Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxidation and hydrogenation of Pd: suppression of oxidation by prolonged H2 exposure
Takehiro Tamaoka†
 ab,
Hideto Yoshida
ab,
Hideto Yoshida *a and
Seiji Takedaa
*a and
Seiji Takedaa
aThe Institute of Scientific and Industrial Research, Osaka University, 8-1 Mihogaoka, Ibaraki, Osaka 567-0047, Japan. E-mail: h-yoshida@sanken.osaka-u.ac.jp
bDepartment of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
First published on 19th March 2019
Abstract
We investigate the phase transition of a Pd surface in both oxidizing and reducing environments by environmental transmission electron microscopy (ETEM). ETEM allows us to study sequential exposure of Pd to O2 and H2 in the same TEM conditions. First, under ETEM observation, oxidation occurs at step edges but it can also occur at terraces. Second, as the most important result, we observed a novel process where previous exposure to H2 suppresses new oxidation of the Pd surface. Third, we show by electron energy loss spectroscopy (EELS) that this process, suppression of oxidation by previous exposure to H2, is not due to the formation of bulk β-phase Pd hydride. We also demonstrate that this process is not present in Pt. Finally, we discuss the hypothesis to explain this phenomenon: formation of surface–Pd–hydride suppresses the new oxidation. This observation, suppression of oxidation by H2 exposure, may eventually lead to new breakthroughs.
Introduction
Studying reaction processes at gas–metal interfaces are important in research fields such as catalysis and gas sensing. However, the reaction processes at the atomic scale remain poorly understood because these reactions happen in the atomic scale, they are fast, and they are complex. Additionally, obtaining high resolution images and spectra at reaction environments is difficult. To this day, in order to understand atomic-scale reaction processes at the interface, a lot of methodologies have been developed such as STM,1,2 AFM,3 EELS,4,5 and XPS.2 Among these techniques, environmental transmission electron microscopy (ETEM) is a powerful tool because of its high spatial and temporal resolution under a reaction environment. Although ETEM requires high energy electrons that may damage the specimen, this technique has been extremely useful in elucidating many reaction processes.6–9In this study, we focus on the oxidation and reduction of Pd surfaces. The interface between Pd and gases, such as O2 and H2, is important because Pd is used in various applications such as catalysis,10–12 hydrogen sensing,13,14 and hydrogen storage.15,16 Therefore, there have been studies about adsorbed states of the gas molecules,1,17 surface reconstructions,18,19 and surface phase transitions.20 These results have been useful, but the research field can clearly benefit from dynamic information, and this opens opportunities for ETEM studies. Zhang et al. studied individual Pd nanoparticles under O2 and suggested that the oxidation starts from step edges.21 Baldi et al. combined E(S)TEM and EELS to study hydrogenation of individual Pd nanoparticles and found that the gradual increase of hydrogen concentration during the formation of β-phase Pd hydride was occurred in nanoparticles below 15 nm and that the hydrogenation pressure became lower with decreasing particle size.22 Therefore, there is opportunity to continue to explore this system; for example, by investigating both oxidation and hydrogenation in the same measurement conditions, and furthermore with interchangeable capability.
Here, we investigate the phase transition of Pd in both oxidizing and reducing environments. ETEM allows us to study, for the first time, sequential exposure of Pd to O2 and H2 in the same TEM conditions. First, our results confirm that, under TEM observation, oxidation occurs at step edges but we also show that it can occur at terraces. Second, and perhaps the most important result, we observed a novel process where previous exposure to H2 suppresses new oxidation of the Pd surface. Third, we show by EELS that this process, suppression of oxidation by previous exposure to H2, is not due to the formation of bulk β-phase Pd hydride. And we also demonstrate that this process is not present in Pt. Finally, we discuss the hypothesis to explain this phenomenon: formation of surface–Pd–hydride suppresses the new oxidation. Observation of this suppression process may lead to new interesting discoveries about this important metal–gas interface.
Experimental
For ETEM study, we prepared wedge-shape Pd by electrochemical polishing and argon ion milling of Pd wires (99.95% and 0.2 mm of diameter). For the electrochemical polishing, we used Au-ring electrode as an anode and mixed solution of hydrochloric acid and ethanol. After the electrochemical polishing, we heated the Pd wires at 423 K to remove any remaining solution, and then, we further polished the Pd wires by argon ion milling. For comparison, we also studied wedge-shaped platinum which was prepared by electrochemical polishing and argon ion milling of Pt wires (99.95% and 0.25 mm of diameter). For the electrochemical polishing of Pt wires, we used mixed solution of CaCl2, H2O, and acetone. The ETEM (Titan G2 ETEM) is equipped with a corrector for the spherical aberration of the objective lens and an EELS spectrometer (Gatan GIF Tridiem 863). The acceleration voltage was 300 kV. The specimens were studied at room temperature under O2 and H2. We recorded images using an electron current density of 10 A cm−2 by Thermo-Fisher Ceta 16M CMOS camera with a temporal resolution of 0.5 s and a frame resolution of 4096 pixels. The Pd samples were observed with an electron beam parallel to the [110] zone axis of crystalline fcc Pd. We also performed TEM–EELS measurements under H2 by the ETEM operated at 300 kV without a monochromator at a dispersion of 0.02 eV per channel. Five samples were studied and the results were consistent across.Results and discussions
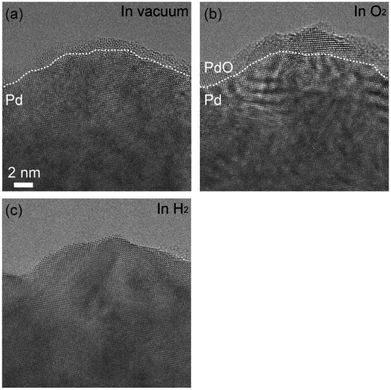
Fig. 1(a) shows as-prepared Pd foil. The surface of Pd foils was covered by hydrocarbon contamination. The contamination was eliminated by intensive electron irradiation in O2. After the elimination, the Pd surface was covered by crystalline material of several nanometers (Fig. 1(b)). Measurement of the distances between crystal planes showed that the crystalline material was Pd monoxide.18 After exhaustion of O2, the PdO film still remained, but after introduction of H2, the PdO was immediately and completely reduced to Pd (Fig. 1(c)). EELS measurement showed that there was no bulk Pd hydride.After the PdO film was reduced, we exhausted H2 and then introduced O2. The duration of H2 exposure was about 10 minutes. After exposure to O2 in 305 seconds, we observed oxidation happening in both step edges and terraces (Fig. 2(a)–(c)). Then, the oxidation proceeded on the Pd surface (Fig. 2(d)), and finally, the Pd surface was completely covered by the PdO film (Fig. 2(e)). It should be mentioned that electron beam induced the oxidation, as demonstrated by a previous study.21 It should also be pointed out that TEM images are projections, and therefore, the geometry of the sample perpendicular to (or behind) the electron beam is unknown. Therefore, there is inherent limitation to knowing the geometry of the area where the oxidation started. For example, the area we viewed as a terrace could have been a step edge in the direction perpendicular to the beam. Nonetheless, as more data becomes available for this system then this conclusion could become stronger.
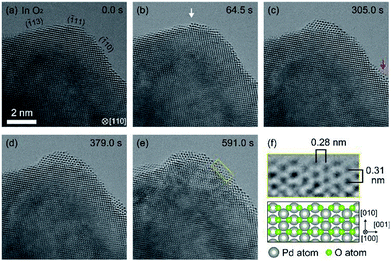 | ||
| Fig. 2 Oxidation processes of the Pd surface in O2 after 10 minutes exposure to H2 (Fig. 1(c)). (a) shows the sample just after starting observation. (b) shows an instance, after 64.5 s of oxygen exposure, where the oxidation started at a step edge (white arrow). (c) shows an instance where the oxidation started at a terrace (red arrow). The oxidation proceeded on the Pd surface as shown in (d), and finally, the Pd surface was completely covered by PdO after 591.0 s. (f) shows enlarged ETEM image of yellow rectangle region in (e) and schematic illustration of PdO (tetragonal, a = 3.051 Å, c = 5.495 Å).18 The gray and green balls in the schematic illustration represent Pd atoms and O atoms, respectively. | ||
In the hydrogenation–oxidation sequence, we also studied the effect of the duration of the H2 exposure. We found that there is a dependence of hydrogen exposure on the oxidation of Pd. After short H2 exposure (10 minutes), Pd can be oxidized as shown in Fig. 2. However, after long exposure (over 90 minutes), the oxidation of the Pd surface was not possible (Fig. 3). To our knowledge, suppression of oxidation by prolonged exposure to H2 has never been reported.
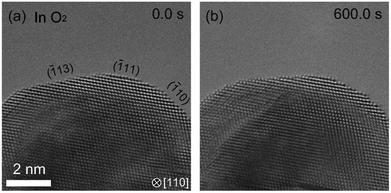 | ||
| Fig. 3 Dependence of history of hydrogen exposure on the oxidation of palladium. After the sample was kept under H2 in 90 minutes, we observed the Pd surface in O2. (a) shows the Pd surface just after starting observation. (b) shows that the Pd surface is not oxidized in 600 s where the surface of Pd is completely covered by PdO after the short exposure to H2 (Fig. 2). | ||
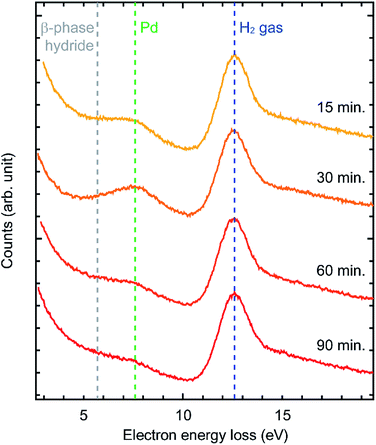
Our initial hypothesis to explain this phenomenon was that the bulk Pd was hydrogenated into bulk β-phase hydride which then suppressed further oxidation. However, in the literature, at room temperature, hydrogenation of Pd requires much higher H2 pressure than that in our experiments.22 In order to verify bulk β-phase hydride, we performed EELS measurements (Fig. 4). EEL spectra were acquired from the area of about 50 nm in diameter including the surface of Pd sample which is selected by a spectrometer entrance aperture. In the spectra, two peaks were observed at 7.7 eV and 12.6 eV. Previous studies showed that the peak at 7.7 eV is derived from Pd and the peak at 12.6 eV is derived from H2 gas.22 As for the Pd peak, previous studies showed that when β-phase hydride is formed, the peak at 7.7 eV shifts to about 5.7 eV.22 However, in our data, the position of the Pd peak was not shifted even after 90 minutes. This suggests that the formation of bulk β-phase hydride did not occur. Therefore, the formation of bulk β-phase hydride is not the mechanism for the suppression of the oxidation.
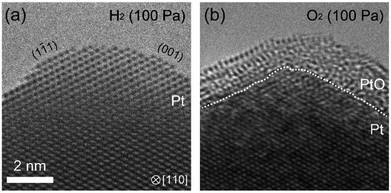
Another interesting question was to see the suppression of oxidation by prolonged exposure to H2 existed in other metals. To answer this question, we studied platinum using the same ETEM conditions and protocol (reduction–oxidation sequences). Fig. 5 shows the ETEM results. In H2, metallic Pt was observed (Fig. 5(a)). In O2, after 90 minutes exposure to H2, the Pt surface was covered by amorphous Pt oxide (Fig. 5(b)) as previously seen in ref. 23. This result shows that the oxidation of Pt was not suppressed even after 90 minutes exposure to H2. Therefore, it is clear that H2 is interacting with Pd in a process that is suppressing subsequent oxidation, and that this process is not existent in Pt. This is very important because it is well known in the literature that hydrogen atoms penetrate palladium but not platinum because the heat of solution of hydrogen in platinum is very large.24 And this is remarkably consistent with our observations of suppression in Pd but not in Pt.
As mentioned before, the formation of bulk β-phase hydride is not the process by which the oxidation was suppressed. However, surface hydride, as a barrier that prevent further oxidation, is possible. And this corresponds to our hypothesis on the process responsible. However, hydrogenation limited to the surface of Pd cannot be observed neither by ETEM nor EELS. It is possible that the dilute bulk α-phase hydride exists in addition to surface hydride. It is very likely that interstitial hydrogen atoms in the bulk α-phase hydride could diffuse to the surface and/or subsurface and contribute to suppress the oxidation of the Pd surface in O2. However, confirmation of our hypothesis is outside the scope of our measurement methods and this work.
We can however look to the literature for information about the surface hydrogenation of Pd. Adsorption of H atoms at the subsurface of Pd has been studied by means of temperature programmed desorption (TPD),25 low energy electron diffraction (LEED),25 and other methods.26 These studies showed that adsorption of H atoms on the subsurface is possible and depends on the crystal plane, coverages of adsorbed H2 molecules, and temperature.25 Conrad et al.25 showed that even at low pressures (<1 Pa), the work function of the Pd(110) and Pd(111) surfaces increases due to the negative charge27,28 of the adsorbed H atoms. They showed that the adsorption of hydrogen can occur at room temperature and at very low pressures. Annealing sequence measurements also showed that some hydrogen penetrates the bulk, and that after short times the hydrogen can come back to the surface as adsorbed atoms.25 In these conditions, bulk diffusion calculations yield an average penetration distance of about 200 microns in 1000 s of exposure.25,29 Further proof of the subsurface occupation of hydrogen atoms was given by Behm et al. who showed that on Pd (110), subsurface occupation with coverages larger than 1 monolayer can occur even at lower temperatures (170 K).30 Similarly, neutron scattering measurements showed that even at very low pressures hydrogen can occupy subsurface sites in Pd.31 Therefore, our hypothesis is consistent with the literature: our experimental conditions in the ETEM have a hydrogen pressure (100 Pa) that is high enough to cause surface adsorption and subsurface occupation of hydrogen atoms.
Conclusions
The observation of suppression of oxidation by prolonged exposure to H2 raises very interesting questions about the gas–metal interface in Pd under O2 and H2. This process is further made more interesting by the absence of bulk β-phase hydride and the absence of this phenomenon in Pt under the same experimental conditions. This observation could eventually lead to the discovery of new surface process involving the surface hydrogenation of Pd. Though in this work, we focused on the discovery, systematic experiments for understanding conditions are needed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This works was supported in part by a Grant-in-Aid for Scientific Research (A), Grant No. 25246003 and “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).Notes and references
- T. Mitsu, M. K. Rose, E. Fomin, D. F. Ogletree and M. Salmeron, Nature, 2003, 422, 705–707 CrossRef PubMed.
- Z. Zhu, F. Tao, F. Zheng, R. Chang, Y. Li, L. Heinke, Z. Liu, M. Salmeron and G. A. Somorjai, Nano Lett., 2012, 12, 1491–1497 CrossRef CAS PubMed.
- Y. S. Gong, C. Lee and C. K. Yang, J. Appl. Phys., 1995, 77, 5422–5425 CrossRef CAS.
- M. S. Chen and D. W. Goodman, Science, 2004, 306, 252–255 CrossRef CAS PubMed.
- H. Tan, J. Verbeeck, A. Abakumov and G. Van Tendeloo, Ultramicroscopy, 2012, 116, 24–33 CrossRef CAS.
- G. Zhou, L. Luo, L. Li, J. Ciston, E. A. Stach and J. C. Yang, Phys. Rev. Lett., 2012, 109, 235502 CrossRef PubMed.
- Y. Kuwauchi, H. Yoshida, T. Akita, M. Haruta and S. Takeda, Angew. Chem., Int. Ed., 2012, 51, 7729–7733 CrossRef CAS PubMed.
- L. Li, L. Luo, J. Ciston, W. A. Saidi, E. A. Stach, J. C. Yang and G. Zhou, Phys. Rev. Lett., 2014, 113, 136104 CrossRef PubMed.
- H. Yoshida, K. Matsuura, Y. Kuwauchi, H. Kohno, S. Shimada, M. Haruta and S. Takeda, Appl. Phys. Express, 2011, 4, 065001 CrossRef.
- M. R. Lyubovsky and L. D. Pfefferle, Catal. Today, 1999, 47, 29–44 CrossRef CAS.
- J. G. McCarty, Catal. Today, 1995, 26, 283–293 CrossRef CAS.
- M. Lyubovsky, L. Pfefferle, A. Datye, J. Bravo and T. Nelson, J. Catal., 1999, 187, 275–284 CrossRef CAS.
- F. Favier, E. C. Walter, M. P. Zach, T. Benter and R. M. Penner, Science, 2001, 293, 2227–2231 CrossRef CAS PubMed.
- X. Li, Y. Liu, J. C. Hemminger and R. M. Penner, ACS Nano, 2015, 9, 3215–3225 CrossRef CAS PubMed.
- M. Yamauchi, R. Ikeda, H. Kitagawa and M. Takata, J. Phys. Chem. C, 2008, 112, 3294–3299 CrossRef CAS.
- G. Li, H. Kobayashi, J. M. Taylor, R. Ikeda, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Toh, S. Matsumura and H. Kitagawa, Nat. Mater., 2014, 13, 802–806 CrossRef CAS PubMed.
- F. P. Leisenberger, G. Koller, M. Sock, S. Surnev, M. G. Ramsey, F. P. Netzer, B. Klo and K. Hayek, Surf. Sci., 2000, 445, 380–393 CrossRef CAS.
- J. Rogal, K. Reuter and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 075421 CrossRef.
- W. Dong, V. Ledentu, P. Sautet, G. Kresse and J. Hafner, Surf. Sci., 1997, 379, 56–61 CrossRef.
- E. Lundgren, G. Kresse, C. Klein, M. Borg, J. N. Andersen, M. De Santis, Y. Gauthier, C. Konvicka, M. Schmid and P. Varga, Phys. Rev. Lett., 2002, 88, 246103 CrossRef CAS PubMed.
- D. Zhang, C. Jin, H. Tian, Y. Xiong, H. Zhang, P. Qiao, J. Fan, Z. Zhang, Z. Y. Li and J. Li, Nanoscale, 2017, 9, 6327–6333 RSC.
- A. Baldi, T. C. Narayan, A. L. Koh and J. A. Dionne, Nat. Mater., 2014, 13, 1143–1148 CrossRef CAS PubMed.
- H. Yoshida, H. Omote and S. Takeda, Nanoscale, 2014, 6, 13113–13118 RSC.
- Y. Ebisuzaki, W. J. Kass and M. O'Keeffe, J. Chem. Phys., 1968, 49, 3329–3332 CrossRef CAS.
- H. Conrad, G. Ertl and E. E. Latta, Surf. Sci., 1974, 41, 435–446 CrossRef.
- W. Eberhardt, S. G. Louie and E. W. Plummer, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 465–477 CrossRef CAS.
- R. Suhrmann, G. Welder and G. Shumicki, Naturwissenschaften, 1959, 46, 600 CrossRef.
- R. Suhrmann, G. Schumicki and G. Wedler, Z. Naturforsch., 1964, 19a, 1208–1216 CAS.
- D. N. Jewett and A. C. Makrides, Trans. Faraday Soc., 1964, 61, 932–939 RSC.
- R. J. Behm, K. Christmann and G. Ertl, Surf. Sci., 1980, 99, 320–340 CrossRef CAS.
- J. M. Nicol, J. J. Rush and R. D. Kelley, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 9315–9317 CrossRef CAS.
Footnote |
| † Present address: Department of Applied Quantum Physics and Nuclear Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, 819-0395, Japan. |
| This journal is © The Royal Society of Chemistry 2019 |