Open Access Article
Open Access ArticleMicrowave absorption properties of steelmaking dusts: effects of temperature on the dielectric constant (ε′) and loss factor (ε′′) at 1064 MHz and 2423 MHz
Mamdouh Omran *ab,
Timo Fabritiusa,
Guo Chen*c and
Aoxi Hec
*ab,
Timo Fabritiusa,
Guo Chen*c and
Aoxi Hec
aProcess Metallurgy Research Group, Faculty of Technology, University of Oulu, P. O. Box: 4300, Oulu, Finland. E-mail: addressesmamdouh.omran@oulu.fi; mamdouh_nasr82@yahoo.com
bMineral Processing and Agglomeration Laboratory, Central Metallurgical Research and Development Institute, Cairo, Egypt
cKey Laboratory of Resource Clean Conversion in Ethnic Regions of Education Department of Yunnan, Yunnan Minzu University, Kunming 650500, P. R. China. E-mail: guochen@kmust.edu.cn
First published on 27th February 2019
Abstract
The microwave absorption properties of a material depend largely on the dielectric properties of the material being heated. Therefore, the influences of temperature on the dielectric constant (ε′), loss factor (ε′′), loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) and penetration depth (DP) of steelmaking dust at frequencies of 1064 MHz and 2423 MHz were measured. Three steelmaking dust samples were studied. The effects of temperature on the dielectric properties of the samples were insignificant at temperatures below 600 °C. However, above this temperature, a rapid rise in the values of the dielectric properties of the samples was observed. Comparing the thermogravimetric analysis and differential scanning calorimetry (TGA-DSC) results and mass spectra (MS) of the dusts with their dielectric properties revealed that the changes in the dielectric values of the dusts were associated with the thermal decomposition of calcium carbonate and the release of CO/CO2 gases. Furthermore, the increase in the electrical conductivity of the samples at high temperature resulted in increased dielectric values. The behavior of the loss tangent of the samples with increasing temperature coincided with the behavior of the loss factor. The penetration depth decreased with an increase in temperature at both frequencies, while an increase in the dielectric properties caused a significant decrease in the penetration depth. The results indicated that steelmaking dusts have good microwave absorbing properties owing to their carbon and iron oxide contents.
δd) and penetration depth (DP) of steelmaking dust at frequencies of 1064 MHz and 2423 MHz were measured. Three steelmaking dust samples were studied. The effects of temperature on the dielectric properties of the samples were insignificant at temperatures below 600 °C. However, above this temperature, a rapid rise in the values of the dielectric properties of the samples was observed. Comparing the thermogravimetric analysis and differential scanning calorimetry (TGA-DSC) results and mass spectra (MS) of the dusts with their dielectric properties revealed that the changes in the dielectric values of the dusts were associated with the thermal decomposition of calcium carbonate and the release of CO/CO2 gases. Furthermore, the increase in the electrical conductivity of the samples at high temperature resulted in increased dielectric values. The behavior of the loss tangent of the samples with increasing temperature coincided with the behavior of the loss factor. The penetration depth decreased with an increase in temperature at both frequencies, while an increase in the dielectric properties caused a significant decrease in the penetration depth. The results indicated that steelmaking dusts have good microwave absorbing properties owing to their carbon and iron oxide contents.
1. Introduction
Steelmaking dusts are waste byproducts generated through a secondary steelmaking process in an electric arc furnace (EAF) and are considered hazardous waste in most industrialized countries.1,2 Steelmaking dust from converters and EAF is a fine-grained material containing significant amounts of zinc and iron in addition to variable amounts of calcium, manganese, magnesium, silicon, and chromium.3,4 Zinc oxide (ZnO) and zinc ferrite (ZnFe2O4) are the major compounds in the dust.5The use of microwaves, a form of electromagnetic radiation, as a heat source in material processing applications has become increasingly frequent over the past three decades.6–9 Microwave heating is fundamentally different from conventional heating because microwaves take the form of electromagnetic energy and can penetrate deep into the sample. This allows sample heating to be initiated volumetrically as opposed to conventional thermal heating, which heats the sample from the outside in via standard heat-transfer mechanisms, i.e., through convection, conduction, and radiation.10 Compared to conventional heating, microwave heating offers several advantages such as selective heating, rapid heating, and volumetric heating.11,12 The dielectric properties of materials define how well they absorb microwaves and whether they can be heated under microwave irradiation.13,14
Therefore, to make microwave heating more efficient, it is important to know the dielectric properties, especially the dielectric constant (ε′) and dielectric loss factor (ε′′), of a material. There is a paucity of information on the dielectric properties of steelmaking dusts. Previous work focused on the removal of zinc from dust using microwave energy as a heating source.8,9,15 No detailed studies have been reported regarding the effect of temperature on the dielectric constant (ε′) and loss factor (ε′′) of steelmaking dust.
Al-harahsheh et al.8,15 measured the dielectric properties of EAF dust (EAFD) at different temperatures. It was found that both the dielectric constant and the loss factor of the EAF dust increase as the temperature rises; a sharper increase of both values was noticed when the temperature exceeded 300 °C. The authors did not provide an interpretation of the changes in the dielectric properties of the EAF dust with temperature. Zhang et al.16 measured the dielectric properties of zinc oxide dust in different apparent densities. They concluded that the dielectric properties of zinc oxide dust increase when the apparent density increases. Liu et al.17 studied the dielectric properties of low-grade Panzhihua ilmenite ore at 2.45 GHz at temperatures ranging from 20 °C to 100 °C. They indicated that both the dielectric constant (ε′) and the loss factor (ε′′) of the ilmenite significantly increase with temperature. Jiang et al.18 studied the dielectric properties and oxidation roasting of molybdenite concentrate by using microwave heating. They concluded that the dielectric constant increased as the temperature increased, while the loss factor presented an opposite trend.
This study aims to examine the dielectric properties of steelmaking dusts to understand their heating behavior under microwave irradiation. The influence of temperature on the dielectric constant (ε′), loss factor (ε′′), loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) and penetration depth (DP) at frequencies of 1064 MHz and 2423 MHz were investigated.
δd) and penetration depth (DP) at frequencies of 1064 MHz and 2423 MHz were investigated.
1.1 Dielectric properties
Several techniques, for examples, cavity perturbation,19 open-ended coaxial probes,20 waveguide transmission line,21 and free-space methods,22 have been employed to measure the dielectric properties of materials. The open-ended coaxial probe method is the most widely used technique owing to the flexible requirements for the sample shape and the high accuracy of measurements.23The interaction of a material with a microwave field is determined by its dielectric properties (i.e., the complex permittivity, ε) of the material.13,24
The complex permittivity ε is expressed as:
| ε = ε′ − jε′′ | (1) |
The real permittivity (ε′) is conventionally termed the dielectric constant, and the imaginary permittivity (ε′′) is conventionally termed the dielectric loss factor. The dielectric constant expresses the ability of the material to absorb electromagnetic radiation within its structure, whereas the loss factor represents the ability of a material to dissipate the adsorbed radiation into heat.13,25 The heating of a material depends greatly on the ratio of its loss factor to the dielectric constant. Materials with a high loss factor are easily heated by microwave energy.14
The loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) is an important factor that provides an indication of how well a material dissipates stored energy into heat.25 The loss tangent (tan
δd) is an important factor that provides an indication of how well a material dissipates stored energy into heat.25 The loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) is expressed as:
δd) is expressed as:
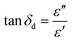 | (2) |
The penetration depth (DP) is an important criterion for designing and scaling up any microwave heating system. The penetration depth (DP, cm) is defined as the distance from the material surface where the absorbed electric field falls to 1/e of the electric field at the surface.16,25 DP is given by the following eqn (3):16
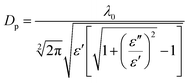 | (3) |
2. Experimental procedure
2.1 Materials
Three steelmaking dust samples were used in this study:- Ferrochrome converter (CRC) and EAF stainless steel (EAFSS) dusts were obtained from the Outokumpu Tornio stainless steel plantin Finland.
- EAF carbon steel (EAFCS) dust was obtained from Ovako Imatra, Finland.
Each is a representative sample of that dust's average composition.
2.2 Characterization methods
The mineral phases of steelmaking dusts were determined using X-ray diffraction (D/Max 2200, Rigaku, Japan). The measurements were performed with a cobalt tube in the 2θ range from 4 to 90°. The chemical composition of the steelmaking dust samples were measured using X-ray fluorescence (XRF) spectrometer (Bruker AXS S4 Pioneer).The carbon contents were determined using a LECO carbon analyzer. The microanalyses of samples were conducted using a Zeiss ULTRA plus field-emission scanning electron microscope (FE-SEM) attached to an energy-dispersive X-ray spectroscopy (EDS) unit for chemical analysis. The thermal behaviors of the steelmaking dusts were examined using a Netzsch STA409 PC Luxx under air atmosphere. Approximately 23.84 mg of sample was placed in a platinum crucible on a pan of the microbalance at a heating rate of 10 °C min−1. The temperature range was 20–1400 °C. The characterization were performed at the Center of Microscopy and Nanotechnology (CMNT), University of Oulu, Finland.
2.3 Dielectric property measurements
In this study, the dielectric properties of the steelmaking dusts were measured using an open-ended coaxial probe. In the experiments, the sample powder was sealed in a resonant cavity (inner diameter 80 mm, length 100 mm) made of stainless steel and heated to the desired temperature by an electric furnace placed inside the holder cavity. The probe was inserted into the sample powder for a full contact. The open-ended coaxial probe was connected to an Agilent PNA5230 network analyzer to measure the signals reflected from the sample. The reflected signals contained information related to the dielectric properties of the sample. At least three duplicate measurements were carried out for each sample. The details of the procedure are described in Liu et al.17The effects of temperature on the dielectric constant (ε′) and dielectric loss factor (ε′′) of the samples were measured under the temperature range from 25 to 1100 °C and at frequencies of 1064 MHz and 2423 MHz.
2.4 Microwave heating experiments
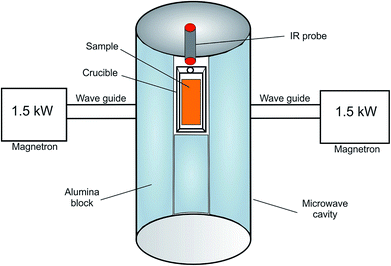
The microwave heating profiling of the dust samples was carried out in a microwave device developed by the Key Laboratory of Unconventional Metallurgy, Kunming University of Science and Technology, China. A schematic diagram of the microwave heating system is shown in Fig. 1.The power supply of the microwave device was two magnetrons (1.5 kW power, 2.45 GHz frequency), and it was cooled via water circulation. The cylindrical microwave cavity was insulated with alumina blocks.
The microwave heating experiments were carried out at microwave power of 1.1 kW under air atmosphere. 100 gram sample was placed in a microwave transparent alumina crucible, which was positioned at the center of the microwave irradiation. The temperature of the test sample was measured using an infra-red IR probe. At least two duplicate measurements were carried out for each sample.
3. Results and discussion
3.1 Materials characterization
The chemical compositions of the steelmaking dusts are listed in Table 1. The major elements of the dusts were Zn, Fe, Cr, Ca, Si and Mg.| Analyses | CRC | EAFSS | EAFCS |
|---|---|---|---|
| a x − Sign mean the order of abundance, xxxx – means major, and x – means minor. | |||
| Chemical composition | Concentration (wt%) | ||
| C | 0.3 | 0.5 | 1.5 |
| Fe | 18.74 | 23.70 | 23.50 |
| Zn | 10.83 | 19.84 | 35.76 |
| Cr | 20.88 | 3.19 | 0.47 |
| CaO | 14.27 | 11.91 | 5.93 |
| MgO | 9.76 | 7.21 | 1.07 |
| MnO | 1.56 | 5.82 | 3.99 |
| SiO2 | 9.99 | 8.75 | 3.13 |
| K2O | 0.74 | 1.49 | 3.21 |
| Phases | Abundance | ||
| Franklinite (ZnFe2O4) | — | xxxx | xxxx |
| Zincite (ZnO) | xx | xx | xxxx |
| Chromite (FeCr2O4) | xxxx | — | — |
| Lime (CaO) | x | x | x |
| Periclase (MgO) | x | x | x |
| Magnetite | x | x | x |
| Particle size analysis | Value (μm) | ||
| Median (d50) | 3.15 | 2.37 | 1.63 |
| d25 | 0.84 | 0.31 | 0.48 |
| d75 | 4.84 | 3.94 | 2.96 |
| d100 | 15.42 | 13.00 | 14.62 |
| Physical properties | Value | ||
| pH | 10 | 10–11 | 10–11 |
| Moisture content (%) | 0.22 | 0.30 | 0.54 |
| Density | 1.059 | ||
The contents of iron in the CRC, EAFSS, and EAFCS dusts were 18.74, 23.70, and 23.50 wt%, respectively, whereas the contents of zinc were 10.83, 19.84, and 35.76 wt%, respectively (Table 1). The contents of chromium in the CRC, EAFSS, and EAFCS dusts were 20.88, 3.19, and 0.47 wt%, respectively. The dusts contained high amounts of calcium oxide and magnesium oxide owing to the dolomitic lime added to the steelmaking furnace.1 The CaO contents in the CRC, EAFSS, and EAFCS dusts were 14.27, 11.91, and 5.93 wt%, respectively (Table 1).
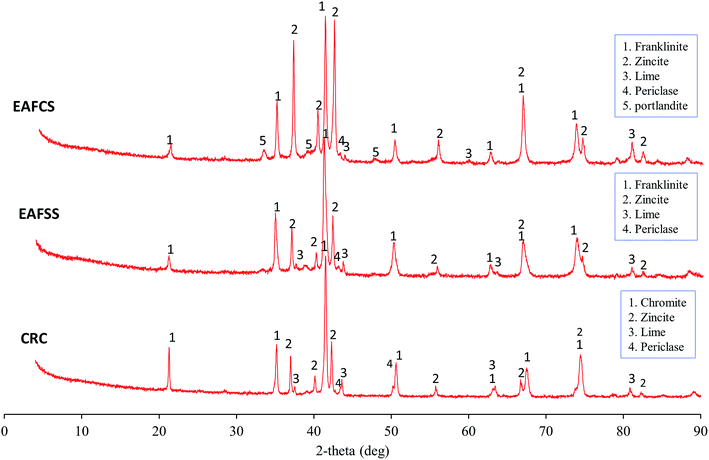
The mineral phases in the CRC, EAFSS, and EAFCS dusts are shown in Fig. 2. In the CRC dust, chromite (FeCr2O4) represented the main phase with a spinel structure, and zincite (ZnO) was the zinc-bearing phase. The EAFSS and EAFCS dusts consisted mainly of franklinite (ZnFe2O4) and zincite (ZnO). In addition to the main phases, portlandite (Ca(OH)2), lime (CaO), and periclase (MgO) were identified. The samples also contained magnetite (Fe3O4), but the peaks of magnetite overlapped with the peaks of franklinite and chromite. SEM coupled with EDS was used for the identification of these phases; the results were published in a previous study.4
The SEM image of CRC dust showed that chromite exists as irregular particles, whereas zincite appears as a monocrystalline sphere (Fig. 3A). The EAFSS dust was dominated by encapsulation particles; franklinite was enclosed in calcium-iron-silicate glass sphere (Fig. 3B). Based on the SEM image of EAFCS dust, the dust was dominated by franklinite and zincite spheres (Fig. 3C). The median (d50) particle sizes for the CRC, EAFSS, and EAFCS dusts were 3.15, 2.37, and 1.63 μm, respectively (Table 1).
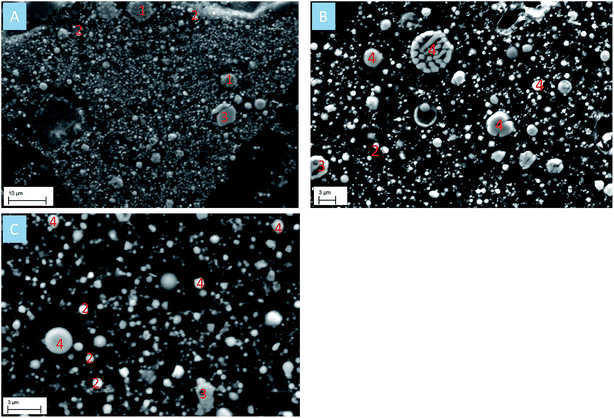 | ||
| Fig. 3 (A) SEM image of CRC dust; (B) SEM image of EAFSS dust; (C) SEM image of EAFCS dust. (1) Chromite; (2) zincite; (3) magnetite; (4) franklinite. | ||
3.2 Dielectric properties and thermal analysis
The dielectric constant (ε′) and loss factor (ε′′) of the studied materials at room temperature and frequencies of 1064 MHz and 2423 MHz are listed in Table 2.| Property | CRC | EAFSS | EAFCS |
|---|---|---|---|
| At 2423 MHz frequency | |||
| Dielectric constant (ε′) | 1.78 | 1.80 | 1.77 |
| Loss factor (ε′′) | 0.028 | 0.041 | 0.022 |
| Loss tangent (δd) | 0.016 | 0.022 | 0.012 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| At 1064 MHz frequency | |||
| Dielectric constant (ε′) | 1.89 | 1.95 | 1.89 |
| Loss factor (ε′′) | 0.033 | 0.0417 | 0.0216 |
| Loss tangent (δd) | 0.0174 | 0.0214 | 0.0113 |
At room temperature, the dielectric values of all steelmaking dusts (CRC, EAFSS, and EAFCS) were almost in the same range, which indicates that the dusts have similar microwave absorbing properties. At a frequency of 1064 MHz, the dielectric constant (ε′) values ranged from 1.89 to 1.95, and the loss factor (ε′′) values ranged from 0.216 to 0.417. In contrast, at a frequency of 2423 MHz, the dielectric constant (ε′) values ranged from 1.77 to 1.80, and the loss factor (ε′′) values ranged from 0.22 to 0.41. Similar dielectric values were also reported by Al-harahsheh et al.8 for EAF dust.
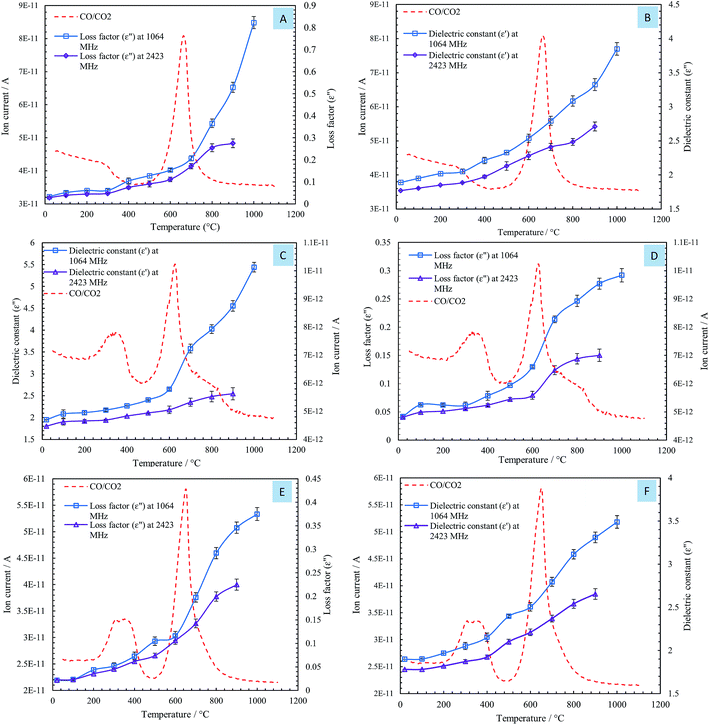
The influences of temperature on the dielectric constant (ε′) and loss factor (ε′′) of the steelmaking dusts at frequencies of 1064 MHz and 2423 MHz are shown in Fig. 4.
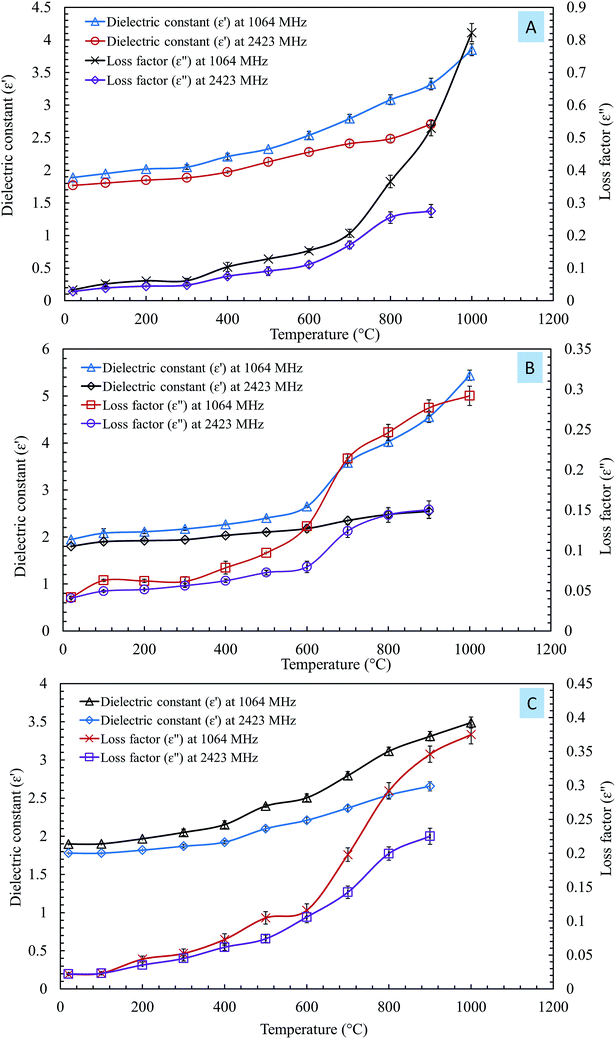 | ||
| Fig. 4 Influences of temperature on the dielectric constant (ε′) and loss factor (ε′′) of the steel making dust at frequencies of 1064 MHz and 2.423 GHz. (A) CRC dust; (B) EAFSS dust; (C) EAFCS dust. | ||
The changes in the dielectric constant (ε′) of dusts were insignificant with an increase in temperature from 20 to 600 °C, as shown in Fig. 4. When the temperature exceeded 600 °C, a rise in the values of the dielectric constant of the dusts was observed. The dielectric constants of the CRC, EAFSS, and EAFCS dusts increased from 2.53, 2.65, and 2.50 to 3.85, 5.44, and 3.49, respectively, with an increase in temperature from 600 to 1000 °C at a frequency of 1064 MHz.
The loss factors (ε′′) of the dusts increased slightly with an increase in the temperature and then rapidly increased when the temperature exceeded 600 °C. At a frequency of 1064 MHz, the loss factors of CRC, EAFSS, and EAFCS dusts increased from 0.15, 0.13, and 0.12 to 0.82, 0.29, and 0.37, respectively, with an increase in the temperature from 600 to 1000 °C. At a frequency of 2423 MHz, the loss factors of the CRC, EAFSS, and EAFCS dusts increased from 0.11, 0.08, and 0.11 to 0.27, 0.15, and 0.22, respectively, with an increase in temperature from 600 to 1000 °C.
The curves showed that the temperature had a more significant effect on the loss factor than on the dielectric constant of the dusts. Fig. 4 shows that the changes in the dielectric were much lower at 2423 MHz than at 1064 MHz.
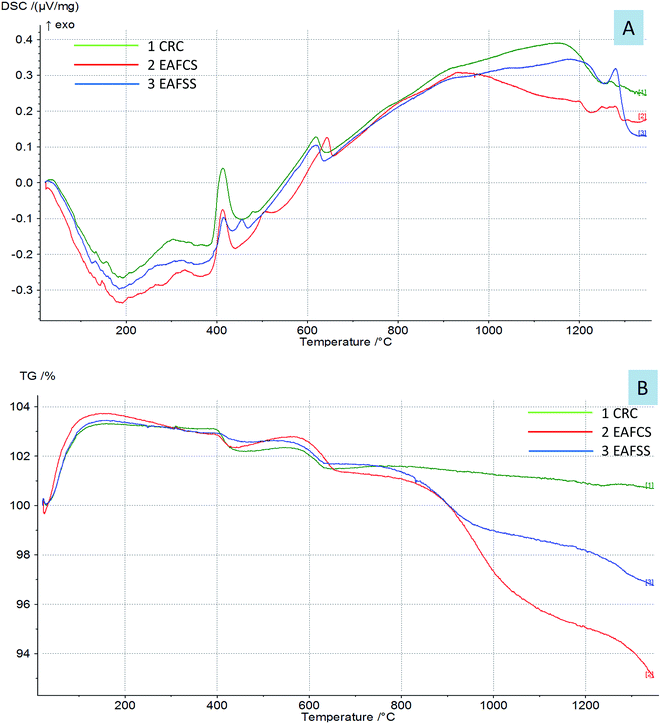
The thermal behavior of the dusts is shown in Fig. 5. The DSC-TG curves of the CRC, EAFSS and EAFCS dusts showed that there were two main reactions: one between 415 and 418 °C and another between 622 and 645 °C; these are related to the dehydroxylation of calcium hydroxide and the decomposition of calcium carbonate, respectively, according to the following reactions.4,26,27
| Ca(OH)2 → CaO + H2O | (4) |
| CaCO3 → CaO + CO2 | (5) |
Both Ca(OH)2 and CaCO3 existed originally as free lime in the dusts, which, in turn, may have hydrated and carbonated on exposure to ambient moisture and CO2.4,28 H2O and CO2 gases from the samples at these temperatures were detected with their mass spectra. The mass losses of the dusts at temperatures above 900 °C were related to the volatilization of Zn, Pb and K.5,26 The carbon contained in the dust could react with zinc, which evaporated into zinc vapor.4 The reactions at high temperatures above 1222–1297 °C resulted from the sintering of the samples and the formation of calcium ferrite. At high temperatures, the calcium carbonate contained in the sample began to decompose, and the free calcium oxide combined with the free iron oxide to form calcium ferrites.29
By comparing the DSC curves of the steelmaking dusts with their dielectric properties, we can observe that the changes in the dielectric properties of the dusts were correlated with the reactions in the range of 622–645 °C related to the thermal decomposition of calcium carbonate into calcium oxide. Fig. 6 shows that the changes occurring in the dielectric constant (ε′) and loss factor (ε′′) of the dusts coincided with the mass spectra of the CO/CO2 gases evaporated from the calcium carbonate decomposition. The phases of decomposition and transformation inside the material resulted in a change in its electrical and ionic conductivity that is proportional to the loss factors under high temperatures.18 The composition of the dusts changed during microwave heating owing to the thermal decomposition of CaCO3 into CaO and the evaporation of zinc that resulted in the release of CO/CO2 gases. Lovas et al.13 obtained the same result with magnesium carbonate. They observed that the value of the imaginary permittivity increases at 950 °C due to the thermal decomposition of MgCO3into MgO. Al-harahsheh et al.8,15 showed that both the dielectric constant and the loss factor of EAF dust increase as the temperature rises, but an explanation was not provided.
The increase in the dielectric properties at high temperatures above 900 °C was attributed to the increase in the electrical conductivity owing to sample melting. The rate of microwave energy absorption by a material is controlled either by its electronic or ionic conductivity or by the number of free permanent dipolar molecules per unit volume.14,30 As the temperature increased, the polarization capability of steelmaking molecules increased as well.
3.3 Loss tangent and penetration depth
Eqn (2) was used to calculate the values of the loss tangent, and the results are shown in Fig. 7. From eqn (2), an increase in the loss tangent was the result of a more rapid increase in ε′′ than in ε′.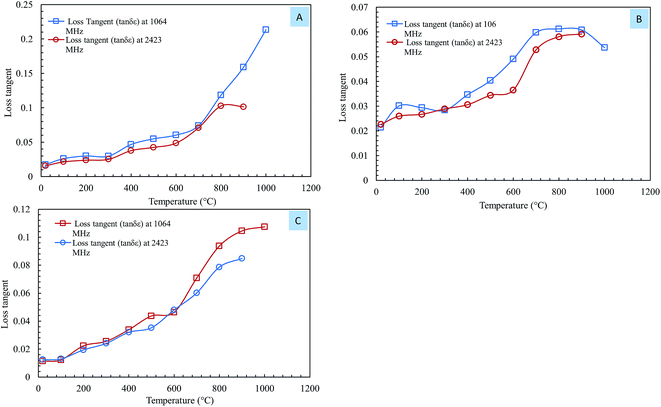 | ||
| Fig. 7 The loss tangent of steelmaking dusts as a function of temperature at frequencies of 1064 MHz and 2.423 GHz. (A) CRC dust; (B) EAFSS dust; (C) EAFCS dust. | ||
The loss tangents of the steelmaking dusts at room temperature are listed in Table 2. At a frequency of 1064 MHz, the loss tangents (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) of the CRC, EAFSS, and EAFCS dusts were 0.0174, 0.0214 and 0.0113, respectively; at a frequency of 2423 MHz, the loss tangents (tan
δd) of the CRC, EAFSS, and EAFCS dusts were 0.0174, 0.0214 and 0.0113, respectively; at a frequency of 2423 MHz, the loss tangents (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δd) of the CRC, EAFSS, and EAFCS dusts were 0.016, 0.022 and 0.012, respectively.
δd) of the CRC, EAFSS, and EAFCS dusts were 0.016, 0.022 and 0.012, respectively.
Fig. 7 shows the loss tangents of the steelmaking dusts as a function of temperature at 1064 MHz and 2423 MHz. There was a small increase in the loss tangent of dust in the temperature region of 20–600 °C and a significant increase when the temperature exceeded 600 °C (Fig. 7). The loss tangents of the CRC, EAFSS and EAFCS dusts increased from 0.06, 0.04, and 0.04 to 0.21, 0.66, and 0.11, respectively, with an increase in temperature from 600 to 1000 °C at 1064 MHz. Fig. 7 reveals that the behavior of the loss tangent of the dusts with temperature coincided with the loss factor (ε′′) behavior of the dusts.
Al-harahsheh et al.8,15 observed that above 300 °C, the loss tangent of EAF dust increases considerably with further increases in temperature at 2470 MHz. The authors stated that when the loss tangent value is above 0.05, the material is considered to heat well under microwave irradiation. The loss tangent values of dusts increased with temperature; when the temperature exceeded 600 °C, the loss tangent values rapidly increased, and the values exceeded 0.05 for all samples. The loss tangent values of the steelmaking dusts indicated that they should be a very good microwave absorber and should heat easily and rapidly.8
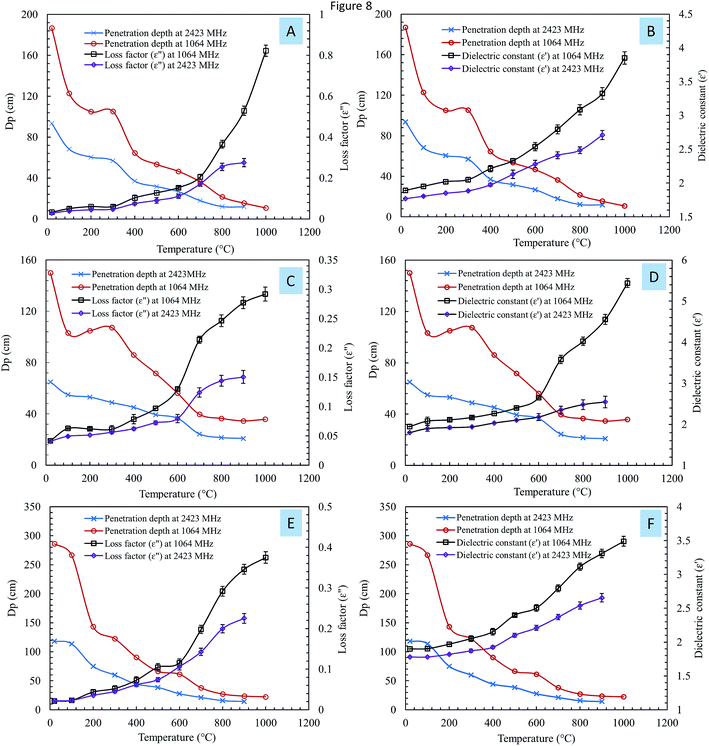
The penetration depths (DP) of steelmaking dusts were calculated using eqn (3). Fig. 8 shows the variation in the penetration depth (DP) as a function of the temperature at frequencies of 1064 MHz and 2423 MHz.
Overall, DP decreased with an increase in temperature at both frequencies. The penetration depth at 1064 MHz was higher than the penetration depth at 2423 MHz; however, as the temperature increased, the differences between the two frequencies decreased. When the temperature exceeded 800 °C, the value of DP was nearly the same at both frequencies. The large penetration depth at low temperature suggests deep microwave penetration in the materials, while at a higher temperature, the surfaces of the materials absorb radiation, resulting in a shallower penetration depth.18 A deeper penetration depth indicates that the material can absorbed more microwave radiation and thus heated more uniformly and quickly.31
Liu et al.17 studied the effect of temperature on the penetration depth of low-grade Panzhihua ilmenite ore at 2450 MHz. They concluded that the penetration depth significantly decreases with temperature. Zhang et al.16 measured the microwave penetration depth of zinc oxide dust at different apparent densities. The results showed that DP decreases when the apparent density increases.
Fig. 8 shows the relationship between the penetration depth of the samples and the continuous changes in the dielectric properties at 1064 MHz and 2423 MHz. Increases in the loss factor and dielectric constant caused a considerable decrease in the penetration depth. The dielectric properties of a material are the internal factors that determine the penetration depth.18 Jiang et al.18 studied the relationship between the penetration depth and the dielectric properties of molybdenite concentrate. They found that an increase in the dielectric loss caused a considerable decrease in the penetration depth. The authors concluded that the interaction between a dielectric material and microwave radiation through dissipation and absorption resulted in a reduced penetration depth.
3.4 Microwave heating behavior
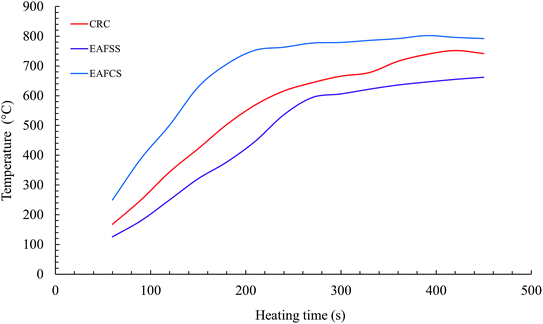
The temperature (T) profiles of steelmaking dusts with time at a microwave power of 1100 W are shown in Fig. 9. After an irradiation time of 240 s, temperatures of 536, 616, and 763 °C were measured for the EAFSS, CRC, and EAFCS dusts, respectively.The heating curve revealed that the temperature increased very rapidly at first and thereafter increased slowly (Fig. 9). In the temperature range from 700 to 900 °C, the rate of increase of the sample temperature was lower than that at temperatures between 100 and 700 °C. Because microwave heating is nonlinear, as is conventional heating, the microwave heating starts from inside.16 The chemical or phase transformations during heating may also affect the heating efficiency of microwaves.32 The composition of the dusts changed during the microwave heating owing to the thermal decomposition of carbonate and the evolution of CO/CO2 gases, which can be attributed to the initial rapid increase in the temperature33 (Fig. 9).
In contrast to conventional heating, microwaves provide selective material heating. Therefore, the mineralogical composition of the sample affects the heating efficiency of microwaves.32,33 Table 3 gives the microwave heating properties of the mineral phases in the dusts. Different dielectric phases are contained in the dusts. In particular, magnetite and carbon heat up rapidly (hyperactive materials), zincite and franklinite heat up slowly (difficult to heat), and lime does not heat (inactive material).11,34 When dusts are irradiated by microwave radiation, the components with high dielectric properties can absorb more electromagnetic energy from microwave radiation and cause an increase in the temperature. In contrast, components with low dielectric properties are heated by mass and heat transfer. Fig. 10 shows the sintering of the EAFCS dust following microwave heating at 1100 W for 5 min.
| Phase | Abundance | Microwave heating | ||
|---|---|---|---|---|
| CRC | EAFSS | EAFCS | ||
| Franklinite (ZnFe2O4) | — | xxxx | xxxx | Difficult to heat [ref. 11 and 34] |
| Zincite (ZnO) | xx | xx | xxxx | Difficult to heat [ref. 11 and 34] |
| Chromite (FeCr2O4) | xxxx | x | — | Heats readily [ref. 11 and 34] |
| Lime (CaO) | x | x | x | Inactive [ref. 11 and 34] |
| Periclase (MgO) | x | x | — | Difficult to heat [ref. 11 and 34] |
| Magnetite (Fe3O4) | Minor | Minor | Minor | Hyperactive materials [ref. 11 and 34] |
| Carbon | 0.3 | 0.5 | 1.5 | Hyperactive materials [ref. 11 and 34] |
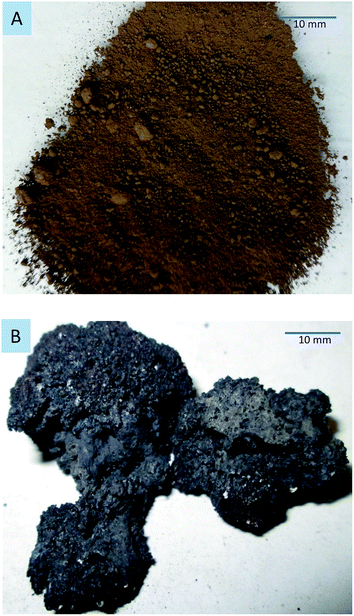 | ||
| Fig. 10 EAF dust following microwave heating at 1100 W for 5 min. (A) Raw sample; (B) after microwave heating. | ||
The good microwave heating properties of steelmaking dusts are attributed to the contents of carbon and iron oxides, which are classified as excellent microwave absorbers.11,34 Sun et al.35 indicated that EAF dust is an excellent microwave absorbing material. When 20 g of EAF dust was placed alone in a microwave furnace and irradiated at 1100 W for 5 min., the temperature of the EAF dust reached 907 °C.
4. Conclusion
The effects of temperature on the microwave absorption properties of steelmaking dusts at frequencies of 1064 MHz and 2423 MHz have been studied in this paper.The main conclusion that can be drawn based on the results of the experiments is that the dielectric values of CRC, EAFSS, and EAFCS dusts at room temperature were approximately within the same range. The effect of temperature on the dielectric properties was found to be minor at temperatures below 600 °C. Above this temperature, sharp rises in the values of both the dielectric constant and the loss factor were observed. This change can be attributed to the thermal decomposition of the dusts during microwave heating.
The behavior of the loss tangent of the dusts with increasing temperature coincided with the loss factor (ε′′) behavior of the dusts. While the penetration depth (DP) decreased with an increase in the loss factor of the dusts. The DP decreased with an increase in the temperature at both frequencies.
The findings of this paper indicate that the steelmaking dusts have good microwave heating owing to the contents of carbon and iron oxides, which are classified as excellent microwave absorbers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support of the CECIRE project (2430207011). The authors acknowledge Outokumpu Tornio and Ovako Imatra in Finland for the supply of industrial samples.References
- J. G. M. S. Machado, F. A. Brehm, C. A. M. Moraes, C. A. dos Santos, A. C. F. Vilela and J. B. M. da Cunha, Chemical, physical, structural and morphological characterization of the electric arc furnace dust, J. Hazard. Mater., 2006, B136, 953–960 CrossRef PubMed.
- D. K. Xia and C. A. Pickles, Microwave caustic leaching of electric arc furnace dust, Miner. Eng., 2000, 13, 79–94 CrossRef CAS.
- T. Sofilić, A. Rastovčan-Mioč, Š. Cerjan-Stefanović, V. Novosel-Radović and M. Jenko, Characterization of steel mill electric-arc furnace dust, J. Hazard. Mater., 2004, 109, 59–70 CrossRef PubMed.
- M. Omran and T. Fabritius, Effect of steelmaking dust characteristics on suitable recycling process determining: ferrochrome converter (CRC) and electric arc furnace (EAF) dusts, Powder Technol., 2017, 308, 47–60 CrossRef CAS.
- P. Oustadakis, P. E. Tsakiridis, A. Katsiapi and S. Agatzini-Leonardou, Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD) part I: characterization and leaching by diluted sulphuric acid, J. Hazard. Mater., 2010, 179, 1–7 CrossRef CAS PubMed.
- M. Omran, T. Fabritius, A. Elmahdy, N. A. Abdel-Khalek, M. El-Aref and A. Elmanawi, XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore, Appl. Surf. Sci., 2015, 345, 127–140 CrossRef CAS.
- M. Omran, T. Fabritius, A. Elmahdy, N. A. Abdel-Khalek and S. Gornostayev, Improvement of phosphorus removal from iron ore using combined microwave pretreatment and ultrasonic treatment, Sep. Purif. Technol., 2015, 156, 724–737 CrossRef CAS.
- M. Al-harahsheh, S. Kingman, L. Al-Makhadmah and I. E. Hamilton, Microwave treatment of electric arc furnace dust with PVC: dielectric characterization and pyrolysis-leaching, J. Hazard. Mater., 2014, 274, 87–97 CrossRef CAS PubMed.
- K. Nishioka, T. Maeda and M. Shimizu, Dezincing Behavior from Iron and Steelmaking Dusts by Microwave Heating, ISIJ Int., 2002, 42, S19–S22 CrossRef CAS.
- M. Omran, T. Fabritius and R. Mattila, Thermally assisted liberation of high phosphorus oolitic iron ore: a comparison between microwave and conventional furnaces, Powder Technol., 2015, 269, 7–14 CrossRef CAS.
- K. E. Haque, Microwave energy for mineral treatment processes-a brief review, Int. J. Miner. Process., 1999, 57(1), 1–24 CrossRef CAS.
- G. Roussy, and J. A. Pearce, Foundations and Industrial Applications of Microwave and Radiofrequency Fields-Physical and Chemical Processes, Wiley, 1995. ch. 10–12 Search PubMed.
- M. Lovas, M. Kovacova, G. Dimitrakis, S. Čuvanova, I. Znamenaćkova and Š. Jakabsky, Modeling of microwave heating of andesite and minerals, Int. J. Heat Mass Transfer, 2010, 53, 3387–3393 CrossRef.
- M. Omran, T. Fabritius, A. Elmahdy, N. A. Abdel-Khalek, M. El-Aref and A. Elmanawi, Effect of microwave pretreatment on the magnetic properties of iron ore and its implications on magnetic separation, Sep. Purif. Technol., 2014, 136, 223–232 CrossRef CAS.
- M. Al-harahsheha, S. Kingman and I. Hamilton, Microwave treatment of electric arc furnace dust with tetrabromobisphenol A: dielectric characterization and pyrolysis-leaching, J. Anal. Appl. Pyrolysis, 2017, 128, 168–175 CrossRef.
- L. Zhang, A. Ma, Ch. Liu, W. Qu, J. Peng, Y. Luo and Y. Zuo, Dielectric properties and temperature increase characteristics of zinc oxide dust from fuming furnace, Trans. Nonferrous Met. Soc. China, 2014, 24, 4004–4011 CrossRef CAS.
- C. Liu, L. Zhang, J. Peng, B. Liu, H. Xia, X. Gu and Y. Shi, Effect of temperature on dielectric property and microwave heating behavior of low grade Panzhihua ilmenite ore, Trans. Nonferrous Met. Soc. China, 2013, 23, 3462–3469 CrossRef CAS.
- J. Yonglin, L. Bingguo, L. Peng, P. Jinhui and Z. Libo, Dielectric Properties and Oxidation Roasting of Molybdenite Concentrate by Using Microwave Energy at 2.45 GHz Frequency, Metall. Mater. Trans. B, 2017, 48B, 3047–3057 CrossRef.
- K. T. Matthew and U. Raveendranath, Cavity perturbation techniques for measuring dielectric parameters of water and other allied liquids, Sens. Update, 2000, 7(1), 185–210 CrossRef.
- S. O. Nelson and P. G. Bartley, Measuring frequency-and temperature-dependent dielectric properties of food materials, IEEE Trans. Instrum. Meas., 2002, 51(4), 589–592 CrossRef.
- M. D. Deshpande, C. J. Reddy, P. I. Tiemsin and R. Cravey, A new approach to estimate complex permittivity of dielectric materials at microwave frequencies using waveguide measurements, IEEE Trans. Microwave Theory Tech., 1997, 45(3), 359–365 CrossRef CAS.
- I. S. Seo, W. S. Chin and D. G. Lee, Characterization of electromagnetic properties of polymeric composite materials with free space method, Compos. Struct., 2004, 66(1–4), 533–542 CrossRef.
- N. I. Sheen and I. M. Woodhead, An open-ended coaxial probe for broad-band permittivity measurement of agricultural products, J. Agric. Eng. Res., 1999, 74(2), 193–202 CrossRef.
- C. A. Pickles, Microwave in extractive metallurgy: part 1 review of fundaments, Miner. Eng., 2009, 22(13), 1102–1111 CrossRef CAS.
- M. Al-Harahsheh, S. Kingman, A. Saeid, J. Robinson, G. Dimitrakis and H. Alnawafleh, Dielectric properties of Jordanian oil shales, Fuel Process. Technol., 2009, 90, 1259–1264 CrossRef CAS.
- S. A. Mikhail and A.-M. Turcotte, Thermal reduction of steel-making secondary materials I basic-oxygen-furnace dust, Thermochim. Acta, 1998, 311, 113–119 CrossRef CAS.
- S. A. Mikhail, A.-M. Turcotte and J. Aota, Thermoanalytical study of EAF dust and its vitrification product, Thermochim. Acta, 1996, 287, 71–79 CrossRef CAS.
- C. Navarro, M. Díaz and M. Villa-García, Physico-Chemical Characterization of Steel Slag. Study of its Behavior under Simulated Environmental Conditions, Environ. Sci. Technol., 2010, 44, 5383–5388 CrossRef CAS PubMed.
- C. A. Pickles, Thermodynamic analysis of the selective carbothermic reduction of electric arc furnace dust, J. Hazard. Mater., 2008, 150, 265–278 CrossRef CAS PubMed.
- R. K. Amankwah, C. A. Pickles and W.-T. Yen, Gold recovery by microwave augmented ashing of waste activated carbon, Miner. Eng., 2005, 18, 517–526 CrossRef CAS.
- M. Tripathi, J. N. Sahu, P. Ganesan and T. K. Dey, effect of temperature on dielectric properties and penetration depth of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS, Fuel, 2015, 153, 257–266 CrossRef CAS.
- N. Standish and W. Huang, Microwave application in carbothermic reduction of iron ores, ISIJ Int., 1991, 31(3), 241–245 CrossRef CAS.
- J. A. Aguilar and I. Gomez, Microwaves applied to carbothermic reduction of iron ore pellets, J. Microwave Power, 1997, 32(2), 67–73 Search PubMed.
- D. A. Jones, T. P. Lelyveld, S. D. Mavrofidis, S. W. Kingman and N. J. Miles, Microwave heating applications in environmental engineering-a review, Conserv. Recycl., 2002, 34, 75–90 CrossRef.
- X. Sun, J. Hwang and X. Huang, The Microwave Processing of Electric Arc Furnace Dust, JOM, 2008, 60(10), 35–39 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |