Open Access Article
Open Access ArticleSynthesis of discrete catalytic oligomers and their potential in silica-supported cooperative catalysis†
Prakash Chandraa,
Alain M. Jonas a and
Antony E. Fernandes
a and
Antony E. Fernandes *ab
*ab
aInstitute of Condensed Matter and Nanosciences, Université catholique de Louvain, 1348 Louvain-la-Neuve, Belgium
bCertech, Rue Jules Bordet 7180 Seneffe, Belgium. E-mail: antony.fernandes@certech.be
First published on 7th May 2019
Abstract
Cooperative catalysis on solid surfaces relies primarily on two or more catalytic partners being close enough to each other to sustain the catalytic cycle. We describe here the synthesis and preliminary investigation of discrete homo-oligomers as flexible scaffolds to inflect the intersite distance and blur the compositional heterogeneity in a silica-grafted catalytic triad.
Supported multifunctional catalysts provide significant advantages over their parent homogeneous systems for more sustainable and efficient approaches in cooperative and cascade catalysis.1–12 However, although it is now becoming possible to combine most activation modes on a single support and compete with the original molecular catalysts, controlling the surface distribution of the different functional groups remains challenging.13–18 As exemplified by biological catalysts, the perfect spatial preorganization of catalytic centers within a controlled and confined environment is central to enable optimal synergistic interactions. Hence, the current limitation of grafting strategies to random, entropy-controlled systems is an important issue that prevents the fully rational design of active multifunctional surfaces.
The utilization of mixed monolayers for the preparation of silica-supported multifunctional catalysts intrinsically leads to a statistical distribution of the active components on surface.19 This eventually limits the potential of these systems since it creates a statistical distribution of pairings and spacings between the functional groups, with some catalytic partners being found at unmatched stoichiometry and distance within the mixed monolayer. Hence, it becomes important to develop methods to optimize the spatial positioning of functional groups in supported multifunctional catalysts to overcome the limitations associated with random functionalization. So far, only a few strategies have been proposed to reach a more homogeneous spatial distribution of multiple functional groups on surface. Molecular imprinting or imprint coating methods have allowed to better control the relative arrangement of surface-bound functional groups.20–25 For instance, Davis demonstrated that paired thiol/sulfonic acid obtained by molecular imprinting on silica surpassed the same randomly distributed cooperative catalytic system.23 In another way, the complementary functional groups can be assembled on a single molecular scaffold prior to immobilization.26 In this direction, we recently reported the use of short sequence-defined oligomers for the preorganization of the (pyta)Cu/TEMPO/imidazole (pyta = pyridyltriazole, TEMPO = 2,2,6,6-tetramethylpiperidine-N-oxyl) catalytic triad on mesoporous silica and their superior activity in the aerobic oxidation of alcohols compared to a mixed monolayer of the triad.27,28
The homogeneous (bpy)Cu/TEMPO/NMI catalyst system (bpy = bipyridine, NMI = N-methyl-imidazole) developed by Stahl and co-workers,29–32 provides a practical, efficient and environmentally benign alternative compared to common methods based on stoichiometric and toxic oxidizing reagents for the transformation of alcohols to aldehydes and ketones.33 The mechanism involves the formation of a (bpy)CuI(NMI) complex that is then oxidized by molecular oxygen to provide the active oxidizing CuII species. The role of TEMPO is still unclear as whether it interacts directly or not with the Cu center;34–36 however, the formation of the (bpy)CuI(NMI) complex remains central in the catalytic cycle. Hence, in our approach, it is important to promote interactions between the supported Cu and imidazole sites while the TEMPO is located in close vicinity of the Cu complex to enable high turnovers. Here, we present another approach to minimize the effect of local compositional heterogeneity in randomly-mixed monolayers, based on the use of multivalent flexible short chains which dynamically blur the local composition of the grafted layer, and thereby achieve a statistically more efficient pairing between functional units.
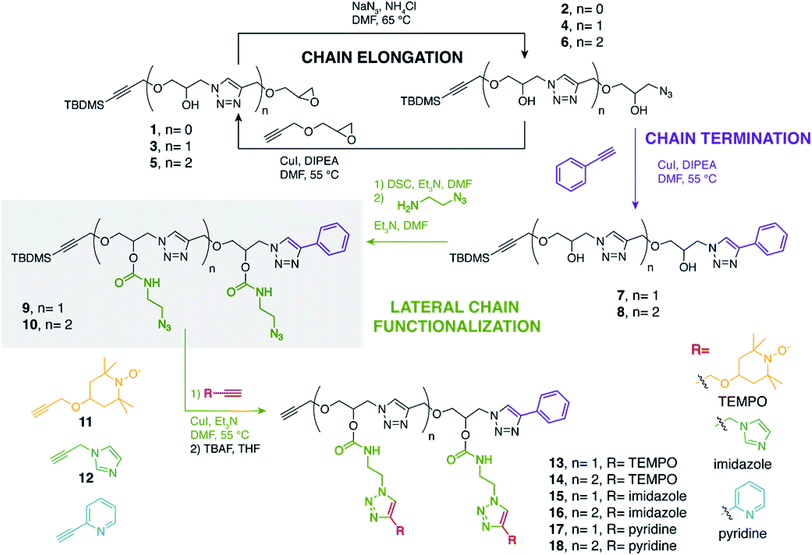
In that aim, we first describe the simple iterative synthesis of azide-functionalized monodisperse oligomers as versatile platforms for the anchoring of the three catalytic units (pyta)Cu, TEMPO and imidazole. After grafting a stoichiometric mixture of the oligomers on mesoporous silica, we find that the catalytic activity per unit amount of catalytic center is strongly enhanced for longer oligomeric chains. This is ascribed to the larger volume explored by longer chains, resulting in a more efficient coupling between the three elements of the catalytic triad despite the grafting heterogeneity of the layers. The functional homo-dimers 13, 15 and 17 and homo-trimers 14, 16 and 18 bearing pendant TEMPO, imidazole and pyta active centers, respectively, share the identical dimeric and trimeric precursors 9 and 10, respectively (Scheme 1). The oligomeric platforms 9 and 10 were straightforwardly synthesized from racemic propargyl glycidyl ether 1 following an iterative epoxide opening/CuAAC (copper-catalyzed azide–alkyne cycloaddition) sequence.27,37,38 Precisely, the opening of the oxirane ring in 1 (n = 0) with the azide anion releases a secondary hydroxyl function that would later serve for the installation of the catalytic units. The azide functionality in 2 subsequently allows the re-introduction of the epoxide motif by CuAAC reaction with propargyl glycidyl ether. Reiterating this elongation sequence allows to access monodisperse oligomers with lateral –OH handles.
The “living” oligomers 4 and 6 were terminated by reaction of the end-chain azide with phenylacetylene under CuAAC conditions to afford dimer 7 and trimer 8, respectively. In order to install the catalytic components of the triad, the lateral hydroxyl groups were treated with N,N′-disuccinimidyl carbonate (DSC) followed by coupling with 2-azidoethylamine, to provide the dimeric (9) and trimeric (10) platforms with accessible azide groups. The later were reacted either with TEMPO 11, imidazole 12, or 2-ethynylpyridine under CuAAC conditions to provide the TBDMS-protected multivalent homo-oligomers that were ultimately treated with TBAF (tetra-n-butylammonium fluoride) to afford the alkyne-terminated oligomers 13–18.
The two-step post-polymerization modification strategy developed here for the installation of lateral functional groups mirrors previous approaches based on labile ester bounds.27,37 Precisely, the lateral carbamate bounds ensure here enhanced chemical stability compared to ester analogues that are partly or totally cleaved during the TBAF-mediated alkyne deprotection while the carbamate variants remain unaltered.
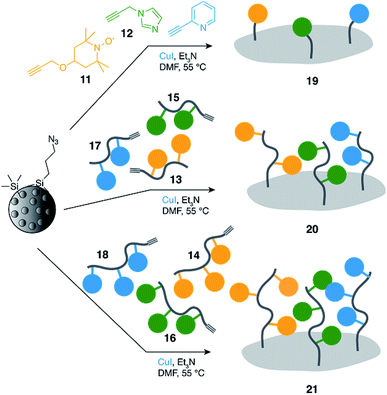
Trifunctional (pyta)Cu/TEMPO/imidazole supported catalysts of increasing valency were prepared using our typical CuAAC protocol by reacting stoichiometric mixtures of the corresponding alkyne-terminated catalytic groups with azide-functionalized mesoporous silica in presence of Cu(I) (Scheme 2); the Cu(I) used for the CuAAC grafting also serving for the realization of the (pyta)Cu sites of the supported catalysts.39,40
In order to further study the effect of the oligomeric scaffolds, the more traditional “monomeric” supported catalyst 19 was prepared from 11, 12 and 2-ethynylpyridine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and azide silica (0.29 ± 0.04 mmol azide per g) in presence of CuI and Et3N.
1 molar ratio) and azide silica (0.29 ± 0.04 mmol azide per g) in presence of CuI and Et3N.
Similarly, catalyst 20 was obtained by reacting a mixture of dimers 13, 15 and 17 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with azide silica; catalyst 21 was in turn prepared from a stoichiometric mixture of trimers 14, 16 and 18.
1 molar ratio with azide silica; catalyst 21 was in turn prepared from a stoichiometric mixture of trimers 14, 16 and 18.
The grafting reactions were monitored by thermogravimetric analysis (TGA) and FT-IR. TGA (ESI, Fig. S41a†) provided loadings of 0.27 ± 0.01, 0.15 ± 0.01 and 0.12 ± 0.01 mmol g−1 for 19, 20 and 21, respectively, which corresponds to grafting efficiencies of 89 ± 11, 53 ± 11 and 43 ± 9% with respect to the initial azide loading. The DTG curves (ESI, Fig. S41b†) also showed the persistence of thermal events corresponding to the azide layer for catalysts 20 and 21, also indicative of partial grafting. As can be expected, the grafting efficiency decreased with the length and bulkiness of the oligomers as steric hindrances enter into consideration for the surface CuAAC reaction. FT-IR confirmed this observation with the residual azide signal (2100 cm−1) being more prominent with the length of the immobilized oligomers, synonymous with incomplete CuAAC grafting (ESI, Fig. S42†). The apparition of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration band at 1730 cm−1 in the FT-IR spectra of 20 and 21 provided additional evidence for the covalent grafting of the multifunctional oligomers bearing carbamate bounds (Fig. S42†). The difference between the loadings of di- and trimeric chains is small, which results from their (unperturbed) radii of gyration being close (the theoretical ratio being (2/3)1/2 = 0.82). In contrast, the “monomeric” units are essentially rigid rods that can be grafted to much higher densities. The content of Cu for each silica catalysts was quantified by ICP-AES, giving Cu loadings of 0.08, 0.09 and 0.10 mmol g−1 for 19, 20 and 21, respectively. The experimental Cu contents fit well with the theoretical values calculated from the measured grafting density and the expected formation of a 1
O vibration band at 1730 cm−1 in the FT-IR spectra of 20 and 21 provided additional evidence for the covalent grafting of the multifunctional oligomers bearing carbamate bounds (Fig. S42†). The difference between the loadings of di- and trimeric chains is small, which results from their (unperturbed) radii of gyration being close (the theoretical ratio being (2/3)1/2 = 0.82). In contrast, the “monomeric” units are essentially rigid rods that can be grafted to much higher densities. The content of Cu for each silica catalysts was quantified by ICP-AES, giving Cu loadings of 0.08, 0.09 and 0.10 mmol g−1 for 19, 20 and 21, respectively. The experimental Cu contents fit well with the theoretical values calculated from the measured grafting density and the expected formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pyta/Cu complex (0.09, 0.10 and 0.12 mmol g−1 for 19, 20 and 21, respectively). Noticeably, owing to the reduction of grafting efficiencies with chain length, catalysts 19–21 have nearly identical Cu loadings. XPS analysis (ESI, Fig. S43†) of supported catalysts 19–21 confirmed the presence of Cu(I) species with Cu 2p1/2 and Cu 2p3/2 peaks at ca. 952.4 and 932.5 eV, respectively (Fig. S44†). In addition, analysis of the high-resolution XPS C 1s region showed an increase of the high-energy components at ca. 286.4 eV and the apparition of a peak of higher energy at 289.0 eV in catalysts 20 and 21 compared to 19, which are attributed to the extra electron deficient C–O and C
1 pyta/Cu complex (0.09, 0.10 and 0.12 mmol g−1 for 19, 20 and 21, respectively). Noticeably, owing to the reduction of grafting efficiencies with chain length, catalysts 19–21 have nearly identical Cu loadings. XPS analysis (ESI, Fig. S43†) of supported catalysts 19–21 confirmed the presence of Cu(I) species with Cu 2p1/2 and Cu 2p3/2 peaks at ca. 952.4 and 932.5 eV, respectively (Fig. S44†). In addition, analysis of the high-resolution XPS C 1s region showed an increase of the high-energy components at ca. 286.4 eV and the apparition of a peak of higher energy at 289.0 eV in catalysts 20 and 21 compared to 19, which are attributed to the extra electron deficient C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbons of the oligomers, respectively (ESI, Fig. S44†).
O carbons of the oligomers, respectively (ESI, Fig. S44†).
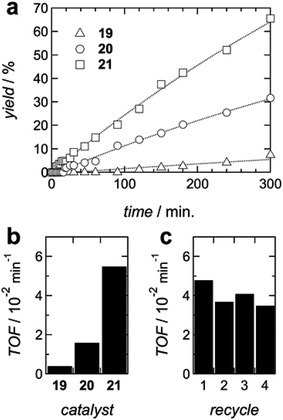
The supported catalysts were tested in the model aerobic oxidation of benzyl alcohol29,30 using a 5 mol% Cu loading (Fig. 1a). Catalyst 21, with the mixed homo-trimer monolayer, showed the highest activity with an initial turnover frequency (TOF) more than one order of magnitude superior to the more traditional mixed monolayer catalyst 19 (Fig. 1b).
Catalyst 20 showed itself a 4-fold increase in initial TOF compared to 19. Catalyst 21 could be easily recovered and reused for five consecutive runs with moderate erosion of its activity (Fig. 1c).
The remarkable increase of catalytic efficiency of the oligomeric chains can only be explained by the local mixing of the three catalytic units, which is provided by the flexibility of the chains combined with the larger volume of space statistically sampled by the random fluctuations of longer chains. In contrast, the rigid monomeric units do not permit an optimal pairing of the catalytic centers, resulting in a strongly depreciated TOF.
Conclusions
In conclusion, we described here the synthesis of short discrete oligomers using an iterative epoxide opening/CuAAC chemistry. Derivatization of the resulting lateral hydroxyl groups into azide handles provided unique platforms for the integration of catalytic units. Homo-dimers and -trimers bearing the (pyta)Cu, TEMPO and imidazole active centers were obtained and grafted on mesoporous silica. The supported oligomeric catalysts surpassed the activity of a more conventional mixed monomeric version of the triad, with the trimeric catalysts showing a 14-fold enhanced TOF in the model aerobic oxidation of benzyl alcohol.The utilization of oligomeric scaffolds opens opportunities in supported cooperative catalysis by affording ways to expand the probability of synergistic interactions within confined spaces. Further refining the tacticity of the chains and the relative arrangement of the pendant functional groups is under investigation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the European Regional Development Fund (ERDF) and Wallonia (Operational Program “Wallonia-2020.EU”), the Belgian Federal Science Policy (IAP P7/05) and the Fonds de la Recherche Scientifique – FNRS and the Fonds Wetenschappelijk Onderzoek under EOS project no. 30650939 for financial support. Anne Iserentant, Cécile D'Haese and François Devred are acknowledged for ICP-AES, XPS and physisorption measurements, respectively. Gabriella Barrozino-Consiglio is acknowledged for help with some NMR measurements.Notes and references
- J. M. Notestein and A. Katz, Chem.–Eur. J., 2006, 12, 3954–3965 CrossRef CAS PubMed.
- E. L. Margelefsky, R. K. Zeidan and M. E. Davis, Chem. Soc. Rev., 2008, 37, 1118–1126 RSC.
- F.-X. Felpin and E. Fouquet, ChemSusChem, 2008, 1, 718–724 CrossRef CAS.
- J.-K. Lee, M. Kung and H. Kung, Top. Catal., 2008, 49, 136–144 CrossRef CAS.
- S. Shylesh and W. R. Thiel, ChemCatChem, 2011, 3, 278–287 CrossRef CAS.
- C. Yu and J. He, Chem. Commun., 2012, 48, 4933–4940 RSC.
- U. Diaz, D. Brunel and A. Corma, Chem. Soc. Rev., 2013, 42, 4083–4097 RSC.
- N. A. Brunelli and C. W. Jones, J. Catal., 2013, 308, 60–72 CrossRef CAS.
- K. Motokura, ChemCatChem, 2014, 6, 3067–3068 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra and M. J. Sabater, ACS Catal., 2014, 4, 870–891 CrossRef CAS.
- D. Jagadeesan, Appl. Catal., A, 2016, 511, 59–77 CrossRef CAS.
- Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC.
- A. Puglisi, R. Annunziata, M. Benaglia, F. Cozzi, A. Gervasini, V. Bertacche and M. C. Sala, Adv. Synth. Catal., 2009, 351, 219–229 CrossRef CAS.
- C.-H. Tsai, H.-T. Chen, S. M. Althaus, K. Mao, T. Kobayashi, M. Pruski and V. S. Y. Lin, ACS Catal., 2011, 1, 729–732 CrossRef CAS.
- N. A. Brunelli, K. Venkatasubbaiah and C. W. Jones, Chem. Mater., 2012, 24, 2433–2442 CrossRef CAS.
- N. A. Brunelli, S. A. Didas, K. Venkatasubbaiah and C. W. Jones, J. Am. Chem. Soc., 2012, 134, 13950–13953 CrossRef CAS.
- S. Shylesh, D. Hanna, J. Gomes, S. Krishna, C. G. Canlas, M. Head-Gordon and A. T. Bell, ChemCatChem, 2014, 6, 1283–1290 CAS.
- H. Noda, K. Motokura, Y. Wakabayashi, K. Sasaki, H. Tajiri, A. Miyaji, S. Yamaguchi and T. Baba, Chem.–Eur. J., 2016, 22, 5113–5117 CrossRef CAS.
- R. Mouawia, A. Mehdi, C. Reye and R. J. P. Corriu, J. Mater. Chem., 2008, 18, 4193–4203 RSC.
- A. Katz and M. E. Davis, Nature, 2000, 403, 286 CrossRef CAS.
- V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2003, 125, 9403–9413 CrossRef CAS.
- J. D. Bass and A. Katz, Chem. Mater., 2006, 18, 1611–1620 CrossRef CAS.
- E. L. Margelefsky, A. Bendjériou, R. K. Zeidan, V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2008, 130, 13442–13449 CrossRef CAS.
- Y. Peng, J. Wang, J. Long and G. Liu, Catal. Commun., 2011, 15, 10–14 CrossRef CAS.
- J. E. Lofgreen and G. A. Ozin, Chem. Soc. Rev., 2014, 43, 911–933 RSC.
- X. Yu, X. Yu, S. Wu, B. Liu, H. Liu, J. Guan and Q. Kan, J. Solid State Chem., 2011, 184, 289–295 CrossRef CAS.
- P. Chandra, A. M. Jonas and A. E. Fernandes, J. Am. Chem. Soc., 2018, 140, 5179–5184 CrossRef CAS PubMed.
- P. Chandra, A. M. Jonas and A. E. Fernandes, ACS Catal., 2018, 8, 6006–6011 CrossRef CAS.
- J. M. Hoover and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901–16910 CrossRef CAS.
- J. M. Hoover, J. E. Steves and S. S. Stahl, Nat. Protoc., 2012, 7, 1161–1166 CrossRef CAS.
- J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357–2367 CrossRef CAS.
- J. M. Hoover, B. L. Ryland and S. S. Stahl, ACS Catal., 2013, 3, 2599–2605 CrossRef CAS.
- J.-E. Bäckvall, Modern Oxidation Methods, Wiley-VCH Verlag & Co. KGaA, 2nd edn, 2010 Search PubMed.
- B. L. Ryland, S. D. McCann, T. C. Brunold and S. S. Stahl, J. Am. Chem. Soc., 2014, 136, 12166–12173 CrossRef CAS.
- R. C. Walroth, K. C. Miles, J. T. Lukens, S. N. MacMillan, S. S. Stahl and K. M. Lancaster, J. Am. Chem. Soc., 2017, 139, 13507–13517 CrossRef CAS.
- J. Rabeah, U. Bentrup, R. Stosser and A. Bruckner, Angew. Chem., Int. Ed. Engl., 2015, 54, 11791–11794 CrossRef CAS.
- J. C. Barnes, D. J. C. Ehrlich, A. X. Gao, F. A. Leibfarth, Y. Jiang, E. Zhou, T. F. Jamison and J. A. Johnson, Nat. Chem., 2015, 7, 810–815 CrossRef CAS.
- Y. Jiang, M. R. Golder, H. V. T. Nguyen, Y. Wang, M. Zhong, J. C. Barnes, D. J. C. Ehrlich and J. A. Johnson, J. Am. Chem. Soc., 2016, 138, 9369–9372 CrossRef CAS.
- A. E. Fernandes, O. Riant, A. M. Jonas and K. F. Jensen, RSC Adv., 2016, 6, 36602–36605 RSC.
- A. E. Fernandes, O. Riant, K. F. Jensen and A. M. Jonas, Angew. Chem., Int. Ed., 2016, 55, 11044–11048 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00847k |
| This journal is © The Royal Society of Chemistry 2019 |