rhee and J. Kim, Nanoscale, 2013, 5, 8711–8717 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01018a |
| This journal is © The Royal Society of Chemistry 2019 |